Int. J. Dev. Biol. 66: 305 - 309 (2022)
Correlation between CDK1 protein and CDK1 mRNA during oocyte maturation in mouse
Short Communication | Published: 2 May 2022
Abstract
The aim of this study was to investigate the correlation between CDK1 protein and CDK1 mRNA during oocyte maturation in vivo in mouse. GV, GVBD, MI and MII oocytes were obtained from mice, respectively. Western blot validated that the CDK1 protein expression increased continuously and significantly with oocyte maturation in vivo (P<0.05). Real-time qRT-PCR showed that CDK1 mRNA expression was down-regulated significantly during transformation from GV to MI stages (P<0.05), and up-regulated significantly during transformation from MI to MII stages (P<0.05). The level of CDK1 mRNA peaked at MII stages. Spearman correlation analysis indicated that CDK1 protein expression was poor correlation with CDK1 mRNA expression during oocyte maturation in vivo (R=0.200). This finding suggested that the increase of CDK1 protein during oocyte maturation in vivo was not entirely caused by the change of transcription level. The results provide new food for thought for further research on the molecular mechanism of oocyte maturation in vivo.
Keywords
mouse, oocyte maturation in vitro (IVM), maturation-promoting factor (MPF), cyclin dependent kinases 1 (CDK1), germinal vesicle breakdown (GVBD)
Introduction
In assisted reproduction, oocyte maturation in vitro (IVM) serves as an alternative therapy to avoid ovarian hyperstimulation syndrome (OHSS). However, due to the lack of understanding of oocyte molecular level, IVM does not fully implement change of nucleus and cytoplasm as physical ovulation stimulation. Studies have shown that maturation in vitro affects gene expression profile of human oocytes, involving several genes that participate in transcriptional regulation, embryogenesis, epigenetics, development and cell cycle (Virant-Klun et al., 2018). The meiotic maturation rates, fertilization rates and blastocyst formation rates of oocytes cultured in vitro were lower than those of oocytes matured in vivo (Khaliliet al., 2013). A number of oocytes cannot be matured in vitro. One needs to understand the regulatory mechanism of oocyte maturation in vivo. Oocyte maturation refers to the meiotic process that takes place from germinal vesicle (GV) stage to metaphase II (MII) stage. The first indication of this process is the disappearance of germinal vesicle as observed under a light microscope, a phenomenon called germinal vesicle breakdown (GVBD). After GVBD, oocytes pass through metaphase of the first meiotic division (MI), followed by the first polar body extrusion and entry into the second meiotic division. Thereafter, oocytes are arrested at metaphase of the second meiotic division until fertilization takes place. GVBD is regulated by the activation of maturation-promoting factor (MPF). MPF is a heterodimer composed of catalytic subunit-dependent cell cycle protein kinase 1 (CDK1, also known as p34cdc2 or cdc2) and regulatory subunit-cell cycle regulatory proteins B (cyclinB) (Gavet and Pines, 2010). The catalytic subunit has the kinase activity, and the regulatory subunit decides the substrate specificity of catalytic subunit.
CDK1, also known as p34cdc2 or cdc2, has a molecular weight of 34 kda and was the first member of the cyclin dependent kinase (CDK) family to be discovered. CDK1 protein plays a crucial role in the acquisition of oocyte meiotic competence (Adhikari et al., 2012). The relocation of CDK1 proteins associated with cyclinB from cytoplasm to geminal vesicle (nuclear) is closely related to geminal vesicle breakdown (GVBD) in oocyte maturation (Mitra and Schultz, 1996). As a catalytic subunit, CDK1 protein induces oocyte maturation by binding with its regulatory subunit cyclinB (Dedieu et al., 1998). In this study, CDK1 protein and CDK1 mRNA levels in GV, GVBD, MI and MII oocytes, respectively, were detected to verify variation tendency and correlation of CDK1 protein and CDK1 mRNA during oocyte maturation in vivo in mouse.
Results
Change of CDK1 protein during oocyte maturation in vivo in mouse
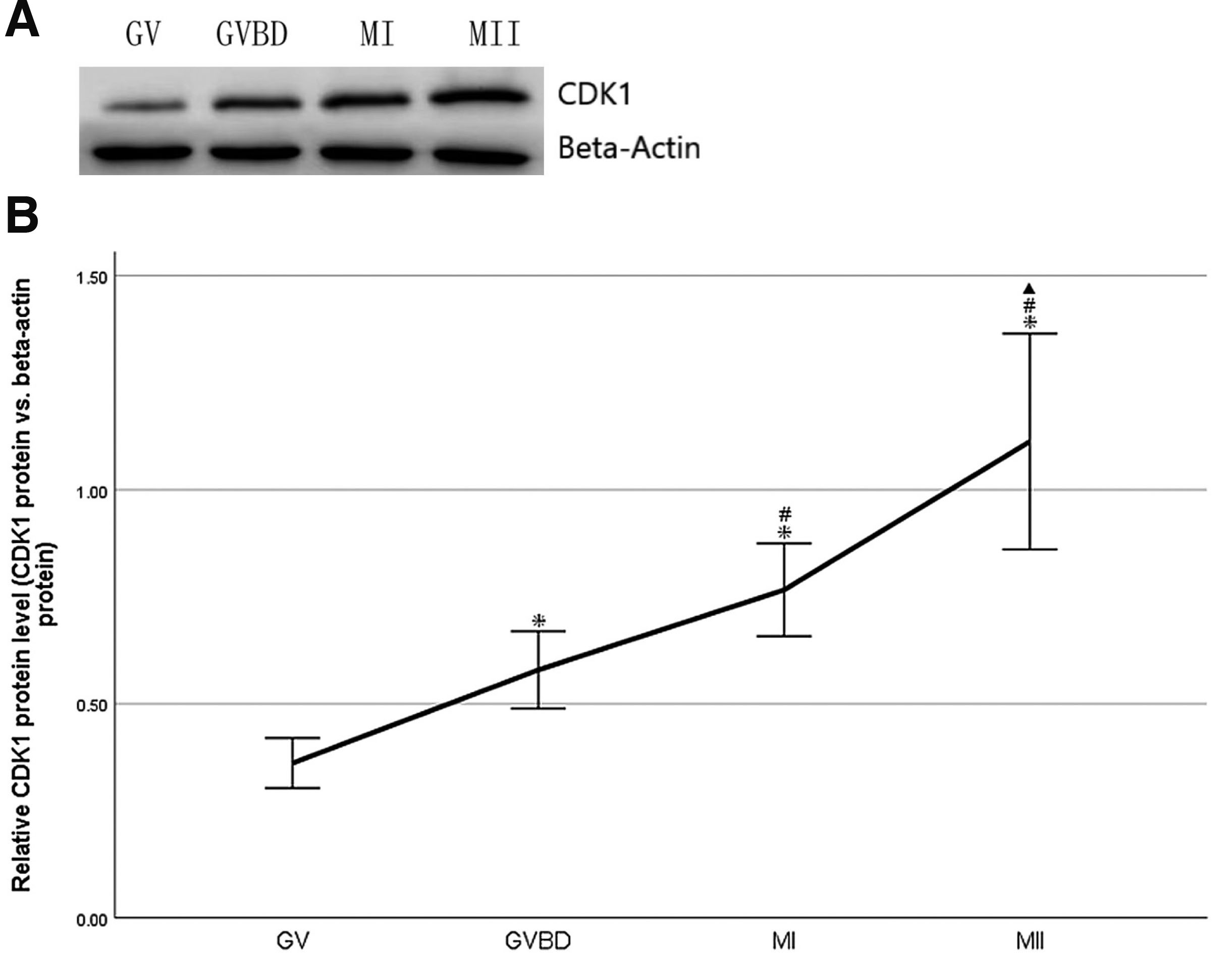
A total of 200 oocytes at GV, GVBD, MI and MII stages, respectively. were collected from mice to study CDK1 protein expression. Beta-actin was used as loading control. Western blot showed that CDK1 protein was expressed at GV, GVBD, MI and MII stages. (Fig. 1A). CDK1 protein expression increased continuously and significantly with oocyte maturation in vivo (P<0.05, Fig. 1B).
Fig. 1. Change of CDK1 protein during oocyte maturation in vivo in mouse.
(A) Expression of CDK1 protein in GV, GVBD, MI and MII oocytes was detected by Western blot. A total of 200 oocytes at GV, GVBD, MI and MII stages, respectively, were collected from mice, and beta-actin was used as loading control. The molecular weights of CDK1 and beta-actin were 34 and 42 kD respectively. (B) Densitometric quantification of CDK1 protein relative to beta-actin was shown. Data were presented as means and standard error of three replicates (*P<0.05: GVBD, MI, MII vs.GV; #P<0.05: MI, MII vs.GVBD; ▲P<0.05: MII vs.MI).
Expression of CDK1 mRNA during oocyte maturation in vivo in mouse
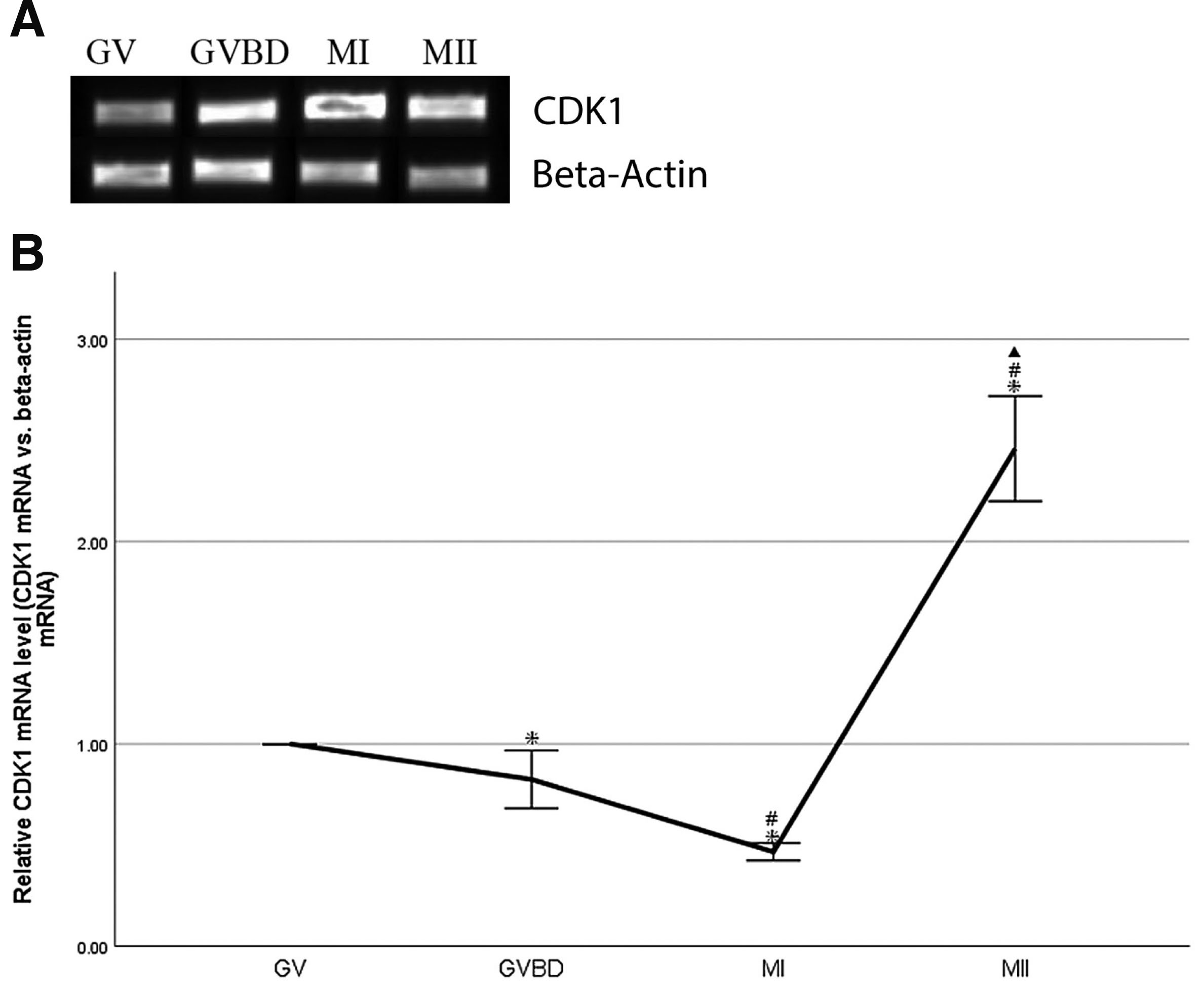
A total of 50–100 oocytes at GV, GVBD, MI and MII stages, respectively, were collected from mice. CDK1 mRNA was analyzed by real-time qRT-PCR. The amplified beta-actin mRNA by real-time qRT-PCR was used as control. CDK1 mRNA was expressed at GV, GVBD, MI and MII stages. (Fig. 2A). CDK1 mRNA expression was down-regulated significantly during transformation from GV to MI stages (P<0.05), and up-regulated significantly during transformation from MI to MII stages (P<0.05, Fig. 2B). The level of CDK1 mRNA peaked at MII stages. (Fig. 2B).
Fig. 2. Expression of CDK1 mRNA during oocyte maturation in vivo in mouse.
(A) CDK1 real-time qRT-PCR products in GV, GVBD, MI and MII oocytes. A total of 50–100 oocytes at GV, GVBD, MI and MII stages, respectively, were collected from mice, and beta-actin was used as loading control. (B) Quantification of CDK1 mRNA relative to beta-actin. Data were presented as means and standard error of three replicates (*P<0.05: GVBD, MI, MII vs.GV; # P<0.05: MI, MII vs.GVBD; ▲P<0.05: MII vs.MI).
Correlation between CDK1 protein and CDK1 mRNA during oocyte maturation in vivo in mouse
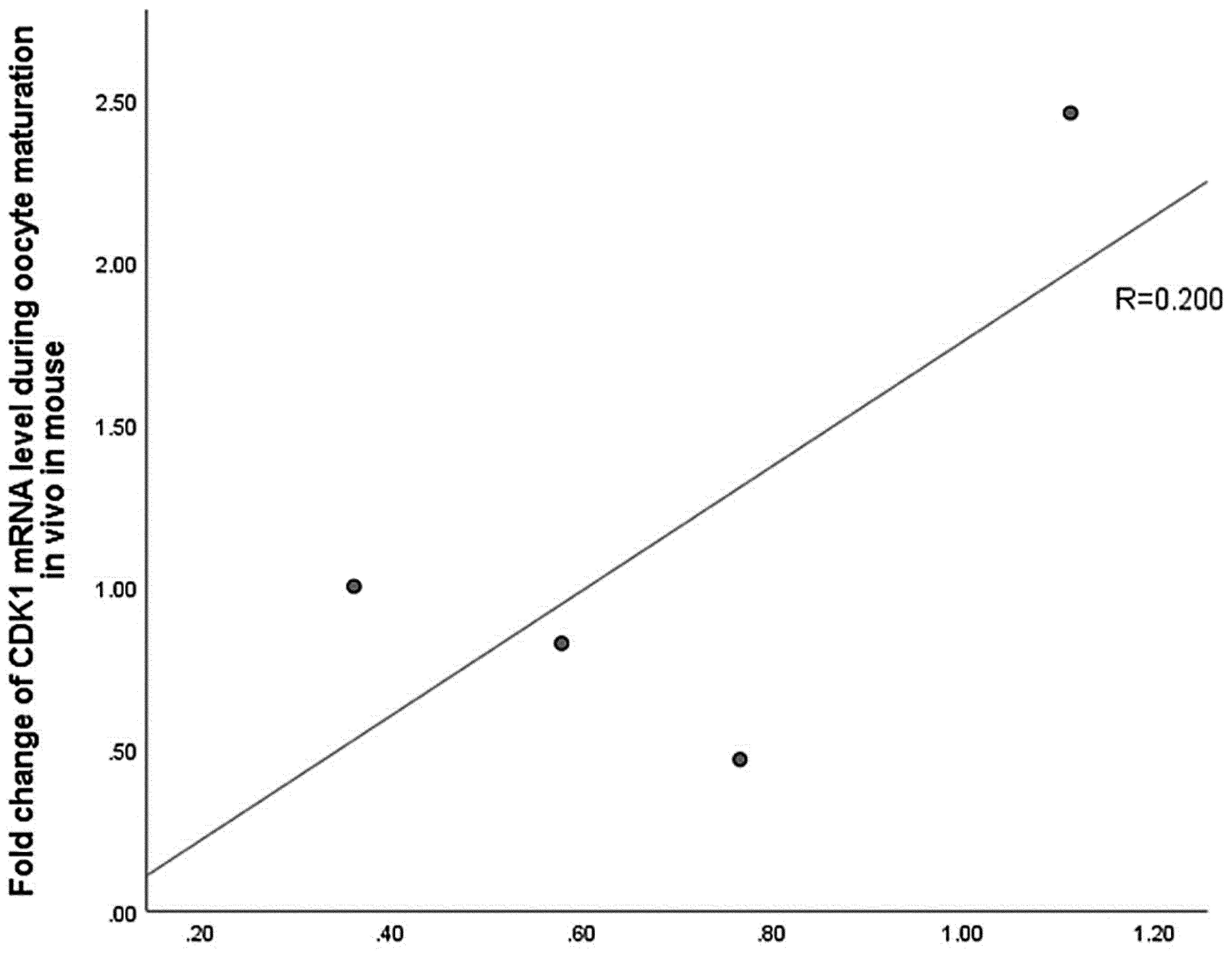
Spearman correlation analysis was used to determine correlation between CDK1 protein and mRNA changes. The results indicated that CDK1 protein expression was poor correlation with CDK1 mRNA expression during oocyte maturation in vivo in mice (R=0.200, Fig. 3). This finding suggested that the increase of CDK1 protein during oocyte maturation in vivo was not entirely caused by the change of transcription level.
Discussion
In the present study, GV, GVBD, MI and MII oocytes, respectively, were collected from hyperovulatory mice in vivo. During transformation from GV to MI stages, CDK1 protein expression increased continuously and significantly. In the meantime, CDK1 mRNA expression was down-regulated continuously and significantly. During transformation from MI to MII stages, CDK1 protein expression increased significantly, and CDK1 mRNA expression was also up-regulated significantly. The accumulation of CDK1 protein and CDK1 mRNA of oocytes peaked at MII stage. Spearman correlation analysis showed that the increase in the amount of CDK1 protein during oocyte maturation was not consistent with the change of CDK1 mRNA. We first demonstrate the dynamic changes of CDK1 protein levels and mRNA levels during oocyte maturation in vivo in mice. The downside of this study is that it does not compare differences between in vivo and in vitro oocyte cultivation.
Several previous studies have identified that CDK1 is essential to drive resumption of meiosis in mouse oocytes. CDK1 maintains the phosphorylation status of protein phosphatase 1 and lamin A/C in oocytes in order for meiosis resumption to occur, and the lack of CDK1 leads to female infertility due to a failure of the resumption of meiosis in the oocyte (Adhikari et al., 2012). Because of the loss of CDK1, Cyclin B1 is destroyed in late prometaphase, while CDK1-bound cyclin B1 is destroyed only during metaphase, ensuring a period of prolonged CDK1 activity sufficient to achieve optimal chromosome alignment and prevent aneuploidy (Levasseur et al., 2019). In 2021, Li et al., demonstrated that the cyclin B2/CDK1 complex conservatively regulates separase activity via inhibitory phosphorylation of separase in both meiosis I and meiosis II of mouse oocytes (Li et al., 2021).
As the oocyte progresses in growth and development, it acquires maternal stores (mRNAs and proteins) that are essential to support the development of the embryo during the early cleavage stages (Li et al., 2010; Zhang and Smith, 2015). In mouse oocytes, the concentration of CDK1 protein increased with the ability to resume and complete meiosis. CDK1 was present at very low levels in incompetent oocytes (50-, 55-, 60-, and 65-mm diameter) and accumulated abruptly in competent oocytes (80-mm diameter) (de Vantéry et al., 1996). Firmani et al., found that the ability of oocytes to mature, and oocyte CDK1 protein levels, were dependent on follicle size, Real-time PCR showed that the levels of CDK1 mRNA were similar in oocytes retrieved from follicles of different sizes (Firmani et al., 2018). Results from a previous study using immunoblotting indicated that the concentration of CDK1 in the incompetent oocyte (obtained from 10-day-old prepubertal mice) was 17.5% of that in the competent oocyte (collected from 6-week-old, PMSG primed female mice). Nevertheless, meiotically incompetent and competent oocytes contained essentially similar concentrations of transcripts, and the relative rate of synthesis of CDK1 in meiotically competent oocytes is approximately three times greater than that in meiotically incompetent oocytes, whereas the stability of newly synthesized CDK1 is essentially the same in each cell type (Mitra and Schultz, 1996). Thus, these changes in protein concentration that occur during oocyte growth likely reflect changes in the translational efficiency of their mRNAs. However, these previous studies only elucidated the changes of CDK1 during GV oocyte growth. In mice, full-grown oocytes are transcriptionally quiescent and mRNAs are remarkably stable in oocytes due to the RNA-binding protein MSY2, which stabilizes mRNAs, and low activity of the 5' and 3' RNA degradation machinery (Svoboda et al., 2015). But the trend of CDK1 mRNA change during oocyte maturation is still unclear.
We indicated that increased transcription is unlikely to account for higher amounts of CDK1 protein in oocytes. Xing et al., reported that Knockdown of the tRNA methytransferase NSun2 decreased the CDK1 protein level, while overexpression of NSun2 elevated it without altering CDK1 mRNA levels. Their studies also revealed that NSun2 enhanced CDK1 translation by methylating CDK1 mRNA in cells and promoted cell growth (Xing et al., 2015). Other regulation mechanisms of CDK1 translation level can be further analyzed.
In conclusion, the accumulation of CDK1 protein and CDK1 mRNA of oocytes peaked at MII stage, and CDK1 protein level may be not correlated with CDK1 mRNA level during oocyte maturation in vivo in mouse. Extensive and profound research is required to gain further insights into oocyte maturation in vivo and in vitro in mice.
Materials and Methods
Acquisition of GV, GVBD, MI and MII oocytes
SPF Female ICR mice aged 6–8 weeks (Laboratory Animal Center, Medical College, Xi'an Jiaotong University) were intraperitoneally injected with 10IU of pregnant equine serum gonado-tropin (PMSG) (Nanjing Aibei Biotechnology Co., Ltd., M2620). GV oocytes were acquired as follows: 48h after being intraperitoneally injected with PMSG, the mice were sacrificed with cervical dislocation. Their ovaries were exposed under sterile conditions. The entire ovary was placed into preheated M2 medium (Nanjing Aibei Biotechnology Co., Ltd., M1250), rinsed twice, and quickly torn into < 1 mm3 pieces using a sterile syringe needle. GV oocytes with the correct size and regular shape were selected under the stereo microscope. Impurities such as granulosa cells were removed mechanically, and GV oocytes were collected into a 1.5 ml sterile centrifuge tube. GVBD oocytes and MI oocytes were acquired respectively as follows: 48h after being intraperitoneally injected with PMSG, the mice were intraperitoneally injected with 10IU of human chorionic gonadotropin (HCG) (Nanjing Aibei Biotechnology Co., Ltd., M2520). Four hours later, GVBD oocytes were collected. Eight hours later, MI oocytes were collected. MII oocytes were acquired as follows: 48h after being intraperitoneally injected with PMSG, the mice were intraperitoneally injected with 10IU of HCG. Twelve hours later, the mice were sacrificed with cervical dislocation. Their fallopian tubes were exposed under sterile conditions and placed into preheated M2 medium, rinsed twice, and quickly torn using a sterile syringe needle. MII oocytes were selected under the stereo microscope. Impurities such as granulosa cells were removed using hyaluronidase (Nanjing Aibei Biotechnology Co., Ltd., M2215), and MII oocytes were collected into a 1.5 ml sterile centrifuge tube.
Western blot analysis
Total protein was extracted from 200 GV, GVBD, MI and MII oocytes, respectively, using 150ul RIPA buffer (Beyotime Biotechnology, P0013B) containing 1 mM PMSF (Beyotime Biotechnology, ST506). BCA Protein Quantification Kit (Multi Sciences, PQ0012) was used to estimate the concentration of each protein sample. Approximately 10μg of protein extracts were fractionated using 10% SDS-PAGE under reducing condition and then transferred into PVDF membranes (Merck Millipore Ltd., R0BB30223). The membranes were then blocked with 5% skimmed milk in TBST (Beyotime Biotechnology, ST673-500ml) for 1h at room temperature. Primary antibodies were diluted in TBST following the manufacturer’s instructions and incubated with the membranes overnight at 4°C. Secondary antibodies were diluted in TBST and incubated with the membranes at room temperature for 30min. An ECL reagent, Meilunbio® fg super sensitive ECL luminescence reagent (Dalian Meilun biotechnology Co., Ltd., MA0186) was used for chemiluminescence signal detection. Images were acquired with the ChemiDocTM Imaging System (Bio-RAD, chemidoc MP), and band intensities were quantified using ImageJ software and normalized to beta actin for each sample. Each experiment was performed in triplicate using different biological replicates.
Antibodies
Antibodies used for this study were purchased from Cell Signaling Technology and Proteintech. CDK1 antibody (Cat.no.77055) were purchased from Cell Signaling Technology and used in 1:1500 dilution. Beta actin Rabbit Polyclonal antibody (Cat.no.20536-1-AP) was obtained from Proteintech and used in 1:7500 dilution. HRP-linked goat anti-rabbit IgG antibody (Cat.no.7074) was purchased from Cell Signaling Technology and used in 1:4500 dilution.
RNA preparation, reverse transcription, and real-time qRT-PCR
Every 50–100 GV, GVBD, MI and MII oocyte was disrupted respectively by adding 350ul Buffer RLT Plus (Qiagen, 74034) including 2mM DTT (BBI, B645939) and 4ng/ul carrier RNA (Qiagen, 74034). Then total RNA was extracted using RNeasy Plus Micro kit (Qiagen, 74034) following the Qiagen official protocol. Total RNA was reverse transcribed into cDNA following the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, RR047A). Reverse transcription primers of CDK1 and beta actin were RT Primer Mix. A negative control for reverse transcription reactions without the reverse transcriptase was carried out to confirm no genomic DNA contamination in cDNA. Real-time qRT-PCR was performed as TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, RR820A) using CFX96 Real-Time PCR Detection System. CDK1 Primers (F:agt tca tgg att ctt cac tcg t, R:gtg tgt aca ctc gta tcg gta t) in real-time qRT-PCR were designed and synthesized by BBI, Beta actin primers as an internal reference in real-time qRT-PCR were purchased from BBI (B661202). The cycling program was set as follows: 95°C, 30s (predenaturing), 1 cycle only; 95°C, 5s (denaturing), 60°C, 30s (annealing and elongating), 40 cycles. Expression of beta actin was used as an endogenous control for CDK1. The amount of mRNA was normalized relative to the amount of beta actin by using the standard 2−ΔΔCt relative expression method. Samples were analyzed in triplicates from three biological replicates. Amplification bands of real-time qRT-PCR products adding InstantViewTM red fluorescence DNA loading buffer (6×, BeyoRed) (Beyotime Biotechnolog, D0081-1ml) were visualized in 2% agarose gel to confirm the specificity.
Statistical analysis
Each experiment was repeated three times using different biological samples which was plotted as mean±standard deviation (mean±SD). All statistical analyses were carried out using SPSS software (version26.0). Differences between four groups were compared by using one-way ANOVA, and p<0.05 was considered to be significant. Spearman correlation coefficient was used for correlation analysis.
Acknowledgements
This research was funded by Yuncheng Central Hospital, Shanxi Province (Project number 2019014). The authors thank Miss Yaoyao An for her help in the western blot experiment.
Abbreviations
CDK, cyclin dependent kinase ; CDK1, dependent cell cycle protein kinase 1 ; cyclinB, cell cycle regulatory proteins B ; GV, germinal vesicle ; GVBD, germinal vesicle breakdown ; HCG, human chorionic gonadotropin ; IVM, maturation in vitro ; MI, metaphase of the first meiotic division ; MII, metaphase II ; MPF, maturation-promoting factor ; OHSS, ovarian hyperstimulation syndrome ; PMSF, Phenylmethanesulfonyl fluoride ; PMSG, pregnant mare serum gonadotropin ;References
Adhikari D., Zheng W., Shen Y., Gorre N., Ning Y., Halet G., Kaldis P., Liu K. (2012). Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Human Molecular Genetics 21: 2476-2484.
de Vantéry C., Gavin A.C., Vassalli J.D., Schorderet-Slatkine S. (1996). An Accumulation of p34cdc2at the End of Mouse Oocyte Growth Correlates with the Acquisition of Meiotic Competence. Developmental Biology 174: 335-344.
Dedieu T., Gall L., Hue I., Ledan E., Crozet N., Ruffini S., Sevellec C. (1998). p34cdc2 expression and meiotic competence in growing goat oocytes. Molecular Reproduction and Development 50: 251-262.
Firmani L. D., Uliasz T. F., Mehlmann L. M. (2018). The switch from cAMP-independent to cAMP-dependent arrest of meiotic prophase is associated with coordinated GPR3 and CDK1 expression in mouse oocytes. Developmental Biology 434: 196-205.
Gavet O., Pines J. (2010). Progressive Activation of CyclinB1-Cdk1 Coordinates Entry to Mitosis. Developmental Cell 18: 533-543.
Khalili M. A., Nottola S. A., Shahedi A., Macchiarelli G., (2013). Contribution of human oocyte architecture to success of in vitro maturation technology. Iranian journal of reproductive medicine 11: 1-10.
Levasseur M. D., Thomas C., Davies O. R., Higgins J. M.G., Madgwick S. (2019). Aneuploidy in Oocytes Is Prevented by Sustained CDK1 Activity through Degron Masking in Cyclin B1. Developmental Cell 48: 672-684.e5.
Li J., Zhang H.Y., Wang F., Sun Q.Y., Qian W.P. (2021). The Cyclin B2/CDK1 Complex Conservatively Inhibits Separase Activity in Oocyte Meiosis II. Frontiers in Cell and Developmental Biology 9: 648053.
Li L., Zheng P., Dean J. (2010). Maternal control of early mouse development. Development 137: 859-870.
Mitra J., Schultz R.M. (1996). Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. Journal of Cell Science 109: 2407-2415.
Svoboda P., Franke V., Schultz R. M. (2015). Sculpting the Transcriptome During the Oocyte-to-Embryo Transition in Mouse. In The Maternal-to-Zygotic Transition. Elsevier.
Virant-Klun I., Bauer C., Ståhlberg A., Kubista M., Skutella T. (2018). Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reproductive BioMedicine Online 36: 508-523.
Xing J., Yi J., Cai X., Tang H., Liu Z., Zhang X., Martindale J. L., Yang X., Jiang B., Gorospe M., Wang W. (2015). NSun2 Promotes Cell Growth via Elevating Cyclin-Dependent Kinase 1 Translation. Molecular and Cellular Biology 35: 4043-4052.
Zhang Kun, Smith George W., (2015). Maternal control of early embryogenesis in mammals. Reproduction, Fertility and Development 27: 880.