Int. J. Dev. Biol. 68: 241 - 249 (2024)
Special Issue: Developmental Biology in Nordic Countries
Knock-in of a 3’ UTR Stop Cassette into the Wnt4 locus increases mRNA expression and leads to ovarian cyst formation
Open Access | Original Article | Published: 4 November 2024
Abstract
Wnt4 signaling is critical for mammalian female sex determination, in female reproductive organ development, in follicular and oocyte maturation, and in steroid hormone production. When Wnt4 function is impaired, female embryos undergo partial female to male sex-reversal. This phenotype is associated with the expression of a set of somatic genes that are typical for the male differentiation pathways such as those of the Leydig cells. Given the roles of the 3`untranslated region (3`UTR) in control of gene expression, we addressed whether a knock-in of a stop cassette to 3`END of the Wnt4 gene would impact female reproductive system development or function. The 3`UTRstop cassette indeed affected Wnt4 gene expression in vivo so that the respective mRNA was upregulated in the ovaries of a three month-old female. The homozygous Wnt4 3`UTRstop mice were noted to be leaner than their wild type (WT) littermate controls. Analysis of the ovarian follicular count at the age of three months revealed increased pre-antral but reduced ovarian corpus luteum follicular counts. Furthermore, two out of five of the homozygous female Wnt4 3`UTRstop mice had ovarian cysts, not noted in WT controls. RT-qPCR and in situ hybridization analysis depicted changes in the expression of a panel of genes which encode enzymes that mediate the synthesis of female steroid hormones or their receptors due to the Wnt4 3`UTRstop knock-in. Thus, female mice which had the homozygous construct exhibited elevated ovarian Wnt4 mRNA expression and the corresponding knock-in was associated with changes in ovarian development and folliculogenesis. Our data reinforce the conclusion that deregulated Wnt4 expression impacts female sex organogenesis, ovary development and function, and that the Wnt4 3`UTRstop knock-in mouse provides a model to explore in more detail the roles of Wnt4 signaling in the process.
Keywords
female fertility, folliculogenesis, steroid hormone synthesis, anovulatory follicle, germ line, meiosis
Introduction
The signals in the Wnt family are involved in the ontogenesis of many organs during embryogenesis, but they exhibit also functions in the adult, such as control of tissue regeneration via stem cells involved in the maintenance of homeostasis (Liu et al., 2022; Nusse and Clevers, 2017). As secreted signaling type of molecules, the Frizzled receptor bound Wnts trigger in part beta-catenin driven downstream signal transduction cascades, which is involved in many processes such as cell differentiation, cell proliferation, cell polarity, and tissue regeneration (Zhang et al., 2021). Deregulated Wnts or their downstream signal transduction components associate with a variety of diseases such as melanoma, leukemia, and breast cancer (Zhan et al., 2017), lung (Guo et al., 2015; Aros et al., 2021), cardiovascular (Foulquier et al., 2018; Akoumianakis et al., 2022; Gay and Towler, 2017), and bone diseases (Baron and Kneissel, 2013; Diegel et al., 2023). These phenotypes point roles for Wnt signaling in control of stemness, metastasis, and inflammation in these diseases.
Of the Wnt family members, Wnt4 has been studied extensively and shown to be functional in several organ systems (Zhang et al., 2021). Wnt4 the first identified signal that was connected to the mammalian female sex determination process (Vainio et al., 1999). In a female indifferent gonad, Wnt4 contributes to femaleness by promoting differentiation of the ovarian estrogen producing female follicular cells and development of the Müllerian duct coordinated by anti- Müllerian hormone signaling. Such evidence on how Wnt4 regulates female ovarian development was based on analyzing the female embryos that were deficient for Wnt4 function in a knockout mouse model. The data showed that the female germ cells, and consequently the oocytes underwent apoptosis later in female sexual development (Vainio et al., 1999). In addition to the germ line cell differentiation, Wnt4 regulated polarity of the follicular cells, basement membrane dynamics, anti-Müllerian hormone expression, and meiosis together with the Wnt5a signaling (Prunskaite-Hyyrylainen et al., 2014; Naillat et al., 2010). Compromised Wnt4 signaling impairs follicle development and leads to premature ovarian failure (Naillat et al., 2010).
Developmental gene expression control involves the untranslated regions (UTRs) in the respective mRNA species. The UTR differ in their sequence length but typically they contain binding sites for the miRNA regulatory elements (Bartel, 2009; Hoffman et al., 2016; Mayr, 2017). Due to this reason, the UTR sequences can contribute to stability of mRNA and impact translation (Berkovits and Mayr, 2015; Ma and Mayr, 2018). The UTRs are connected to such processes as cell proliferation (Sandberg et al., 2008; Gruber et al., 2014), inflammation (Bergant et al., 2023; Jia et al., 2017), and organogenesis (Chekulaeva et al., 2006; Freimer et al., 2018; Yang et al., 2017; Marshall et al., 2021).
To target further the roles of Wnt4 in female sex organogenesis, we generated a novel allele by inserting a specific stop cassette into the gene locus. This cassette was expected to prevent the 3`UTR function to control Wnt4 gene expression. The results depicted that insertion of the Wnt4 3`UTR stop cassette was noted to result in an elevation in Wnt4 mRNA expression in the ovarian follicular cells. Analysis of the Wnt4 3`UTR edited females depicted phenotypes in their sex organs. These data supported a regulatory role for Wnt4 in female folliculogenesis. This seems to occur via control of a panel of genes that are involved in the synthesis of female sex hormones. We conclude that the Wnt4 3`UTRstop mouse may offer a novel model to investigate the impact of the gain of Wnt4 signaling for female fertility.
Results
The Wnt4 3`UTR genomic stop cassette insertion compromises post-natal female body weight
To test whether the inactivation of the 3`UTR stop cassette in the Wnt4 locus would deregulate the protein action, Wnt4 3`UTRstop transgenic mice were made, and bred as depicted in the methods section. The number of homozygous, heterozygous and wild-type (WT) mice obtained from the Wnt4 3`UTRstop and WT genetic crossings followed the Mendelian ratios (Table 1). The values were 24% for the Wnt4 3`UTRstop/Wnt4 3`UTRstop, 53% for Wnt4 3`UTRWT/stop, and 23% for Wnt4 3`UTRWT/WT in line with the reported roles of Wnt4 signaling function during embryogenesis (Zhang et al., 2021; Bartel, 2009; Hoffman et al., 2016; Mayr, 2017; Berkovits and Mayr, 2015; Ma and Mayr, 2018; Sandberg et al., 2008).
Table 1
Mendelian inheritance trail.
| WT | Wnt4 3`UTRWT; Wnt4 3`UTRstop |
Wnt4 3`UTRstop; Wnt4 3`UTRstop |
Total | |
|---|---|---|---|---|
| Female | 8 | 21 | 15 | 54 |
| Male | 8 | 40 | 15 | 63 |
| Total | 26 | 61 | 30 | 117 |
Data were collected from 17 litters. WT refers to wild type genotype, Wnt4 3`UTRWT;Wnt4 3`UTRstop refers to heterozygous genotype, and Wnt4 3`UTRstop;Wnt4 3`UTRstop refers to homozygous genotype.
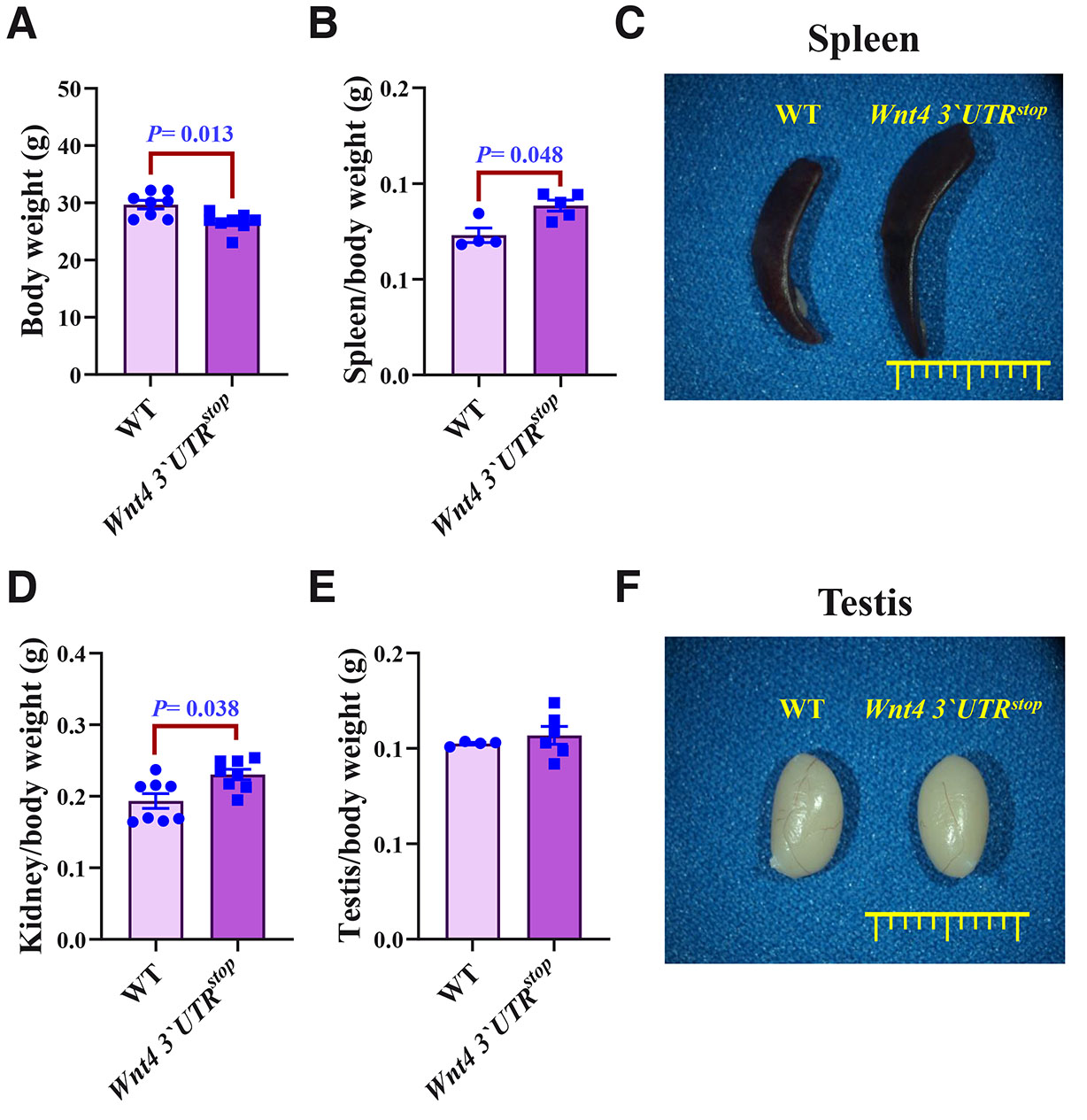
We then assessed from the Wnt4 3`UTRstop intercrosses the overall weight of the mice and their organs. Selected organs such as the kidney, the testis, and the spleen were dissected, and their weight was measured. The overall body weight of the Wnt4 3`UTRstop; Wnt4 3`UTRstop mice was reduced when compared to WT mice (26.18±2.34g versus 29.79±1.27g, respectively; n=8) (Fig. 1A). The spleen and the kidneys of the homozygous Wnt4 3`UTRstop mice were heavier than their normal WT controls being 88.50±6.50 mg / 73.05±7.61 mg and 230.34±20.76 mg / 193.50±30.23 mg, respectively (Fig. 1 B-D). No differences in the weight of the testis of the Wnt4 3`UTRstop and WT mice were noted (Fig. 1 E,F). We conclude that the weight of the homozygous Wnt4 3`UTRstop mice was altered in those organs where Wnt4 has been reported to be functional (Naillat et al., 2010; Vainio et al., 1999; Prunskaite-Hyyryläinen et al., 2016).
Fig. 1. Genetic modification of Wnt4 3`UTR leads to morphological manifestations in organ development.
(A) Total body weight of Wnt4 3`UTRstop;Wnt4 3`UTRstop and WT mice. Data are presented as means ± SEM, n=8 (WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop). (B) Spleen/body weight in Wnt4 3`UTRstop;Wnt4 3`UTRstop and WT mice. Data are presented as means ± SEM, n=4 (WT) and n=5 (Wnt4 3`UTRstop;Wnt4 3`UTRstop). (C) Morphological examination of spleen size in Wnt4 3`UTRstop;Wnt4 3`UTRstop and WT mice. (D) Kidney/body weight in Wnt4 3`UTRstop;Wnt4 3`UTRstop compared to WT mice. Data are presented as means ± SEM, n=8 (WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop). (E) Testis/body weight in Wnt4 3`UTRstop;Wnt4 3`UTRstop versus WT mice. Data are presented as means ± SEM, n=4 (WT) and n=6 (Wnt4 3`UTRstop;Wnt4 3`UTRstop). (F) Morphological examination of kidney size in Wnt4 3`UTRstop;Wnt4 3`UTRstop and WT mice. The two-tailed Student test was used for statistical analysis in (A, B, D, and E). P≤ 0.05 was considered statistically significant.
The Wnt4 3`UTR genomic stop cassette insertion compromises Wnt4 mRNA expression in ovary
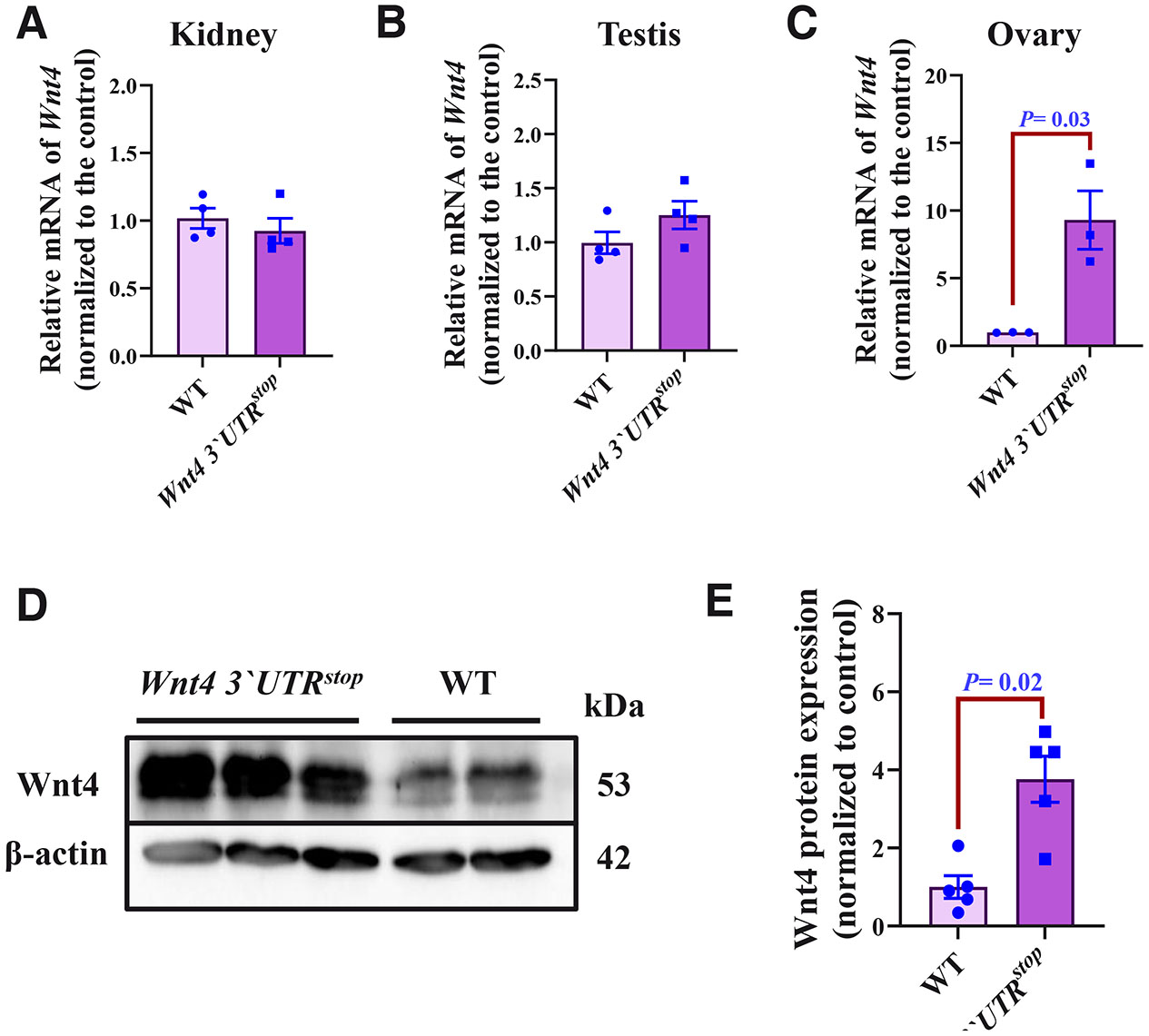
To assess the putative consequences of the inserted 3`UTR stop cassette into the Wnt4 locus, we examined possible changes in the Wnt4 mRNA expression by the RT-QPCR. Messenger RNAs were extracted from the dissected kidney, testis, and ovaries that were prepared from two to three months old mice. Analysis of the Wnt4 mRNA expression level in the kidney of the homozygous Wnt4 3`UTRstop remained unchanged when compared to control (Fig. 2A), while there was a trend for increased Wnt4 expression in the testis (Fig. 2B). A prominent difference in Wnt4 mRNA expression was observed in the ovaries of the Wnt4 3`UTRstop;Wnt4 3`UTRstop females compared to WT female mice samples (Fig. 2C).
Fig. 2. Modification of the Wnt4 3`UTRstop allele results in activation of Wnt4 expression in the ovary of two-three months old mice.
(A-C) Quantification of Wnt4 mRNA by RT-QPCR in kidney (A), testis (B), and ovaries (C). Data are shown as averages after normalization to the control ± SEM, n=4 (WT) and n=3 (Wnt4 3`UTRstop;Wnt4 3`UTRstop); each sample was run in four biological replicates. (D) Western blot Wnt4 analysis of three month-old WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop ovaries. (E) Quantification of Wnt4 protein expression levels in three month-old WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop. The results are shown as averages after normalization to the control ± SEM, n=5 from both genotypes. The two-tailed Student test was used for statistical analysis in (A-E). P≤ 0.05 was considered statistically significant.
To substantiate the obtained results, in situ hybridization approach was applied on the kidney, the testis, and the ovaries of three months old mice. In line with the above RT-QPCR data, no differences in the Wnt4 mRNA expression were observed in the kidney and the testis sections (data not shown), whereas increased Wnt4 mRNA expression was evident in the ovary of the Wnt4 3`UTRstop;Wnt4 3`UTRstop females when compared to WT female samples (Suppl. Fig. 2).
We explored a possible impact of the Wnt4 3`UTRstop knock-in on the Wnt4 protein expression in the ovaries of three month-old female mice in comparison to controls. Western blotting with an anti-Wnt4 antibody revealed, as was the case with the Wnt4 mRNA expression, an increase in the protein level. This was evident also later in the ovaries of three months Wnt4 3`UTRstop knock-in females, compared to ovaries of WT control (Fig. 2 D,E). Together the results suggest that the Wnt4 3`UTRstop knock-in leads to elevated expression of Wnt4 mRNA and protein in the ovary of two to three months old mice.
The Wnt4 3`UTR genomic stop cassette insertion compromises ovarian follicle maturation and female fertility
Given our prior data demonstrating a key role for Wnt4 signaling in the development and function of the female reproductive organs (Vainio et al., 1999; Prunskaite-Hyyrylainen et al., 2014; Naillat et al., 2010; Prunskaite-Hyyryläinen et al., 2016; Veiga-Lopez et al., 2012), and that Wnt4 mRNA expression becomes upregulated in oogenesis, we started to study if the Wnt4 3`UTRstop knock-in allele would impact female fertility.
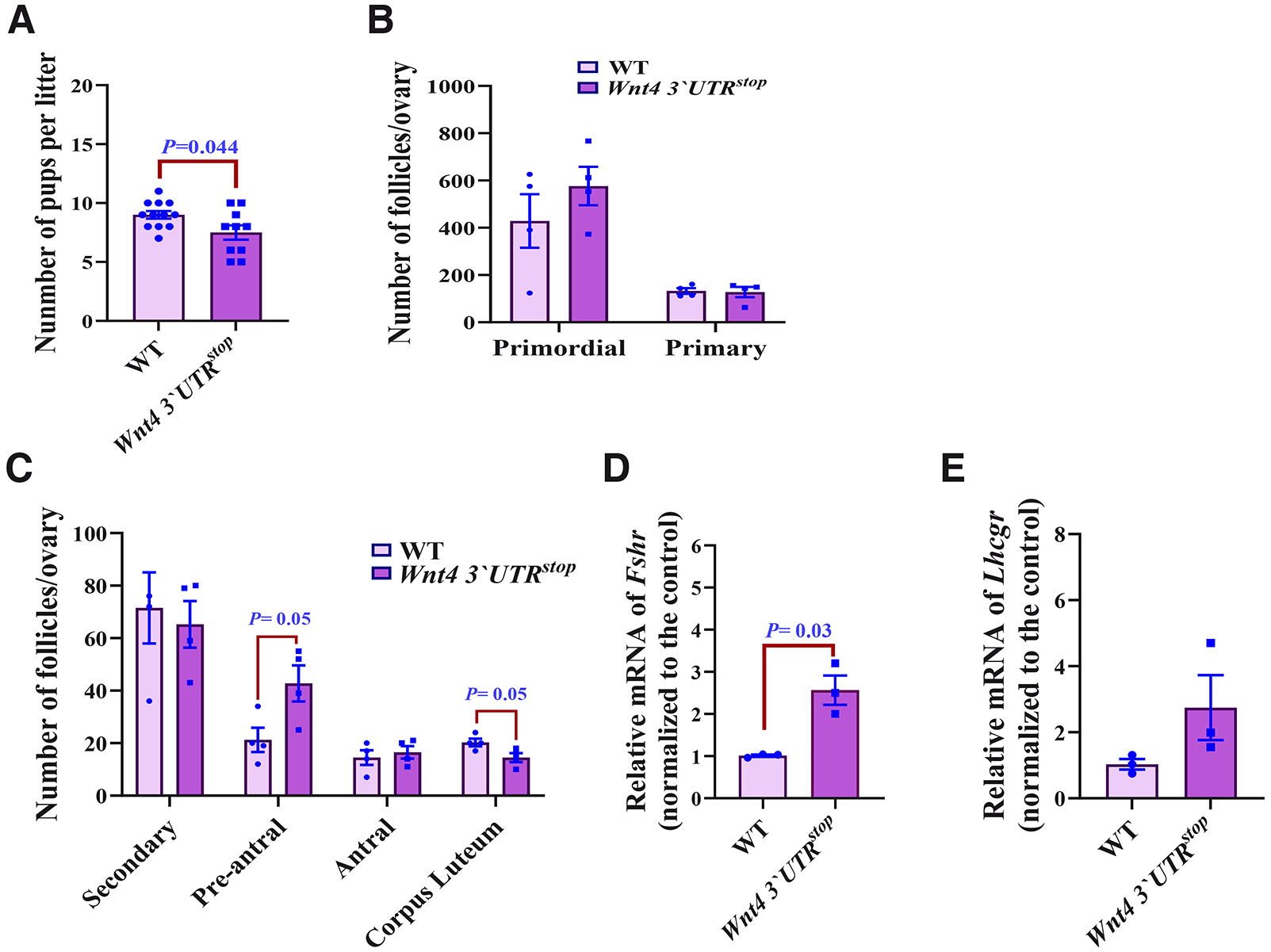
The Wnt4 3`UTRstop;Wnt4 3`UTRstop females and males were crossed with WT mice and the liter sizes were monitored and compared with WT mice breeding. The results revealed that while the average litter size of the bred WT females and males was 9.0±1.1 (n=12), the corresponding number of the Wnt4 3`UTRstop;Wnt4 3`UTRstop female and WT males was 7.5 ± 1.9 (n=10) (Fig. 3A). As expected, no differences in the mean litter size were noted in the crosses of Wnt4 3`UTRstop;Wnt4 3`UTRstop males to WT females being 7.5 ± 1.9 (n=10) (Suppl. Fig. 3).
Fig. 3. Wnt4 gain of function compromises female fertility.
(A) Number of pups per litter. Data are presented as means ± SEM, n=12 (WT) and n=10 (Wnt4 3`UTRstop;Wnt4 3`UTRstop). (B,C) Follicle counts in WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop, n=4 from both genotypes. Data are presented as means ± SEM. (D) Quantification of Fshr mRNA by RT-QPCR. The results are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. (E) Quantification of Lhcgr mRNA by RT-qPCR. The results are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. The two-tailed Student test was used for statistical analysis in (A-E). P≤ 0.05 was considered statistically significant.
We then counted the number of ovarian follicles based on their maturation stage in the Wnt4 3`UTRstop;Wnt4 3`UTRstop females and WT ones. The follicular counts were the following: the primordial; 576.5±110.1/530.3±227, the primary; 138.6±24.14/127.75±5.5, the secondary; 65.255±17.7/83.3±27.1, and the antral; 16.5±4.7/17±5.6, suggesting no differences between the two genotypes (Fig. 3 B,C). A significant increase, however, in the pre-antral follicle counts 42.7±13.7/24.3±9.2 was accompanied by a reduction in the corpus luteum follicle count 14.5±3.4/20.3±2.8 was noted in Wnt4 3`UTRstop;Wnt4 3`UTRstop when compared to values of WT females (Fig. 3C). We conclude that the Wnt4 3`UTRstop;Wnt4 3`UTRstop genotype impacts ovarian folliculogenesis.
The Wnt4 3`UTR genomic stop cassette insertion compromises the expression of genes involved in ovarian steroid hormone synthesis
We next explored the molecular mechanisms behind the noted changes in ovarian folliculogenesis process. Given the roles of Wnt4 signaling in control of genes that encode for steroid hormone synthesizing enzymes, we assayed putative changes in their expression due to the 3`UTRstop knock-in in the mice. We studied possible changes in the expression of the luteinizing hormone receptor (Lhcgr) and the follicle-stimulating hormone receptor (Fshr) genes by RT-QPCR.
The results revealed that expression of the Fshr mRNA was significantly increased in the ovary of the Wnt4 3`UTRstop;Wnt4 3`UTRstop as compared to WT samples (Fig. 3D). Furthermore, a trend of increase in the expression of Lhcgr mRNA was observed (Fig. 3E).
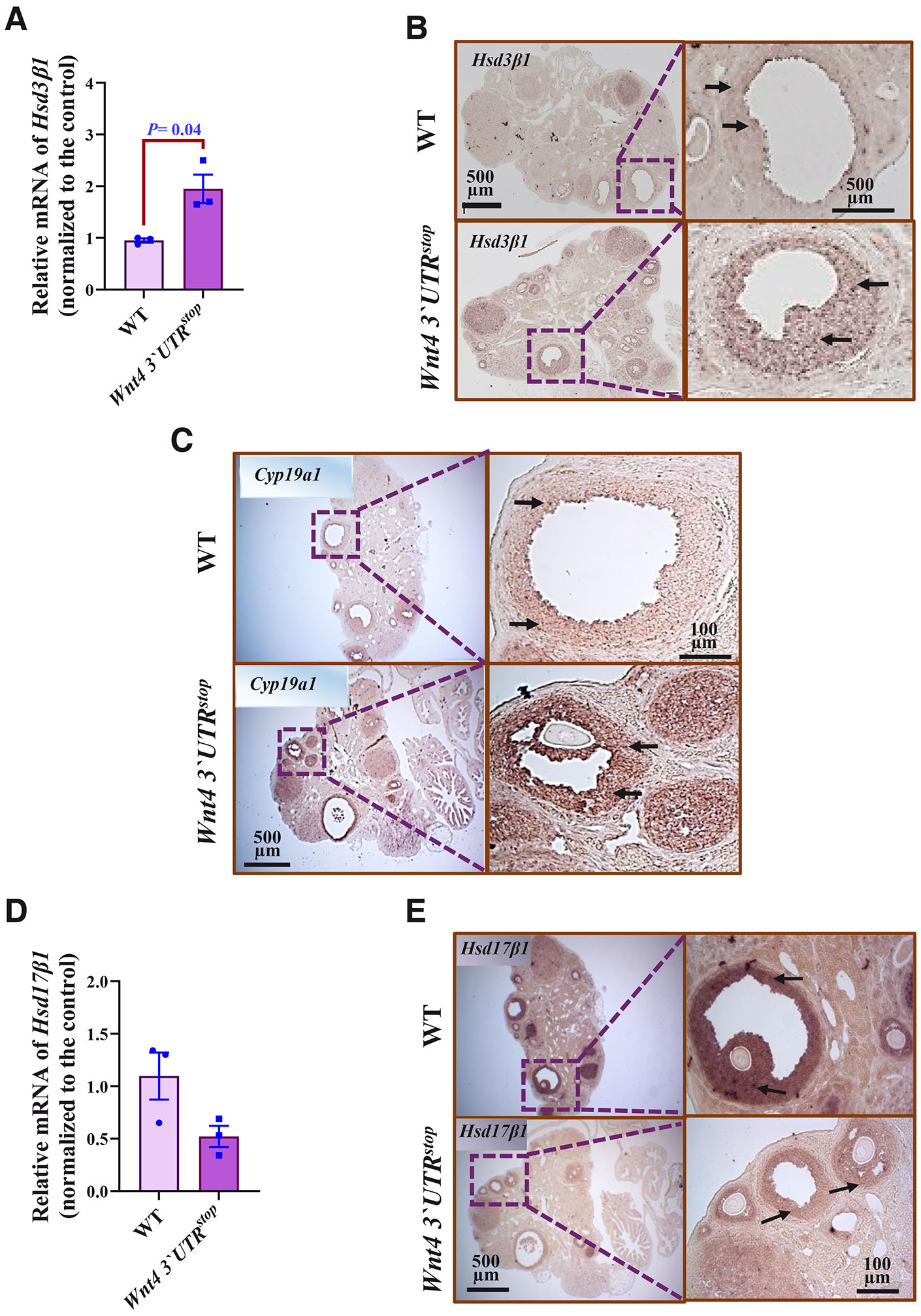
Wnt4 signaling regulates genes that encode steroid hormone synthesis enzymes in the ovary as well (Vainio et al., 1999; Prunskaite-Hyyrylainen et al., 2014; Naillat et al., 2010). Based on this knowledge we conducted RT-QPCR and in situ hybridization to study if the Wnt4 3`UTR knock-in would be associated with changes in the expression of the 3-beta-hydroxysteroid dehydrogenase (Hsd3b1), aromatase (Cyp19a1), and hydroxysteroid 17-beta dehydrogenase-1 (Hsd17b1).
The RT-QPCR studies demonstrated an increase of the Hsd3b1 mRNA expression in the ovaries of the Wnt4 3`UTRstop;Wnt4 3`UTRstop females when compared to control mice (Fig. 4A). Examining the in situ hybridization images, a stronger signal of Hsd3b1 mRNA in the granulosa cells of the Wnt4 3`UTRstop homozygous mice in comparison to that detected in control mice was observed (Fig. 4B). Similarly, Cyp19a1 mRNA expression had changed due to the Wnt4 3`UTR knock-in and increased in the homozygous female samples (Fig. 4C).
Fig. 4. Wnt4 3`UTRstop;Wnt4 3`UTRstop alters gene expression related to hormone synthesis in the ovary.
(A) Quantification of Hsd3b1 mRNA by RT-qPCR. Data are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. (B) Hsd3b1 mRNA in situ hybridization (brown color, black arrows) of three month-old WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop ovaries. Scale bars, 500 µm. (C) Cyp19a1 mRNA in situ hybridization (brown color, black arrows) of three month-old WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop ovaries. Scale bars, 500 and 100 µm. (D) Quantification of Hsd17b1 mRNA by RT-QPCR. Data are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. (E) Hsd17b1 mRNA in situ hybridization (brown color, black arrows) of three month-old WT and Wnt4 3`UTRstop;Wnt4 3`UTRstop ovaries. Scale bars, 500 and 100 µm. The two-tailed Student test was used for statistical analysis in (A,D). P≤ 0.05 was considered statistically significant.
Of the three analyzed hormone synthesis associated genes, the Hsd17b1 was the only one whose expression appeared to be decreased in the ovaries of Wnt4 3`UTRstop;Wnt4 3`UTRstop mice when compared to WT control (Fig. 4D). This result was reinforced with the in situ hybridization analysis, which revealed a diminished signal of the Hsd17b1 mRNA expression in the granulosa cells of the heterozygous compared to WT female samples (Fig. 4E).
Together, the data support a role of Wnt4 signaling in control of the genes that encode steroid hormone synthesis in the ovary, critical for maturation of the ovarian follicular complex.
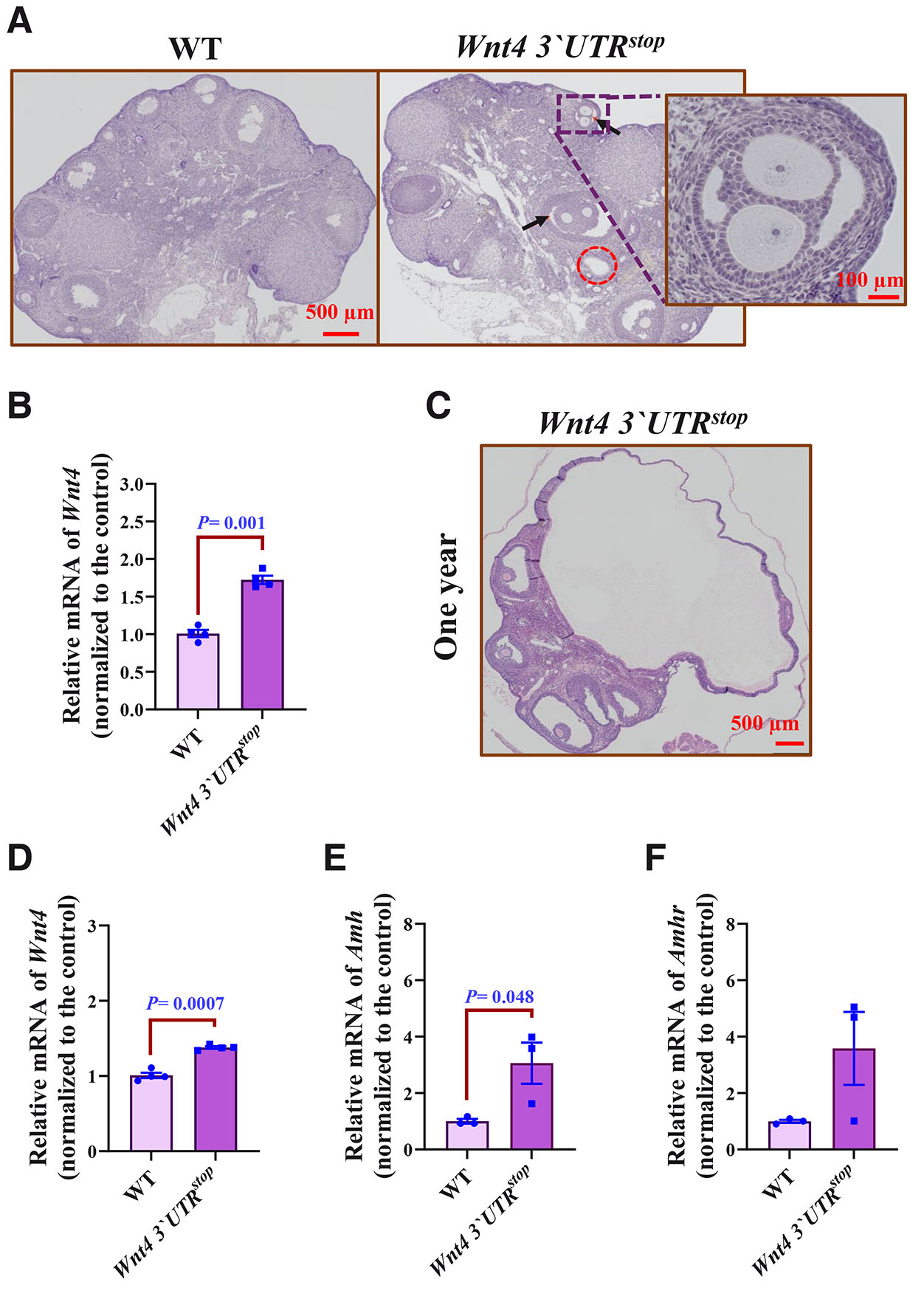
Impaired Wnt4 3`UTR signaling leads to ovarian cyst formation
Given the observed ovarian changes brought by the knock-in expression of the Wnt4 gene by the 3`UTRstop cassette, we focused our attention on monitoring the ovary development of aged females. Histological examination of the ovaries dissected from the Wnt4 3`UTRstop;Wnt4 3`UTRstop females at the age of six months revealed anovulatory follicles containing two oocytes, not noted in control female mice (Fig. 5A). This was associated with a significant elevation of Wnt4 mRNA expression in the females at the age of six months (Fig. 5B). In ovaries derived from one-year-old females 2/5 samples depicted enlarged cysts that were not noted in ovaries of control females (Fig. 5C). As was the case in the females at the age of three and six months, the RT-QPCR analysis demonstrated upregulated Wnt4 mRNA expression at the age of one year when compared with WT female controls (Fig. 5D).
Fig. 5. Wnt4 gain of function enhances cyst formation in the ovary.
(A) Histological examination using eosin/hematoxylin staining demonstrating poly-ovular follicle formation (black arrows) and cyst formation (dotted red circle) in six month-old Wnt4 3`UTRstop;Wnt4 3`UTRstop mice compared with WT. Scale bars, 500 and 100 µm. (B) Quantification of Wnt4 mRNA by RT-qPCR in ovaries of six month-old mice. Data are shown as averages after normalization to the control ± SEM, n=4 from both genotypes. (C) Histological examination using eosin/hematoxylin staining demonstrating cyst size in one year-old Wnt4 3`UTRstop;Wnt4 3`UTRstop ovary. Scale bars, 500 µm. (D) Quantification of Wnt4 mRNA by RT-qPCR in ovaries of twelve month-old mice. Data are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. (E) Quantification of Amh mRNA by RT-qPCR in six month-old mice. Data are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. (F) Quantification of Amhr mRNA by RT-qPCR in six month-old mice. Data are shown as averages after normalization to the control ± SEM, n=3 from both genotypes. The two-tailed Student test was used for statistical analysis in (B) and (D-F). P≤ 0.05 was considered statistically significant.
Wnt4 knock-out has been reported to increase anti-Müllerian hormone levels and gene expression (Prunskaite-Hyyryläinen et al., 2016) in line with the anti-Müllerian hormone as a biomarker of ovary cyst formation (Butt et al., 2022; Oh et al., 2019; Sivanandy and Ha, 2023). Given this, we set out to investigate the putative association of the anti-Müllerian hormone gene (Amh) and the anti-Müllerian hormone receptor (Amhr) expression in the aged mice using RT-QPCR approach. The results demonstrated an increase in expression of the Amh and Amhr genes in the ovaries of the Wnt4 3`UTRstop;Wnt4 3`UTRstop females at the age of six month when compared to controls (Fig. 5 E,F). Thus the anti-Müllerian hormone may be involved in formation of the noted ovarian cysts in the Wnt4 3`UTRstop;Wnt4 3`UTRstop females.
Together, the findings with the novel Wnt4 3`UTRstop;Wnt4 3`UTRstop mouse model provide further evidence for an important role of coordinated regulation of Wnt4 signaling in female sexual development also later in the female reproductive life cycle.
Discussion
In this study, we report a novel mouse model, in which the Wnt4 gene was targeted by inserting a stop cassette in front of the 3`UTR. Such a knock-in mouse model was generated successfully. Analysis of the Wnt4 3`UTRstop cassette knock-in females provided evidence that the level of Wnt4 mRNA and protein expression had become elevated as consequence of the UTR stop cassette insertion into the locus. The examination of the Wnt4 3`UTRstop homozygous mice suggested that the females developed normally but they were smaller in size when compared to WT mice and exhibited increased spleen and kidney weight. Wnt4 serves as a key signal for nephrogenesis during kidney development (Shan et al., 2010; Zhao et al., 2016; DiRocco et al., 2013; He et al., 2018). In the Wnt4 3`UTRstop;Wnt4 3`UTRstop mice, no notable kidney associated phenotypes were found when compared to samples derived from WT controls. This was also the case with the testis. Thus, the 3`UTR mediated regulation on Wn4 expression may not be functional for these organs.
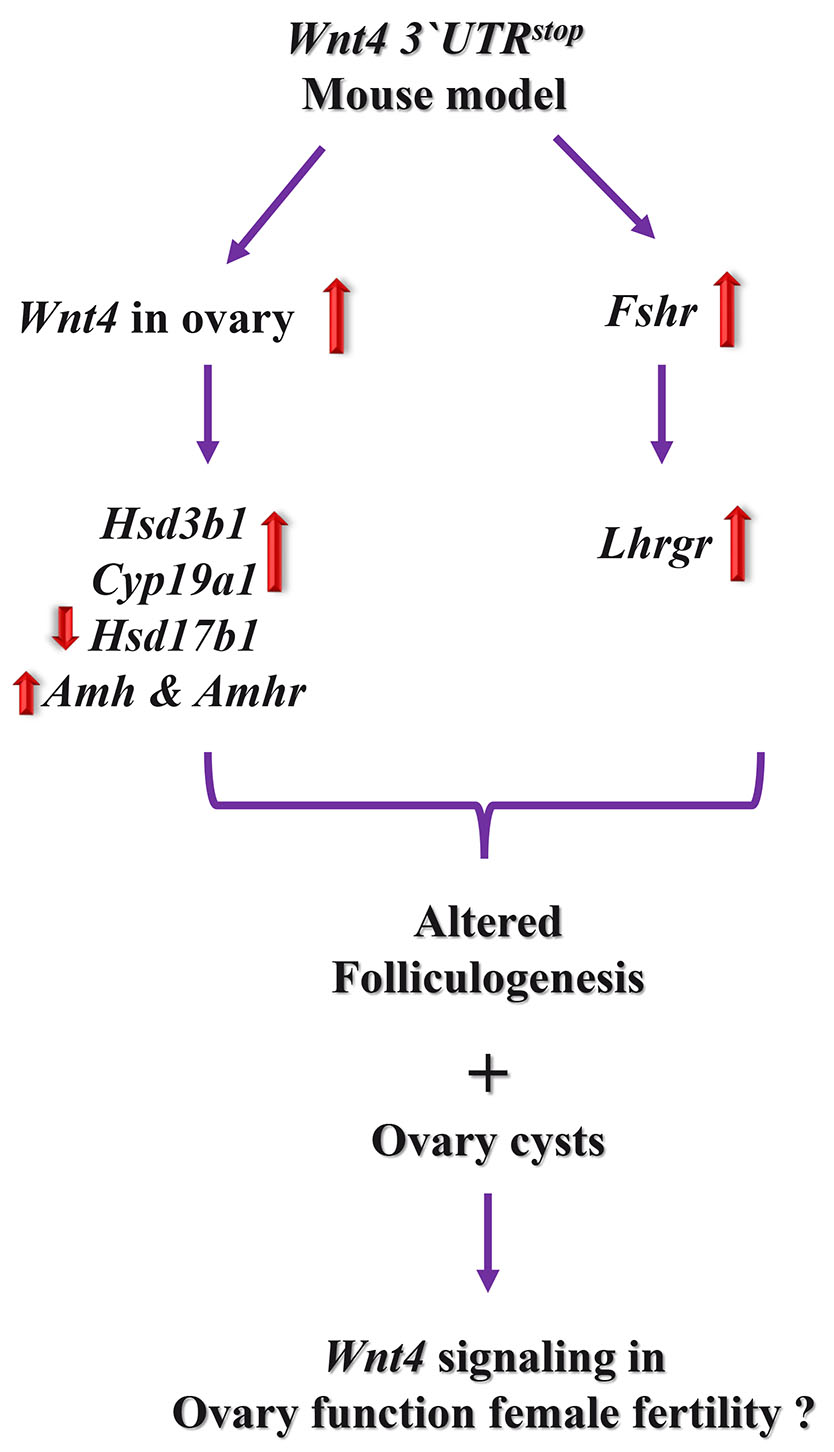
In the novel Wnt4 3`UTRstop;Wnt4 3`UTRstop mouse model, the ovarian folliculogenesis process was notably affected when compared with control females and correlated with upregulation of certain hormone receptors’ expression levels, such as Fshr and Lhcgr. Furthermore, the noted increase in the ovarian Wnt4 gene and protein expression, lead to changes in the expression of genes that are involved in control of estrogens. The Wnt4 3`UTRstop;Wnt4 3`UTRstop females had also cysts in the ovary at six months of age and in line with the anomalies in the follicle maturation in these transgenic mice. Collectively, our data suggest that the established Wnt4 3`UTRstop;Wnt4 3`UTRstop mouse represents a new model to investigate further the roles of Wnt4 signaling in the ovarian function, via controlling the events illustrated in Fig. 6.
Fig. 6. Proposed model of Wnt4 signaling in the female ovary.
Following Wnt4 signaling activation in the ovary of Wnt4 3`UTRstop;Wnt4 3`UTRstop, the expression of a set of genes associated with hormone synthesis is altered leading to changes in follicle counts. Consequently, the Wnt4 3`UTRstop;Wnt4 3`UTRstop female exhibited reduced folliculogenesis which may be linked with cyst development and metabolic changes, namely lipid metabolism.
Previously, we have described the generation and analysis of the hypomorph Wnt4mCh/mCh mouse model, where the Wnt4 and the mCherry encoding sequences were fused together and inserted into the Wnt4 locus to replace the endogenous Wnt4 protein expression. This genetic modification lead to reduced Wnt4 expression in the Wnt4mCh/mCh mice and decreased number of ovarian follicles (Prunskaite-Hyyrylainen et al., 2014). Moreover, the Wnt4mCh/mCh females exhibited few poly-ovular follicles. Now, in our novel Wnt4 3`UTRstop;Wnt4 3`UTRstop experimental allelic model, we noted the opposite, namely an increase in the Wnt4 expression and both at the protein and the mRNA level. This elevation was associated with induction in follicle development to the pre-antral stage. However, after this, a reduction in the amount of corpus luteum was noted. We hypothesize that these phenotypes may be caused in part by the changes in cell polarity and extracellular matrix controlled also Wnt4 signaling as show earlier (Prunskaite-Hyyrylainen et al., 2014).
The FSH is critical for maturation of the pre-antral follicles (Hardy et al., 2017; Wang and Greenwald, 1993; François et al., 2017; Richards and Pangas, 2010). Here, FSH stimulates estradiol (E2) synthesis and associates with induction of the Cyp19b1 expression (François et al., 2017). These regulatory roles are in line with our findings in the Wnt4 3`UTRstop;Wnt4 3`UTRstop females where we observed an increase in the number of pre-antral follicles correlated with upregulation of Fshr and Cyp19b1 expression. In agreement with earlier reports (Casarini et al., 2017; Ziecik et al., 2021; Richards and Pangas, 2010), the positive relationship between the Hsd3b1 and the Cyp19b1 gene expression and also the Amh and the FSH genes in the 3`UTRstop;Wnt4 3`UTRstop females may be connected with the accumulation of pre-antral follicles.
The Hsd17b1 function has been studied by making a Hsd17b1 knockout mouse model. Here, the ovaries undergo rather normal folliculogenesis development, with some reduction in the corpus luteum count (Hakkarainen et al., 2015). In the ovaries of the Wnt4 3`UTRstop;Wnt4 3`UTRstop females, a decrease in the Hsd17b1 expression in the granulosa cells when compared with WT mice was found. This discovered link between Wnt4 signaling is in line with the earlier reports on the roles of Wnt4 to advance hormone synthesis such as the androstenedione conversion to testosterone (Hiltunen et al., 2019; Nokelainen et al., 1996), those of the corpus luteum ones (O’Shaughnessy et al., 2000), and luteinizing hormone (LH) (Hakkarainen et al., 2015; Shehu et al., 2011). Our herein data support the previous observation demonstrating that the Wnt4 signaling is involved in coordination of the steroidogenesis signaling to regulate the follicle complex, thus promoting oocyte maturation (Jiang et al., 2021; Lewis et al., 2019; Alexandre and Mueller, 2023).
Together, the data with the Wnt4 3`UTRstop;Wnt4 3`UTRstop mouse, in which Wnt4 signaling was enhanced via UTR engineering is in line with earlier studies which had indicated important roles for Wnt4 in ovarian ontogenesis and follicle maturation. Thus, the model reported herein provides a new tool with which to study Wnt4 signaling in female reproduction.
Materials and Methods
Cloning of the Wnt4 3`UTRstop transgenic constructs and screening of the Wnt4 3`UTRstop targeted ES cells
The targeting construct was cloned in pBluescript IISK- vector containing a 5’ homologous arm, targeting cassette and a 3’ homologous arm (Suppl. Fig. 1A). An 8.8kb XbaI-SacI genomic fragment corresponding to the nucleotides 38289-47130 was sub-cloned from BAC AL645468 (Shan et al., 2010), which contains the exon3, 4 and 5 of the Wnt4 gene. The 5’ homologous arm corresponding to nucleotides 42352 (KpnI)-43290 (stop codon) covers a part of the intron 4 and the whole exon 5. The 3’ homologous arm corresponding to nucleotides 43291-47130 (SacI) contains a part of the 3`UTR. The targeting construct was linearized by ScaI digestion and electroporated into SV129 ES cells. After selection, 300 ES clones were screened by PCR using primer F1: 5’AGCCGGGCACTCATGAATCTTC3’ and R1: 5’GAAGGAGCCAAGCTGCTA3’. Six putative correct targeted clones were found (Suppl. Fig. 1B). The PCR conditions were a 10-min denaturation at 94 °C followed by 35 cycles of 1 min at 94 °C, 45 s at 60 °C and 1 min at 72 °C, and a final extension of 10 min at 72 °C. Six expended ES clones were verified by Southern blot analysis (Suppl. Fig. 1C). A Genomic fragment XbaI-HindIII corresponding to nucleotides 38289-40137, was used as a probe.
Generation of the Wnt4 3`UTRstop mouse model, genotyping, and breeding
Two out of six positive ES cells were used for blastocyst injection with the standard procedure. Both showed germ line transmission. To avoid any ectopic expression effect on Wnt4 gene, caused by the presence of a PGK promoter in the selected cassette, the PGK-Neo sequence was removed from both lines by Flip-Frt system. The mice were genotyped by PCR analysis using DNA isolated from ear clips. The wild type allele was amplified with primer F2: 5’ATTGACGGCTGCGAGCTACT3’ and R2: 5’TGGGGGTAGGTGGTGGGAGA3’. The targeted allele was amplified with primer F2: 5’ATTGACGGCTGCGAGCTACT3’ and R3: 5’GGTACTCTGTTCTCACCCTTC3’.
The animal care principles and experimental procedures followed here were in accordance with the National Animal Experiment Board (ELLA) for the use of laboratory animals, the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (ETS 123), and EU Directive 86/609/EEC.
Tissue sections and PAS-Staining
The ovaries were dissected in 1× Dulbecco’s PBS and fixed in 4% paraformaldehyde (PFA). The ovaries were then washed, dehydrated, embedded in paraffin, and cut into 6µm sections. These sections were stained with hematoxylin and eosin, and then imaged under the Zeiss Axio Imager motorized histology microscope (Germany). A minimum of 3 ovary-derived sections for each genotype were examined.
RNA extraction and RT-QPCR
The ovary samples were dissected from three- and six month-old Wnt4 3`UTRstop;Wnt4 3`UTRstop and wild type mice, and total RNA was purified with an RNeasy mini kit (Qiagen). 1 µg RNA was converted into cDNA with a First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The cDNA (2 μL), 2.5 μM of the primers, and Brilliant II SYBR® Green QPCR master mix (5 µl; Agilent Technologies) set to a total volume of 10 µl. The primers used are described in Suppl. Table 1. The real-time qPCR program consisted of 40 cycles at 95°C for 30 s and at 60°C for 1 min in a CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad). GAPDH was used for normalization. The real-time qPCR results are presented as means ± SEM (4/4 mice).
Follicular count and fertility
Follicular count and maturation were estimated as described previously (Veiga-Lopez et al., 2012; Myers et al., 2009). To assess the fertility of Wnt4 3`UTRstop;Wnt4 3`UTRstop mice, two month-old female and male mice were housed with WT partners and monitored daily for litters for 3 months (Wnt4 3`UTRstop;Wnt4 3`UTRstop female vs WT male & Wnt4 3`UTRstop;Wnt4 3`UTRstop male vs WT female). Litter size was recorded at birth and the experiment ended 20 days after the removal of the males to allow for a potential final litter. Fertility capacity was judged by litter number and sizes.
Western blotting
The ovaries were collected from two month-old mice and stored at -80℃ until they were used for protein purification. Western blot was conducted as previously described (Veikkolainen et al., 2020). Membranes were incubated with either anti Wnt4 (R&D system # AF475, 1:300) or anti-b-actin (Sigma #5441, 1:3000) antibodies.
Statistics
Statistical analysis was performed with the unpaired, two-tailed Student's test. Values of P≤ 0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
We thank Johanna Kekolahti-Liias and Paula Haipus for their excellent technical assistance for genotyping and animal dissection. We thank Dr. Susanna Kaisto for her help related to mice mating. We thank Docent Florence Naillat for providing the primers for investigating the Wnt4 mRNA using in situ hybridization. This work was supported by the Finnish Research Impact Foundation Tandem Industry Academia grant (NA, SV), the DiHub EAKR (2430461011, NA, SV), PrintoDise (the European Regional Development Fund (EAKR, SV), and the Academy of Finland Flagship GeneCellNano (SV).
Declarations
Conflicts of interest
The authors declare no conflict of interest.
References
Akoumianakis I., Polkinghorne M., Antoniades C., (2022). Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications. Nature Reviews Cardiology 19: 783-797.
Alexandre Y. O., Mueller S. N., (2023). Splenic stromal niches in homeostasis and immunity. Nature Reviews Immunology 23: 705-719.
Aros C. J., Pantoja C. J., Gomperts B. N., (2021). Wnt signaling in lung development, regeneration, and disease progression. Communications Biology 4: 601.
Baron R., Kneissel M., (2013). WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature Medicine 19: 179-192.
Bartel D. P., (2009). MicroRNAs: Target Recognition and Regulatory Functions. Cell 136: 215-233.
Bergant V., Schnepf D., de Andrade Krätzig N., Hubel P., Urban C., Engleitner T., Dijkman R., Ryffel B., Steiger K., Knolle P. A., Kochs G., Rad R., Staeheli P., Pichlmair A., (2023). mRNA 3’UTR lengthening by alternative polyadenylation attenuates inflammatory responses and correlates with virulence of Influenza A virus. Nature Communications 14: 4906.
Berkovits B. D., Mayr C., (2015). Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 522: 363-367.
Butt M. S., Saleem J., Aiman S., Zakar R., Sadique I., Fischer F., (2022). Serum anti-Müllerian hormone as a predictor of polycystic ovarian syndrome among women of reproductive age. BMC Women's Health 22: 199.
Casarini L., Riccetti L., De Pascali F., Gilioli L., Marino M., Vecchi E., Morini D., Nicoli A., La Sala G., Simoni M., (2017). Estrogen Modulates Specific Life and Death Signals Induced by LH and hCG in Human Primary Granulosa Cells In Vitro. International Journal of Molecular Sciences 18: 926.
Chekulaeva M., Hentze M. W., Ephrussi A., (2006). Bruno Acts as a Dual Repressor of oskar Translation, Promoting mRNA Oligomerization and Formation of Silencing Particles. Cell 124: 521-533.
DiRocco D. P., Kobayashi A., Taketo M. M., McMahon A. P., Humphreys B. D., (2013). Wnt4/β−Catenin Signaling in Medullary Kidney Myofibroblasts. Journal of the American Society of Nephrology 24: 1399-1412.
Diegel C. R., Kramer I., Moes C., Foxa G. E., McDonald M. J., Madaj Z. B., Guth S., Liu J., Harris J. L., Kneissel M., Williams B. O., (2023). Inhibiting WNT secretion reduces high bone mass caused by Sost loss-of-function or gain-of-function mutations in Lrp5. Bone Research 11: 47.
Foulquier S., Daskalopoulos E. P., Lluri G., Hermans K. C. M., Deb A., Blankesteijn W. M., (2018). WNT Signaling in Cardiac and Vascular Disease. Pharmacological Reviews 70: 68-141.
François C. M., Petit F., Giton F., Gougeon A., Ravel C., Magre S., Cohen-Tannoudji J., Guigon C. J., (2017). A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Scientific Reports 7: 46222.
Freimer J. W., Krishnakumar R., Cook M. S., Blelloch R., (2018). Expression of Alternative Ago2 Isoform Associated with Loss of microRNA-Driven Translational Repression in Mouse Oocytes. Current Biology 28: 296-302.e3.
Gay A., Towler D. A., (2017). Wnt signaling in cardiovascular disease: opportunities and challenges. Current Opinion in Lipidology 28: 387-396.
Gruber A. R., Martin G., Müller P., Schmidt A., Gruber A. J., Gumienny R., Mittal N., Jayachandran R., Pieters J., Keller W., van Nimwegen E., Zavolan M., (2014). Global 3′ UTR shortening has a limited effect on protein abundance in proliferating T cells. Nature Communications 5: 5465.
Guo Y., Mishra A., Howland E., Zhao C., Shukla D., Weng T., Liu L., (2015). Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1–mediated neutrophilic acute lung inflammation. Blood 126: 2220-2229.
Hakkarainen J., Jokela H., Pakarinen P., Heikelä H., Kätkänaho L., Vandenput L., Ohlsson C., Zhang F.P., Poutanen M., (2015). Hydroxysteroid (17β)-dehydrogenase 1–deficient female mice present with normal puberty onset but are severely subfertile due to a defect in luteinization and progesterone production. The FASEB Journal 29: 3806-3816.
Hardy K., Fenwick M., Mora J., Laird M., Thomson K., Franks S., (2017). Onset and Heterogeneity of Responsiveness to FSH in Mouse Preantral Follicles in Culture. Endocrinology 134-147.
He Y.X., Diao T.T., Song S.M., Wang C.C., Wang Y., Zhou C.L., Bai Y.B., Yu S.S., Mi X., Yang X.Y., Wei Q.J., Li B., (2018). Wnt4 is significantly upregulated during the early phases of cisplatin-induced acute kidney injury. Scientific Reports 8: 10555.
Hiltunen J. K., Kastaniotis A. J., Autio K. J., Jiang G., Chen Z., Glumoff T., (2019). 17B-hydroxysteroid dehydrogenases as acyl thioester metabolizing enzymes. Molecular and Cellular Endocrinology 489: 107-118.
Hoffman Y., Bublik D. R., P. Ugalde A., Elkon R., Biniashvili T., Agami R., Oren M., Pilpel Y., (2016). 3’UTR Shortening Potentiates MicroRNA-Based Repression of Pro-differentiation Genes in Proliferating Human Cells. PLOS Genetics 12: e1005879.
Jia X., Yuan S., Wang Y., Fu Y., Ge Y., Ge Y., Lan X., Feng Y., Qiu F., Li P., Chen S., Xu A., (2017). The role of alternative polyadenylation in the antiviral innate immune response. Nature Communications 8: 14605.
Jiang W., Li Y., Zhang S., Kong G., Li Z., (2021). Association between cellular immune response and spleen weight in mice with hepatocellular carcinoma. Oncology Letters 22: 625.
Lewis S. M., Williams A., Eisenbarth S. C., (2019). Structure and function of the immune system in the spleen. Science Immunology 4: eaau6085.
Liu J., Xiao Q., Xiao J., Niu C., Li Y., Zhang X., Zhou Z., Shu G., Yin G., (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduction and Targeted Therapy 7: 3.
Ma W., Mayr C., (2018). A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3′UTR-Mediated Protein-Protein Interactions. Cell 175: 1492-1506.e19.
Marshall P., Garton D. R., Taira T., Võikar V., Vilenius C., Kulesskaya N., Rivera C., Andressoo J.O., (2021). Elevated expression of endogenous glial cell line‐derived neurotrophic factor impairs spatial memory performance and raises inhibitory tone in the hippocampus. European Journal of Neuroscience 53: 2469-2482.
Mayr C., (2017). Regulation by 3′-Untranslated Regions. Annual Review of Genetics 51: 171-194.
Myers M., Middlebrook B. S., Matzuk M. M., Pangas S. A., (2009). Loss of inhibin alpha uncouples oocyte-granulosa cell dynamics and disrupts postnatal folliculogenesis. Developmental Biology 334: 458-467.
Naillat F., Prunskaite-Hyyryläinen R., Pietilä I., Sormunen R., Jokela T., Shan J., Vainio S. J., (2010). Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Human Molecular Genetics 19: 1539-1550.
Nokelainen P., Puranen T., Peltoketo H., Orava M., Vihko P., Vihko R., (1996). Molecular Cloning of Mouse 17β‐Hydroxysteroid Dehydrogenase Type 1 and Characterization of Enzyme Activity. European Journal of Biochemistry 236: 482-490.
Nusse R., Clevers H., (2017). Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169: 985-999.
Oh S. R., Choe S. Y., Cho Y. J., (2019). Clinical application of serum anti-Müllerian hormone in women. Clinical and Experimental Reproductive Medicine 46: 50-59.
O’Shaughnessy P. J., Baker P. J., Heikkilä M., Vainio S., McMahon A. P., (2000). Localization of 17β-Hydroxysteroid Dehydrogenase/17-Ketosteroid Reductase Isoform Expression in the Developing Mouse Testis—Androstenedione Is the Major Androgen Secreted by Fetal/Neonatal Leydig Cells*. Endocrinology 141: 2631-2637.
Prunskaite-Hyyrylainen R., Shan J., Railo A., Heinonen K. M., Miinalainen I., Yan W., Shen B., Perreault C., Vainio S. J., (2014). Wnt4, a pleiotropic signal for controlling cell polarity, basement membrane integrity, and antimullerian hormone expression during oocyte maturation in the female follicle. The FASEB Journal 28: 1568-1581.
Prunskaite-Hyyryläinen R., Skovorodkin I., Xu Q., Miinalainen I., Shan J., Vainio S. J., (2016). Wnt4 coordinates directional cell migration and extension of the Müllerian duct essential for ontogenesis of the female reproductive tract. Human Molecular Genetics 25: 1059-1073.
Richards J.A. S., Pangas S. A., (2010). The ovary: basic biology and clinical implications. Journal of Clinical Investigation 120: 963-972.
Sandberg R., Neilson J. R., Sarma A., Sharp P. A., Burge C. B., (2008). Proliferating Cells Express mRNAs with Shortened 3' Untranslated Regions and Fewer MicroRNA Target Sites. Science 320: 1643-1647.
Shan J., Jokela T., Skovorodkin I., Vainio S., (2010). Mapping of the fate of cell lineages generated from cells that express the Wnt4 gene by time-lapse during kidney development. Differentiation 79: 57-64.
Shehu A., Albarracin C., Devi Y. S., Luther K., Halperin J., Le J., Mao J., Duan R. W., Frasor J., Gibori G., (2011). The Stimulation of HSD17B7 Expression by Estradiol Provides a Powerful Feed-Forward Mechanism for Estradiol Biosynthesis in Breast Cancer Cells. Molecular Endocrinology 25: 754-766.
Sivanandy M. S., Ha S. K., (2023). The Role of Serum Anti-Mullerian Hormone Measurement in the Diagnosis of Polycystic Ovary Syndrome. Diagnostics (Basel) 13: 907.
Vainio S., Heikkilä M., Kispert A., Chin N., McMahon A. P., (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405-409.
Veiga-Lopez A., Ye W., Padmanabhan V., (2012). Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertility and Sterility 97: 748-756.
Veikkolainen V., Ali N., Doroszko M., Kiviniemi A., Miinalainen I., Ohlsson C., Poutanen M., Rahman N., Elenius K., Vainio S. J., Naillat F., (2020). Erbb4 regulates the oocyte microenvironment during folliculogenesis. Human Molecular Genetics 29: 2813-2830.
Wang X.N., Greenwald G. S., (1993). Hypophysectomy of the Cyclic Mouse. I. Effects on Folliculogenesis, Oocyte Growth, and Follicle-Stimulating Hormone and Human Chorionic Gonadotropin Receptors1. Biology of Reproduction 48: 585-594.
Xu H., Wang W., Liu X., Huang W., Zhu C., Xu Y., Yang H., Bai J., Geng D., (2023). Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduction and Targeted Therapy 8: 202.
Yang Y., Yang C.R., Han S. J., Daldello E. M., Cho A., Martins J. P. S., Xia G., Conti M., (2017). Maternal mRNAs with distinct 3′ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes & Development 31: 1302-1307.
Zhan T., Rindtorff N., Boutros M., (2017). Wnt signaling in cancer. Oncogene 36: 1461-1473.
Zhang Q., Pan Y., Ji J., Xu Y., Zhang Q., Qin L., (2021). Roles and action mechanisms of WNT4 in cell differentiation and human diseases: a review. Cell Death Discovery 7: 287.
Zhao S.L., Wei S.Y., Wang Y.X., Diao T.T., Li J.S., He Y.X., Yu J., Jiang X.Y., Cao Y., Mao X.Y., Wei Q.J., Wang Y., Li B., (2016). Wnt4 is a novel biomarker for the early detection of kidney tubular injury after ischemia/reperfusion injury. Scientific Reports 6: 32610.
Ziecik A. J., Klos J., Gromadzka-Hliwa K., Dietrich M. A., Slowinska M., Likszo P., Knapczyk-Stwora K., Gajewski Z., Kaczmarek M. M., (2021). Endocrine and molecular milieus of ovarian follicles are diversely affected by human chorionic gonadotropin and gonadotropin-releasing hormone in prepubertal and mature gilts. Scientific Reports 11: 13465.