Int. J. Dev. Biol. 68: 9 - 17 (2024)
Understanding megasporogenesis through model plants: contemporary evidence and future insights
Open Access | Review | Published: 2 April 2024
Abstract
The megasporangium serves as a model system for understanding the concept of individual cell identity, and cell-to-cell communication in angiosperms. As development of the ovule progresses, three distinct layers, the epidermal (L1), the subepidermal or the hypodermal (L2) and the innermost layers (L3) are formed along the MMC (megaspore mother cell). The MMC, which is the primary female germline cell, is initiated as a single subepidermal cell amongst several somatic cells. MMC development is governed by various regulatory pathways involving intercellular signaling, small RNAs and DNA methylation. The programming and reprograming of a single nucellar cell to enter meiosis is governed by ‘permissive’ interacting processes and factors. Concomitantly, several nucellar sister cells are prevented from germline fate also by a set of ‘repressive’ factors. However, in certain angiosperms, anomalies in development of the female gametophyte have been observed. The sporophytic tissue surrounding the female gametophyte affects the gametophyte in multiple ways. The role of genes and transcription factors in the development of the MMC and in the regulation of various processes studied in selected model plants such as Arabidopsis is explained in detail in this paper. However, as angiosperms display enormous diversity, it is important to investigate early stages of megasporogenesis in other plant systems as well. Such studies provide valuable insights in understanding the regulation of megasporogenesis and the evolution of the female gametophyte from gymnosperms to flowering plants.
Keywords
arabidopsis, cell-cell signaling, epigenetics, hormonal control, megasporogenesis, programmed cell death (PCD)
Introduction
Development of female gametophyte (FG) exhibits various patterns and processes in the flowering plants. The ovule/megasporangium is a composite of three distinct radial layers L1 (epidermis), L2 (first subepidermal layer), and L3 (innermost layer) enclosing a multicellular nucellus (Vijayan et al., 2021). Studies on model plant Arabidopsis suggest that the FG is a seven celled and eight nucleate structure depicting two-phase development, where megasporogenesis is followed by megagametogenesis. In the first phase, the distal cell in the subepidermal layer (L2) of nucellus develops into megaspore mother cell (MMC) which further undergoes a meiotic division resulting in four haploid megaspores. This established development observed is the manifestation of changes taking place at molecular level and is governed by genes. Studies carried out on Arabidopsis, tomato (Jiang and Zheng, 2022), rice (Ren et al., 2018) and maize (Wu et al., 2011) have revealed plethora of molecules and coordinated mechanisms that play a role in FG development, especially cell specification. Signals arise in the apical L1 cells, the companion cells (the hypodermal nucellus cells in immediate contact with MMC having potential to acquire MMC fate) and the lower L1 cells (Fig. 1). These signals are responsible for a single MMC formation and phase changes (Petrella et al., 2021). Mendes et al., (2020) elucidated the role of regulatory pathways involving small RNAs in differentiation of a single meiocyte during megasporogenesis from a pool of somatic nucellar cells. Post meiosis out of four megaspores formed in the tetrad, only one remains functional and this has been thoroughly investigated and documented. The other three nuclei undergo degeneration or programmed cell death (PCD) which is regulated by hormones (Papini et al., 2011; Schmidt et al., 2015a, 2015b, Pinto et al., 2019, Jiang and Zheng, 2022). Besides hormones, a variety of regulatory factors play a role in megaspore degeneration. Descriptive embryologists have observed variations in the process amongst the members of same family and have attributed it mostly to angiosperm diversity and occasionally to the environmental factors. Signaling molecules such as calcium plays a significant role in the megaspore degeneration as observed in Lactuca sativa (Qiu et al., 2005a, 2005b).
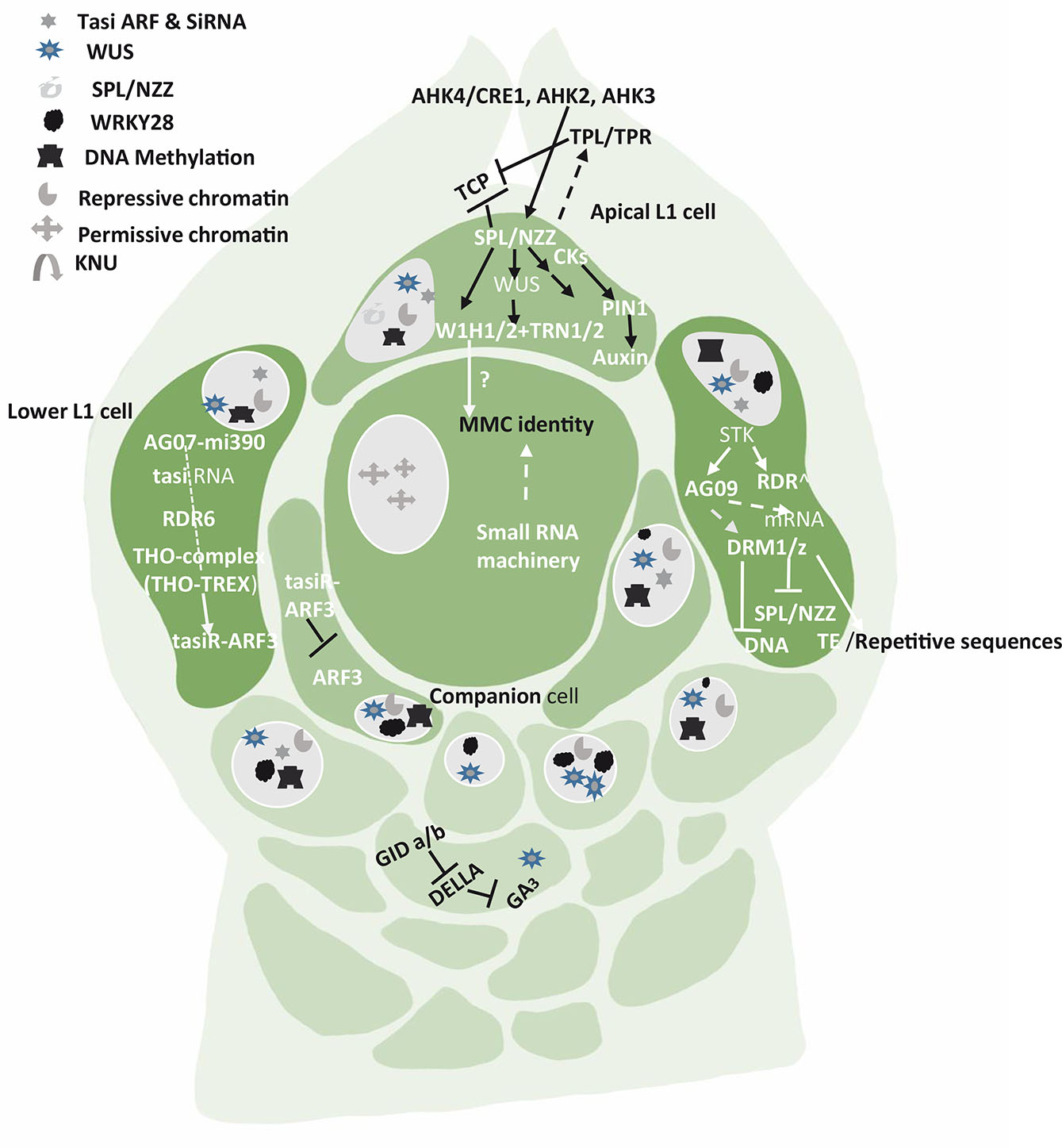
Fig. 1. Pathways, genes and factors that restrict germline fate to only one cell (MMC) while the neighboring cells are in repressive state.
Signals arise in the apical L1 cells, the companion cells (the hypodermal nucellus cells in immediate contact with MMC having potential to acquire MMC fate) and the lower L1 cells. SPL/NZZ pathway in apical L1 cell is the key to differentiation of a distal subepidermal megaspore mother cell. The lower L1 cells show the participation of various RNAs, Transposable elements (TE) and hormones. The chromatin remodeling and reprogramming is an important event in permissive and repressive state of the somatic and germline nucleus.
Fresh insights into megasporogenesis
During megasporogenesis, one cell from the meristematic tissue acquires a unique identity to initiate female germline development. The formation of MMC thus marks the first cell of the germline lineage. The commitment of this cell to switch to a reduction division from equational division is meticulously programmed (Kapil and Bhatnagar, 1980). The callose around the MMC acts as a barrier and a security guard and secludes the developing germline cell from the sporophyte, besides providing nutrients when required. There are many independent aspects which come into action during megasporogenesis, such as regulation of the cell cycle, hormonal interplay, ribosome biogenesis, protein degradation and epigenetic pathways (Simpson, 2019). All these pathways are interconnected and operate via cell-to-cell communication in the developing ovule and its components (Kaur and Koul, 2022). Cell fate transition (somatic cell to germline cell transition) is accompanied by a large-scale chromatin reprogramming which is partially synchronous with meiotic S phase. It is marked by several changes such as chromatin decondensation, reduction in heterochromatin, depletion of linker histones (H1.1, H1.2, LHP1 and H2A.Z), changes in core histone variants and histone modification organization (She et al., 2013). In a normal embryo sac, only one MMC differentiates in the ovule. However, sometimes two or three MMCs are formed. In such a situation, the reproduction is either apomictic or may lead to abnormal female gametophyte development (Schmidt et al., 2014). Remodeling helps MMCs to establish an epigenetic and transcriptional status that is distinct from the pool of the surrounding nucellar tissue. Reprogramming facilitates subsequent meiotic events and post-meiotic events that determine the cell fate. Selection of functional megaspore (FMS) of the tetrad and PCD of the other three (Papini et al., 2011; Singh et al., 2017) lead to development of monosporic embryo sac/FG (Evans and Grossniklaus, 2009). The molecular and genetic basis of this differentiation has been investigated extensively (Schmidt et al., 2015a; Cao et al., 2018).
Single-cell RNA sequencing (scRNA-seq) and other throughput technology has been used to elucidate the role of potential nucellar cells that adapt to a particular germline fate. One MMC develops per ovule and many genes establish or/and maintain germline fate during early ovule development e.g., MULTIPLE ARCHESPORIAL CELLS 1 (MAC1) gene in maize (Sheridan et al., 1996; Wang et al., 2012), and MULTIPLE SPOROCYTES (MSP1) (Nonomura et al., 2003), OsERECTA2 (OsER2) receptor-like kinase gene, OsTDL1A (Zhao et al., 2008) have been identified in rice. However, these genes work in coordination with several other genes, transcription factors, proteins, and hormones during female germline differentiation. The interaction results in maintenance of MMC which is programmed to switch from mitotic divisions to meiotic divisions (Zhao et al., 2020).
Since MMC lies deeply embedded in nucellar (ovular) tissue, it is less accessible and thus understudied compared to pollen mother cells. High throughput techniques that have elucidated the molecular mechanisms underlying developmental program in its counterpart are now being used. Techniques such as gene profiling through bulk RNA sequencing, single - cell type RNA sequencing, laser-assisted micro-dissection, or fluorescence-assisted cell-sorting techniques have helped in isolating deep-seated MMC. Such studies have assisted in revealing the signaling network that regulates specification and differentiation in the female germ line cell (Zhao et al., 2014, Zhao et al., 2020). In high-through put single- cell transcriptomic study, Hou et al., (2021) have analyzed the developmental trajectory of female germ line –associated cell in Arabidopsis thaliana. The studies from pseudo time trajectory of MMC reveal bifurcation of ovule cells at a branch point into two – 1) a germ line branch (GB) and 2) a non-germ line branch (NGB).
Thus, a heterogeneity exists among the cells contributing to ovule primordial and those involved in germline specification (Hou et al., 2021). Such isolation has led to identification of the germ line lineage and the surrounding somatic sub populations. The studies indicate an increased expression of cell cycle genes along the germ line growth. Three germ line-associated cell clusters, AC.1, MMC1.10 and MMC2.6, have been identified with sub clusters (AC- MMC2) nested in them. These sub clusters are enriched for gene expression and macromolecule biosynthesis besides metabolic processes. Many genes and proteins in these are activated during the developmental processes. The germ line sub cluster also revealed enriched GO (Gene Ontology) related to peptide biosynthesis and transport. Such an abundance facilitates peptide-mediated cell communication, an important phenomenon during germ line specification (Zhao et al., 2008, Lieber et al., 2011). At MMC2 stage, the sub cluster shows enrichment of genes involved in ‘chromosome organization’ and microtubule-based processes (Hou et al., 2021). This observation is supported by events such as chromatin remodeling, cell-cell regulation and cell wall composition which are associated with differentiation of MMC (Lora et al., 2017, Nelms and Walbot, 2019, Jiang and Zheng, 2022).
Functional megaspore selection
Recent investigations have revealed that switching over of MMC from mitotic to meiotic division is due to cyclin-dependent kinase (CDK) inhibitor called KIP-RELATED PROTEIN (KRP). The KRP inhibits CDKA:1 and ensures entry of MMC into meiosis. Likewise, CDKA:1 targets RETINOBLASTOMA-RELATED1 (RBR1) in Arabidopsis and inhibits designated MMC from entering mitosis (Zhao et al., 2017). In Arabidopsis, it has been found that ANTIKEVORKIAN (AKV) gene is involved in regulating megaspore survival. The evidence is provided by the akv mutant in which all four megaspores survive in 10% of the ovules. The number and position of surviving megaspores is variable in akv mutants (Yang and Sundaresan, 2000). Calcium is known to play an important role in megaspore degeneration (Qiu et al., 2008a). It is further corroborated that PCD of megaspores in Lettuce is closely related with change in calcium content (Qiu et al., 2008b). Of the four megaspores, a cell that shows differential calcium degeneration and further synthesis develops into a functional megaspore. The decrease in calcium content leads to its degeneration, and increased calcium facilitates the development of functional megaspore. As per Demesa-Arévalo and Vielle-Calzada (2013) selection and survival of functional megaspore in Arabidopsis thaliana is regulated by Arabidogalactan protein 18 (AGP18/AtAGP18). In ovules, where the gene AGP 18 is overexpressed, abnormal megaspores behave as functional megaspores. Further, the AGP18 regulatory region, which is expressed in the abaxial integumentary cells, has auxin, gibberellin, and abscisic acid (ABA) response elements suggesting hormonal control of AGP18 transcription. The degenerating megaspores show fragmented DNA, and express MPS-ONE-BINDER (MOB1) gene encoding for the proteins that regulate apoptosis factors in Drosophila and some mammals (Citterio et al., 2005; Hirabayashi et al.et al., 2008). Callose, a histological marker, accumulates in the MMC and around all the four megaspores and once the process culminates, callase enzyme dissolves it. The decision on which of the four megaspores is supernumerary and is destined to undergo cell death appears to be linked to the callose deposition around the tetrad (Rodkiewicz, 1970; Webb and Gunning, 1990). The callose wall is seen around the degenerating dyad of a bisporic embryo sac, while in tetrasporic embryo sac where all the four meiotically derived nuclei contribute to female gametophyte, callose wall is absent (Webb and Gunning, 1990; Haig, 2020). Callose is known to regulate cell-to-cell communication, and nutrient flow through plasmodesmatal connections (Azim and Buech-Smith, 2020). The reason for appearance and disappearance of callose in megasporogenesis is not well understood and is therefore under scrutiny (Shi et al., 2016). For a deeper understanding, the researchers are elucidating new functions for this molecule using mutants (Tucker and Koltunow 2009; Chen and Kim, 2009; Drews and Koltunow, 2011).
MMC differentiation and the SPL pathway
SPL/NZZ (SPOROCYTELESS) in Arabidopsis, OsSPOROCYTELESS (OsSPL) in rice and AG-NZZ/SPL in pineapple are the genes that promote germline specification (Zhao et al., 2021). SPL/NZZ (SPOROCYTELESS), a key regulator of megasporogenesis is expressed at the tip of the ovule in apical cell of L1 layer and establishes proximal-distal pattern formation within the ovule (Balasubramanian and Schneitz, 2000; Zhao et al., 2020). The SPL signaling pathway includes SPL/NZZ, several genes and factors such as WUSHELL (WUS), WINDHOSE 1 (WH1), WH2, TORNADO 2 (TRN2), and CIN-CINNATA (CIN).
AGAMOUS (AG) activates SPL/NZZ transcription factor in the ovules of pineapple. The homeodomain transcription factor WUSCHELL (WUS) is activated by SPL/NZZ expression in the nucellus, and both promote MMC differentiation. However, the proximity, the breadth and the magnitude of the interaction cascades are least understood. According to Groß-Hardt et al., (2002, 2003), WUS has a role in MMC differentiation, and acts via SPL/NZZ. SPL/NZZ and WUS are promoted by the cytokinin receptors ARABIDOPSIS HISTIDINE KINASE4/CYTOKININ RESPONSE1 (AHK4/CRE1), AHK2 and AHK3. In this pathway, WUS activates expression of the small WINDHOSE 1 and 2 (WIH1/2) peptides. WIH1-2 genes encoding small peptides work together with tetraspanin-type protein (TRN2) and the leucine rich-repeat protein TRN1 to promote megasporogenesis. These two proteins are partners of WIH 1 and WIH 2 and their coordination is required for MMC to function efficiently (Leiber et al., 2011). Auxins also play a key role in MMC specification and YUCCA family of enzymes have been found to control the biosynthesis of auxins. SPL represses the expression of YUCCA genes that are required for auxin biosynthesis (Li et al., 2008). Auxins accumulate at the tip of the nucellus (Ceccato et al., 2013). SPL transcription is repressed in all the nucellar cells of L1 except in the apical cells. In this cell, SPL/NZZ can promote cell enlargement by activation/repression of proteins that will generate a signal for the cell below to enlarge (Fig. 1).
SPL/NZZ activates the expression of the auxin transporter gene PIN-FORMED1 (PIN1), establishing a specific auxin gradient required for germline initiation (Bencivenga et al., 2012). SPL can act as a transcriptional repressor and exert its effect by recruiting transcriptional co-repressor TOPLESS/TOPLESS-RELATED (TPL/TPRs) which suppresses the activities of CINCINNATA (CIN) -like TEOSINTE BRANCHED 1 (TCP1) from maize, CYCLOIDEA (CYC) from snapdragon, PROLIFERATING CELL FACTOR 1 (PCF1) from rice transcription factors. These genes act in coordination to drive the expression of PIN-FORMED1 (PIN1) proteins. Armenta-Medina et al., (2013) reported the role of (IOR) IOREMPTE, a WD40/transducin repeat gene, preferentially expressed in the L1 layer of Arabidopsis ovules. If this gene is misexpressed as seen in mutants, the progression of female gametogenesis is impeded and the L1 layer gets degenerated.
Cytokinins have been found to play a role in cell fate identity during the early developmental stages. Ferreira et al., (2023) demonstrated that mutants lacking the cytokinin synthesis gene Isopentenyltransferase 9 (IPT9) displayed multiple enlarged MMC-like cells. Furthermore, the expression of other IPTs and cytokinin receptors AHK2, AHK3, and AHK4/CRE1 during the MMC stage suggest their involvement in female germline identity (Cai et al., 2023).
Brassinosteroids (BRs) are also participatory hormones in megasporogenesis, and a gradient is established with maximum concentration seen in the L1 layer cells. This helps to restrict the germline fate in the subepidermal cells (Cai et al., 2022). The female germline precursor or the MMC in the normal development program does not show presence of BRs. However, presence of this hormone in the germline cell results in formation of supernumerary MMCs from the subepidermal cells. The key regulator of BR signaling pathway has been identified as BZR1 (BRASSINAZOL-RESISTANT1) transcription factor which interacts with a receptor, BR1 (Brassinosteroid insensitive) and these along with BR activate WRKY23 in the subepidermal somatic cells suppressing the germline fate in these cells (Bishop, 2007). This signaling pathway therefore limits the MMC fate to one per ovule.
MMC differentiation and role of ARGONAUTE
The Arabidopsis genome also contains 10 genes in three clades encoding ARGONAUTE (AGO) proteins that are effectors in all small RNA (sRNA)-related pathways. AGO proteins - AGO9, AGO4, AGO6, AGO8 out of 10 such proteins are known to play crucial role in gametophyte development. They belong to one clade and are involved in MMC formation (Hernández-Lagana et al., 2016). AGO9 is in the basal L1 cells, where it produces a mobile signal that moves to subepidermal cells and restricts megaspore fate. The MMC is known to exhibit a 60% reduction in heterochromatin content. According to Olmedo-Monfil et al.,2010), AGO9 is required to inhibit nucellar cells or the somatic cells around the MMC for adopting germline fate. In maize, AGO 104, a homolog of A. thaliana AGO9, encodes for AGO protein that can silence heterochromatin within young ovule primordia. Though both AGOs are homologs, yet they act in different ways. While AGO104 represses somatic fate in germ cells, AGO9 represses germline fate in somatic cells. A mutation in AGO104 results in the failure of meiosis in MMC and an unreduced embryo sac is formed (Singh et al., 2011). Both the AGO9 and AGO104 proteins are present in the subepidermal companion cells (L2) surrounding the developing MMC but are not reported in the MMC (Fig. 1). It indicates that the surrounding cells produce an AGO104-dependent mobile signal influencing the meiocyte differentiation (Carman, 1997). Asynchronous expression of duplicate genes in angiosperms triggers apomixis, bispory, tetraspory, and polyembryony which can occur on account of certain signals promoting meiosis or repressing further cell divisions in MMC (Singh et al., 2011).
MMC differentiation and epigenetic regulation
The first visible change in the presumptive MMC is marked by cell enlargement and elongation (Schneitz, 1995) and further increase in the size of the nucleus and nucleolus (Schulz and Jensen, 1981; Armstrong and Jones, 2003; Sniezko, 2006). These changes are correlated to the chromatin condensation and heterochromatin formation (Wang et al., 2013). According to She et al., (2013) there is 60% reduction in heterochromatin content and fewer chromocenters of the MMC nucleus in Arabidopsis. This chromatin reprogramming in the MMC promotes post-meiotic competence leading to the development of the pluripotent gametophyte with different types of cells (egg, synergids, central cell and antipodals). Furthermore, the patterns of DNA methyltransferase (enzymes that bring methylation) expression, DNA methylation, and the distribution of histone variants and histone modifications have been reported to enhance the epigenetic differentiation between the somatic cells and the MMC (Ingouff et al., 2007; Schoft et al., 2009; Ingouff et al., 2010; Pillot et al., 2010; Houben et al., 2011; Ibarra et al., 2012; Jullien et al., 2012). Histones H2A, H2B, H3 and H4 along with H1, a linker DNA bring condensation in the nucleosome (Hergeth and Schneider, 2015). In Arabidopsis H1 is encoded by three genes – H1.1, H1.2 and H1.3 (Ascenzi and Gantt, 1997). While H1.1 and H1.2 are down regulated in MMC, H1.3 is mildly detected at the ovular primordia stage, suggesting a shift from the ordinary somatic cell to a cell with germline fate (She et al., 2013). Accompanied with chromatin condensation, histone variants are also markers of germline cells. The variants are proteins substituting for the core canonical histones (H3, H4, H2A, H2B) in nucleosomes of eukaryotes. They often confer specific structural and functional features to nucleosomes and are involved in chromatin remodeling and nucleosome stability (Talbert and Henikoff 2021). Among multiple histone variants, H3.1 is related to inactive transcription (Jacob et al., 2014) and this variant is gradually evicted in MMC, which is indicative of distinguished cell identity. Another variant H2A.Z is also evicted from the early MMC stage but is reincorporated later.
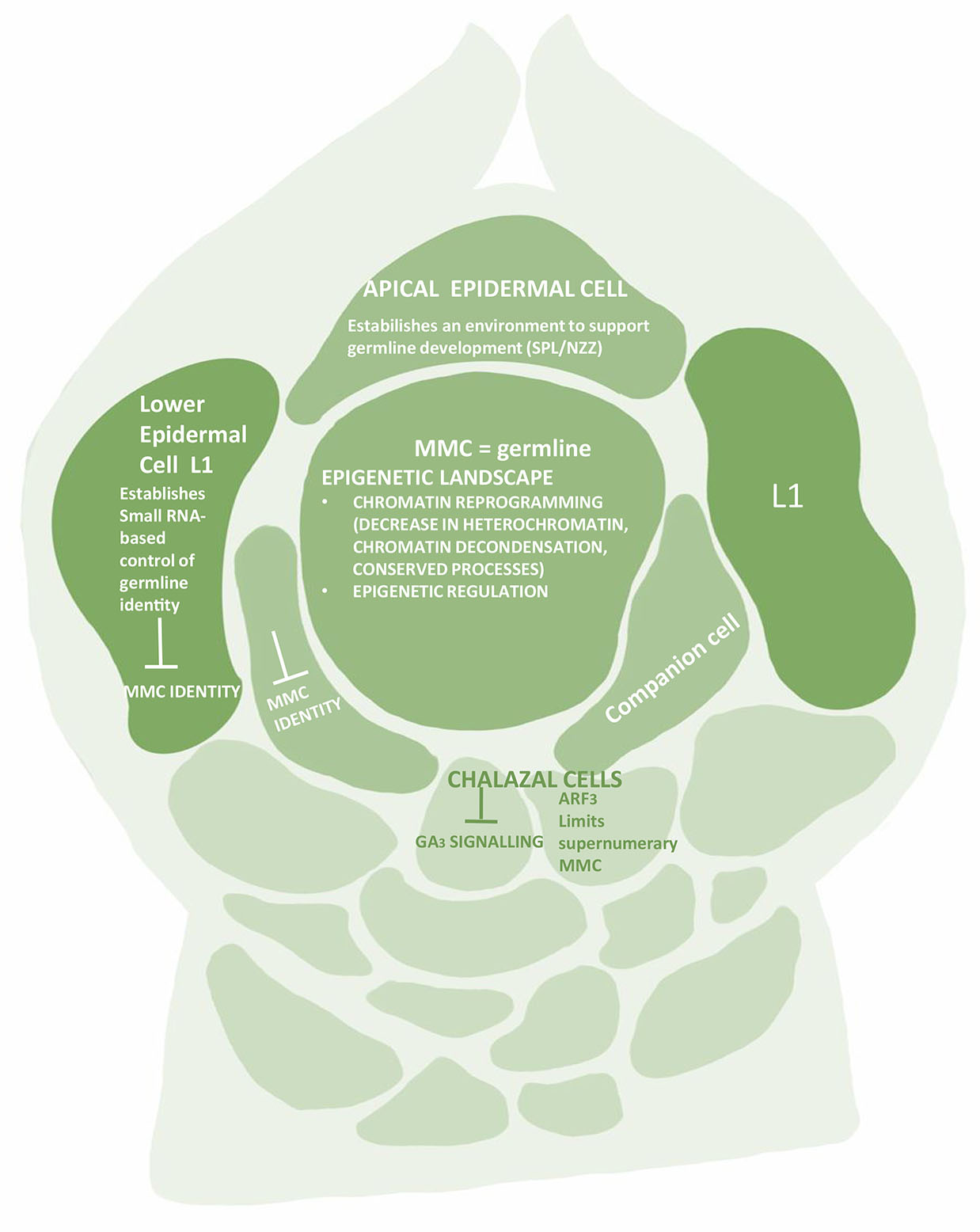
MMC specification is also due to small-RNA-dependent DNA methylation pathways acting in the nucellus (Olmedo-Monfil et al., 2010; Singh et al., 2011). The small RNAs play an important role in MMC formation. The small RNA population usually is divided into three groups – micro-RNA (miRNA), small interfering RNA (Si RNA) and trans-acting small interfering RNA (tasi-RNA) (Borges and Martienssen 2015). The tasi-RNA and siRNA are widely produced during initial ovule development. While the former impedes the surrounding somatic cells to obtain germline identity, the latter strengthens the repressive chromatin status in these cells (Figs. 1 and 2). The tasi-RNA acts through inhibiting ARF3 (AUXIN RESPONSE FACTOR3) expression in nucellar region (Su et al., 2017, 2020), and the siRNA acts by guarding DNA methylation and /or histone modification. The MMC instead shows a higher level of a permissive chromatin marked by active histones. If the activities of siRNA or DNA methylation are disrupted, more MMCs start to differentiate. Thus, epigenetic dimorphism is seen between MMC and the surrounding somatic cells of the nucellus (Fig. 2). The dimorphism is based on differential patterns of DNA methylation, small RNA activities, distribution of histone variants and histone modifications (Jiang and Zheng, 2022). All these regulatory pathways ensure specification and differentiation of single MMC per primordia.
Fig. 2. The ovular primordium showing chromatin landscape of the Megaspore Mother Cell (MMC).
Although SPL/NZZ presents the core transcription factor for germline differentiation, but the differentiation is also associated with epigenetic regulation at multiple layers. The entire battery of histone variants, histone modifications, small RNAs and DNA methylation is involved.
It is equally important for MMC to maintain the acquired permissive chromatin state and for neighboring cells to continue with the repressive state. This heterochromatin state is achieved through decreased active histone modifications such as H1, H3K9me2 and H3K27me3 in somatic cells, while H3K4me3 histone modification is seen in MMC (Jiang and Zeng, 2022). In contrast, a 2.7fold increase is seen in H3K4me3, while H3K27me1, H3K9me2 and H3K27me3 are reduced in MMC (Jiang and Zheng, 2022). Correspondingly a large protein, SET DOMAIN GROUP 2 (SDG2), a writer for H3K4me3 is highly expressed in MMC (Berr et al., 2010, Guo et al., 2010), while a key regulator LHP1 for H3K27me3 is barely expressed (She et al., 2013).
MMC differentiation and the RdDM pathway
The siRNA can trigger DNA methylation through RNA-dependent DNA methylation (RdDM) pathway (Xie and Yu 2015) which confines female germline to a single cell in the nucellar tissue (Aslam et al., 2022). The siRNAs required for MMC formation are produced from double stranded RNAs generated by RDR6. The RdDM pathway proteins, DOMAINS REARRANGED METHYLASES (DRM1 and DRM2) are involved in DNA methylation (Groth et al., 2014). DRM2 is specifically associated with the transcribed locus inducing DNA cytosine methylation (Zhong et al., 2014). In maize, DMT102 and DMT103, the homologs of DRM1 and DRM2 are expressed in a restricted portion of the nucellus during megasporogenesis. This pathway silences transposable elements SPL/NZZ or the repetitive sequences. SPL/NZZ can also be silenced directly by mRNA degradation which is brought about by ARGONAUTE9 (AGO9) and/or by methylation of SPL/NZZ genomic region. While AGO9 is preferentially expressed in the somatic cells of the L1 or companion of the pre-meiotic ovule, it is transiently and sporadically expressed in the nucleus of the MMC (Rodríguez-Leal et al.et al. 2015). The AGO9 also engages with transposable elements (TEs) that are silenced during the process. Another gene, SEEDSTICK (STK), directly regulates AGO9 and RDR6 expression in the ovule and indirectly regulates SPL/NZZ expression and control megasporocyte specification and female gamete formation. MIR822, the AGO9-interactor is involved in regulation of megaspore formation (Tovar-Aguilar et al., 2023).
THO/TREX (Transcription export protein complex) is another important component of the megasporogenesis machinery. It plays an important role in small interfering RNA-dependent processes in plants and its impairment leads to defects in siRNA biogenesis, export, processing and transporting ta-siRNA precursors from the nucleus to the cytoplasm (Jauvion et al., 2010; Yelina et al., 2010) (Francisco-Mangilet et al., 2015). The ta-siRNA restricts the cell specification as MMC by restricting the expression of ARF3 - AUXIN RESPONSIVE FACTOR-3 to the nucellar region (Su et al., 2020). A non-autonomous activity for ARF3 in female germline specification serves as a positive regulator for MMC identity. A protein TEX 1(TRANSCRIPTION EXPORT) is known to prevent somatic cells from adopting MMC fate in a non-cell autonomous manner through Trans Acting Small Interfering RNAs (TAS3)-mediated restriction of ARFs expression. Hence, any mutation in TEX1 leads to the formation of multiple MMCs (Su et al., 2017). Zhao et al., (2017) found that a balance between WUS activation and inactivation is crucial. When activated, WUS specifies MMC in all mutants of the WUS/WIH pathway, in place of the germ cell, while parenchyma-like cells resembling somatic cells are formed (Lieber et al., 2011). However, when RETINOBLASTOMA RELATED1 (RBR1) directly represses WUSCHEL (WUS), germline entry into meiotic division is facilitated. Thus, RBR1 plays a pivotal role in meiocyte differentiation, and its repressive activity affects cell proliferation. Finally, the number of MMCs and subsequent embryo sacs in the Arabidopsis ovule is also regulated by a subset of redundantly acting KIP-RELATED PROTEIN (KRP/ICK) and cyclin-dependent kinase (CDK) inhibitors which restrict one MMC and one FM per ovule. The KRPs act by restricting CDKA;1-dependent inactivation of RBR1(Cao et al., 2018). Once the MMC specification is over, the KRP-CDKA;1-RBR1 pathway ensures that MMC enters meiotic pathway by inhibiting the expansion of WUS into the MMC (Jiang and Zheng, 2022).
Based on the studied mechanisms, Mendes et al., (2020) developed a new model for the genetic and epigenetic control for specification of the single MMC. The model proposes that STK activates RdDM gene expression in the lower L1 layer cells. The siRNAs present in these cells act either via mRNA cleavage/repression or methylation to suppress SPL/NZZ expression and synthesis. One of the pioneer transcription factors, SPL/NZZ and WUS, play an important role in promoting MMC formation. These are synthesized and expressed only at the tip of the ovule primordium/L1 layer. The spatial distribution of SPL, WUS and WRKY28 away from the centred position of the nucellus region is the prerequisite of MMC specification and MMC differentiation, respectively. The specification of a female germline precursor can be achieved via several possible mechanisms. The movement of an effector molecule, possibly through the plasmodesmata, to the subtending L2 cell presumably initiates MMC expansion and eventual MMC specification (Figs. 1 and 2). The nature of this effector, its mode of operation and its target(s) in the L2 cell are still being explored. Hou et al., (2021) provided insights into the molecular mechanisms underlying germline specification in Arabidopsis ovule primordia. They further highlighted the complexity and importance of gene expression, regulation, and cellular processes during the developmental stages. It was observed through serial thin sections at MMC2 stages that cellular differentiation in the germline involves genes linked to chromosome organization and microtubule-based process. Such cell-specific changes in chromatin organization, cell-cycle regulation, and cell wall composition have been related to evolution among angiosperms (Lora et al., 2017).
Conclusions
The first committed cell of female germline lineage is the megaspore mother cell (MMC) which originates from a mass of somatic cells of the nucellar origin. A single MMC is differentiated per ovule and acquires a germline fate. It switches to meiotic divisions from a mitotic cell division plan leading to formation of a tetrad of megaspores; three of these spores (monosporic FG) undergo PCD while the FMS (Functional megaspore) embarks on the second phase of FG formation. The entire development occurs with precision and involves pathways where several genes, factors, hormones, epigenetic mechanisms, and chromosome modelling form the basis.
The MMC first enlarges, and chromosomes and histones undergo various changes. This is followed by MMC specification. For the formation of MMC, inactivation of WUS by RBR1 repressor is mandatory. Disruption of RBR1 and KRP/ICK gene functions leads to supernumerary MMC formation. The expression of another gene, WRKY28 prevents neighboring somatic cells to acquire germline fate. Auxin signaling which acts through ARFs and SPL/NZZ is an important component of MMC specification pathway.
The RdDM pathway with a battery of genes namely, AGO9, DRM1, DRM2, also help to limit the number of MMC to one per ovule. RdDM gene expression in lower L1 cells is activated by STK gene which binds to AGO9 and RDR6, and the complex then silences TE, repetitive sequences and SPL/NZZ. Further, AGO9, DMR1 and DMR2 also silence SPL/NZZ in the subtending cell. The SPL/NZZ essential for MMC differentiation, is synthesized and expressed only in apical L1 but is required for MMC specification in L2 subtending cell. Therefore, SPL/NZZ pathway works in a non- cell autonomous manner. Thus, spatial distribution of SPL (along with WUS and WRKY28) away from L2 subtending presumptive MMC is crucial for germline specification.
Research using modern techniques has largely elucidated the repressive pathways. However, there is paucity of information on mechanisms that promote MMC specification. Considerable amount of variation is displayed in the pattern and pathways operating during megasporogenesis in angiosperms. Such interacting pathways can result in overexpression or underexpression of the genes involved in germline specification and its regulation. Further studies are warranted in this field and encouraging results from single-cell studies on model plant germline trajectory have started to emerge.
Abbreviations
MMC, megaspore mother cell ; RdDM, RNA-dependent DNA methylation ;References
Armenta-Medina A., Huanca-Mamani W., Sanchez-León N., Rodríguez-Arévalo I., Vielle-Calzada J.P. (2013). Functional Analysis of Sporophytic Transcripts Repressed by the Female Gametophyte in the Ovule of Arabidopsis thaliana. PLoS ONE 8: e76977.
Armstrong S. J., Jones G. H. (2003). Meiotic cytology and chromosome behaviour in wild-type Arabidopsis thaliana. Journal of Experimental Botany 54: 1-10.
Ascenzi R., Gantt J. S. (1997). A drought-stress-inducible histone gene in Arabidopsis thaliana is a member of a distinct class of plant linker histone variants. Plant Molecular Biology 34: 629-641.
Aslam M., Fakher B., Qin Y. (2022). Big Role of Small RNAs in Female Gametophyte Development. International Journal of Molecular Sciences 23: 1979.
Azim M. F., Burch-Smith T. M. (2020). Organelles-nucleus-plasmodesmata signaling (ONPS): an update on its roles in plant physiology, metabolism and stress responses. Current Opinion in Plant Biology 58: 48-59.
Balasubramanian S., Schneitz K. (2000). NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule development in Arabidopsis thaliana . Development 127: 4227-4238.
Bencivenga S., Simonini S., Benková E., Colombo L. (2012). The Transcription Factors BEL1 and SPL Are Required for Cytokinin and Auxin Signaling During Ovule Development in Arabidopsis . The Plant Cell 24: 2886-2897.
Berr A., McCallum E. J., Ménard R., Meyer D., Fuchs J., Dong A., Shen W.H. (2010). Arabidopsis SET DOMAIN GROUP2 Is Required for H3K4 Trimethylation and Is Crucial for Both Sporophyte and Gametophyte Development . The Plant Cell 22: 3232-3248.
Bishop G. J. (2007). Refining the plant steroid hormone biosynthesis pathway. Trends in Plant Science 12: 377-380.
Borges F., Martienssen R. A. (2015). The expanding world of small RNAs in plants. Nature Reviews Molecular Cell Biology 16: 727-741.
Cai H., Liu L., Huang Y., Zhu W., Qi J., Xi X., Aslam M., Dresselhaus T., Qin Y., (2022). Brassinosteroid signaling regulates female germline specification in Arabidopsis. Current biology 32: 1102-1114.
Cai H., Liu L., Ma S., Aslam M., Qin Y. (2023). Insights into the role of phytohormones in plant female germline cell specification. Current Opinion in Plant Biology 75: 102439.
Cao L., Wang S., Venglat P., Zhao L., Cheng Y., Ye S., Qin Y., Datla R., Zhou Y., Wang H. (2018). Arabidopsis ICK/KRP cyclin-dependent kinase inhibitors function to ensure the formation of one megaspore mother cell and one functional megaspore per ovule. PLOS Genetics 14: e1007230.
Carman J. G. (1997). Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnean Society 61: 51-94.
Ceccato L., Masiero S., Sinha Roy D., Bencivenga S., Roig-Villanova I., Ditengou F. A., Palme K., Simon R., Colombo L. (2013). Maternal Control of PIN1 Is Required for Female Gametophyte Development in Arabidopsis. PLoS ONE 8: e66148.
Chen X.Y., Kim J.Y. (2009). Callose synthesis in higher plants. Plant Signaling & Behavior 4: 489-492.
Citterio S., Albertini E., Varotto S., Feltrin E., Soattin M., Marconi G., Sgorbati S., Lucchin M., Barcaccia G. (2005). Alfalfa Mob1-like Genes are Expressed in Reproductive Organs during Meiosis and Gametogenesis. Plant Molecular Biology 58: 789-807.
Demesa-Arévalo E., Vielle-Calzada J.P. (2013). The Classical Arabinogalactan Protein AGP18 Mediates Megaspore Selection in Arabidopsis . The Plant Cell 25: 1274-1287.
Drews G. N., Koltunow A. M.G. (2011). The Female Gametophyte. The Arabidopsis Book 9: e0155.
Evans M. M., Grossniklaus U., (2009). The Maize Megagametophyte. In Handbook of Maize: Its Biology. (Ed. Bennetzen J. L., Hake S. C., ) Springer-Science & Business Media, LLC, New York.
Ferreira L. G., Dusi D. M. A., Irsigler A. S. T., Gomes A. C. M. M., Florentino L. H., Mendes M. A., Colombo L., Carneiro V. T. C. (2023). Identification of IPT9 in Brachiaria brizantha (syn. Urochloa brizantha) and expression analyses during ovule development in sexual and apomictic plants. Molecular Biology Reports 50: 4887-4897.
Francisco-Mangilet A. G., Karlsson P., Kim M.H., Eo H. J., Oh S. A., Kim J. H., Kulcheski F. R., Park S. K., Manavella P. A. (2015). THO 2, a core member of the THO / TREX complex, is required for micro RNA production in Arabidopsis . The Plant Journal 82: 1018-1029.
Groß-Hardt R., Laux T. (2003). Stem cell regulation in the shoot meristem. Journal of Cell Science 116: 1659-1666.
Groß-Hardt R., Lenhard M., Laux T. (2002). WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development . Genes & Development 16: 1129-1138.
Groth M., Stroud H., Feng S., Greenberg M. V. C., Vashisht A. A., Wohlschlegel J. A., Jacobsen S. E., Ausin I. (2014). SNF2 chromatin remodeler-family proteins FRG1 and -2 are required for RNA-directed DNA methylation. Proceedings of the National Academy of Sciences 111: 17666-17671.
Guo L., Yu Y., Law J. A., Zhang X. (2010). SET DOMAIN GROUP2 is the major histone H3 lysine 4 trimethyltransferase in Arabidopsis . Proceedings of the National Academy of Sciences 107: 18557-18562.
Haig D. (2020). Poles Apart: Monosporic, Bisporic, and Tetrasporic Embryo Sacs Revisited. Frontiers in Ecology and Evolution 8: 516640.
Hergeth S. P., Schneider R. (2015). The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO reports 16: 1439-1453.
Hernández-Lagana E., Rodríguez-Leal D., Lúa J., Vielle-Calzada J.P. (2016). A Multigenic Network of ARGONAUTE4 Clade Members Controls Early Megaspore Formation in Arabidopsis . Genetics 204: 1045-1056.
Hirabayashi S., Nakagawa K., Sumita K., Hidaka S., Kawai T., Ikeda M., Kawata A., Ohno K., Hata Y. (2008). Threonine 74 of MOB1 is a putative key phosphorylation site by MST2 to form the scaffold to activate nuclear Dbf2-related kinase 1. Oncogene 27: 4281-4292.
Hou Z., Liu Y., Zhang M., Zhao L., Jin X., Liu L., Su Z., Cai H., Qin Y. (2021). High-throughput single-cell transcriptomics reveals the female germline differentiation trajectory in Arabidopsis thaliana. Communications Biology 4: 1149.
Houben A., Kumke K., Nagaki K., Hause G. (2011). CENH3 distribution and differential chromatin modifications during pollen development in rye (Secale cereale L.). Chromosome Research 19: 471-480.
Ibarra C. A., Feng X., Schoft V. K., Hsieh T.F., Uzawa R., Rodrigues J. A., Zemach A., Chumak N., Machlicova A., Nishimura T., Rojas D., Fischer R. L., Tamaru H., Zilberman D. (2012). Active DNA Demethylation in Plant Companion Cells Reinforces Transposon Methylation in Gametes. Science 337: 1360-1364.
Ingouff M., Hamamura Y., Gourgues M., Higashiyama T., Berger F. (2007). Distinct Dynamics of HISTONE3 Variants between the Two Fertilization Products in Plants. Current Biology 17: 1032-1037.
Ingouff M., Rademacher S., Holec S., Šoljić L., Xin N., Readshaw A., Foo S. H., Lahouze B., Sprunck S., Berger F. (2010). Zygotic Resetting of the HISTONE 3 Variant Repertoire Participates in Epigenetic Reprogramming in Arabidopsis. Current Biology 20: 2137-2143.
Jacob Y., Bergamin E., Donoghue M. T. A., Mongeon V., LeBlanc C., Voigt P., Underwood C. J., Brunzelle J. S., Michaels S. D., Reinberg D., Couture J.F., Martienssen R. A. (2014). Selective Methylation of Histone H3 Variant H3.1 Regulates Heterochromatin Replication. Science 343: 1249-1253.
Jauvion V., Elmayan T., Vaucheret H. (2010). The Conserved RNA Trafficking Proteins HPR1 and TEX1 Are Involved in the Production of Endogenous and Exogenous Small Interfering RNA in Arabidopsis . The Plant Cell 22: 2697-2709.
Jenik P. D., Irish V. F. (2000). Regulation of cell proliferation patterns by homeotic genes during Arabidopsis floral development. Development 127: 1267-1276.
Jiang T., Zheng B. (2022). Epigenetic Regulation of Megaspore Mother Cell Formation. Frontiers in Plant Science 12: 826871.
Jullien P. E., Susaki D., Yelagandula R., Higashiyama T., Berger F. (2012). DNA Methylation Dynamics during Sexual Reproduction in Arabidopsis thaliana. Current Biology 22: 1825-1830.
Kapil R. N., Bhatnagar A. K., (1980). Embryology in relation to angiosperm taxonomy. In Glimpses in Plant Research. Vikas Publishing House, New Delhi.
Kaur I., Koul M. (2022). Exploring the sister cells of embryo sac: developmental and functional attributes. The International Journal of Developmental Biology 66: 349-358.
Li L.C., Qin G.J., Tsuge T., Hou X.H., Ding M.Y., Aoyama T., Oka A., Chen Z., Gu H., Zhao Y., Qu L.J. (2008). SPOROCYTELESS modulates YUCCA expression to regulate the development of lateral organs in Arabidopsis . New Phytologist 179: 751-764.
Lieber D., Lora J., Schrempp S., Lenhard M., Laux T. (2011). Arabidopsis WIH1 and WIH2 Genes Act in the Transition from Somatic to Reproductive Cell Fate. Current Biology 21: 1009-1017.
Lora J., Herrero M., Tucker M.R., Hormaza J. I., (2017). The transition from somatic to germline identity shows conserved and specialized features during angiosperm evolution. The New phytologist 216: 495-509.
Mendes M. A., Petrella R., Cucinotta M., Vignati E., Gatti S., Pinto S. C., Bird D. C., Gregis V., Dickinson H., Tucker M. R., Colombo L. (2020). The RNA dependent DNA methylation pathway is required to restrict SPOROCYTELESS/NOZZLE expression to specify a single female germ cell precursor in Arabidopsis. Development 147: dev194274.
Nelms B., Walbot V. (2019). Defining the developmental program leading to meiosis in maize. Science 364: 52-56.
Nonomura K.I., Miyoshi K., Eiguchi M., Suzuki T., Miyao A., Hirochika H., Kurata N. (2003). The MSP1 Gene Is Necessary to Restrict the Number of Cells Entering into Male and Female Sporogenesis and to Initiate Anther Wall Formation in Rice . The Plant Cell 15: 1728-1739.
Olmedo-Monfil V., Durán-Figueroa N., Arteaga-Vázquez M., Demesa-Arévalo E., Autran D., Grimanelli D., Slotkin R. K., Martienssen R. A., Vielle-Calzada J.P. (2010). Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464: 628-632.
Papini A., Mosti S., Milocani E., Tani G., Di Falco P., Brighigna L. (2011). Megasporogenesis and programmed cell death in Tillandsia (Bromeliaceae). Protoplasma 248: 651-662.
Petrella R., Cucinotta M., Mendes M. A., Underwood C. J., Colombo L. (2021). The emerging role of small RNAs in ovule development, a kind of magic. Plant Reproduction 34: 335-351.
Pillot M., Baroux C., Vazquez M. A., Autran D., Leblanc O., Vielle-Calzada J. P., Grossniklaus U., Grimanelli D. (2010). Embryo and Endosperm Inherit Distinct Chromatin and Transcriptional States from the Female Gametes in Arabidopsis. The Plant Cell 22: 307-320.
Pinto S. C., Mendes M. A., Coimbra S., Tucker M. R. (2019). Revisiting the Female Germline and Its Expanding Toolbox. Trends in Plant Science 24: 455-467.
Qiu Y. L., Liu R. S., Xie C. T., Yang Y. H., Ge L. L., Tian H. Q. (2005a). The dynamic of calcium distribution during megasporegenesis of lettuce (Lactuca sativa L.). Zhi wu sheng li yu fen zi sheng wu xue xue bao = Journal of plant physiology and molecular biology 374-382.
Qiu Y. L., Liu R. S., Xie C. T., Yang Y. H., Ge L. L., Tian H. Q. (2005b). The character of calcium distribution in developing anther of lettuce (Lactuca sativa L.). Zhi wu sheng li yu fen zi sheng wu xue xue bao = Journal of plant physiology and molecular biology 377-386.
Qiu Y. L., Liu R. S., Ye L., Tian H. (2008a). Calcium distribution in the central cell of lettuce (Lactuca sativa L.) before and after pollination. Fen zi xi bao Sheng wu xue bao=Journal of Molecular Cell Biology 41: 61-69.
Qiu Y. L., Liu R. S., Xie C. T., Russell S. D., Tian H. Q. (2008b). Calcium changes during megasporogenesis and megaspore degeneration in lettuce (Lactuca sativa L.). Sexual Plant Reproduction 21: 197-204.
Ren L., Tang D., Zhao T., Zhang F., Liu C., Xue Z., Shi W., Du G., Shen Y., Li Y., Cheng Z., (2018). OsSPL regulates meiotic fate acquisition in rice. The New phytologist 218: 789-803.
Rodkiewicz B. (1970). Callose in cell walls during megasporogenesis in angiosperms. Planta 93: 39-47.
Rodríguez-Leal D., León-Martínez G., Abad-Vivero U., Vielle-Calzada J.P. (2015). Natural Variation in Epigenetic Pathways Affects the Specification of Female Gamete Precursors in Arabidopsis. The Plant Cell 27: 1034-1045.
Schmidt A., Schmid M. W., Klostermeier U. C., Qi W., Guthörl D., Sailer C., Waller M., Rosenstiel P., Grossniklaus U. (2014). Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation. PLoS Genetics 10: e1004476.
Schmidt A., Schmid M. W., Grossniklaus U. (2015a). Plant germline formation: common concepts and developmental flexibility in sexual and asexual reproduction. Development 142: 229-241.
Schmid M. W., Schmidt A., Grossniklaus U. (2015b). The female gametophyte: an emerging model for cell type-specific systems biology in plant development. Frontiers in Plant Science 6: e1001155.
Schneitz K., Hülskamp M., Pruitt R. E. (1995). Wild‐type ovule development in Arabidopsis thaliana : a light microscope study of cleared whole‐mount tissue . The Plant Journal 7: 731-749.
Schoft V. K., Chumak N., Mosiolek M., Slusarz L., Komnenovic V., Brownfield L., Twell D., Kakutani T., Tamaru H. (2009). Induction of RNA‐directed DNA methylation upon decondensation of constitutive heterochromatin. EMBO reports 10: 1015-1021.
Schulz P., Jensen W. A. (1981). Pre-fertilization ovule development inCapsella: ultrastructure and ultracytochemical localization of acid phosphatase in the meiocyte. Protoplasma 107: 27-45.
She W., Grimanelli D., Rutowicz K., Whitehead M. W. J., Puzio M., Kotliński M., Jerzmanowski A., Baroux C. (2013). Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development 140: 4008-4019.
Sheridan W. F., Avalkina N. A., Shamrov I. I., Batyea T. B., Golubovskaya I. N. (1996). The mac1 Gene: Controlling the Commitment to the Meiotic Pathway in Maize . Genetics 142: 1009-1020.
Shi X., Han X., Lu T. (2016). Callose synthesis during reproductive development in monocotyledonous and dicotyledonous plants. Plant Signaling & Behavior 11: e1062196.
Simpson M. G. (2019). Plant systematics. Academic press.
Singh M., Goel S., Meeley R. B., Dantec C., Parrinello H., Michaud C., Leblanc O., Grimanelli D. (2011). Production of Viable Gametes without Meiosis in Maize Deficient for an ARGONAUTE Protein. The Plant Cell 23: 443-458.
Singh S. K., Kumar V., Srinivasan R., Ahuja P. S., Bhat S. R., Sreenivasulu Y. (2017). The TRAF Mediated Gametogenesis Progression ( TRAMGaP ) Gene Is Required for Megaspore Mother Cell Specification and Gametophyte Development . Plant Physiology 175: 1220-1237.
Sniezko R., (2006). Meiosis in Plants. In Plant Cell Biology. (Ed. Dashek W. V., Harrison P., ) Science Publisher, New Hampshire.
Su Z., Wang N., Hou Z., Li B., Li D., Liu Y., Cai H., Qin Y., Chen X. (2020). Regulation of Female Germline Specification via Small RNA Mobility in Arabidopsis. The Plant Cell 32: 2842-2854.
Su Z., Zhao L., Zhao Y., Li S., Won S., Cai H., Wang L., Li Z., Chen P., Qin Y., Chen X. (2017). The THO complex non-cell-autonomously represses female germline specification through the TAS3-ARF3 module. Current Biology 27: 1597-1609.
Talbert P. B., Henikoff S. (2021). The Yin and Yang of Histone Marks in Transcription. Annual Review of Genomics and Human Genetics 22: 147-170.
Tovar-Aguilar A., Grimanelli D., Acosta-García G., Vielle-Calzada J. P., Badillo-Corona J. A., Durán-Figueroa N. (2023). The miRNA822 loaded by ARGONAUTE9 modulates the monosporic female gametogenesis in Arabidopsis thaliana. Plant Reproduction In Press: .
Tucker M. R., Koltunow A. M. G. (2009). Sexual and asexual (apomictic) seed development in flowering plants: molecular, morphological and evolutionary relationships. Functional Plant Biology 36: 490.
Vijayan A., Tofanelli R., Strauss S., Cerrone L., Wolny A., Strohmeier J., Kreshuk A., Hamprecht F. A., Smith R. S., Schneitz K. (2021). A digital 3D reference atlas reveals cellular growth patterns shaping the Arabidopsis ovule. eLife 10: e63262.
Wang H., Dittmer T. A., Richards E. J. (2013). Arabidopsis CROWDED NUCLEI (CRWN) proteins are required for nuclear size control and heterochromatin organization. BMC Plant Biology 13: 1-13.
Wang Y., Cheng Z., Huang J., Shi Q., Hong Y., Copenhaver G. P., Gong Z., Ma H. (2012). The DNA Replication Factor RFC1 Is Required for Interference-Sensitive Meiotic Crossovers in Arabidopsis thaliana. PLoS Genetics 8: e1003039.
Webb M.C., Gunning B.E.S. (1990). Embryo sac development in Arabidopsis thaliana. Sexual Plant Reproduction 3: 224-256.
Wu C.C., Diggle P. K., Friedman W. E. (2011). Female gametophyte development and double fertilization in Balsas teosinte, Zea mays subsp. parviglumis (Poaceae). Sexual Plant Reproduction 24: 219-229.
Xie M., Yu B. (2015). siRNA-directed DNA Methylation in Plants. Current Genomics 16: 23-31.
Yang W.C., Sundaresan V. (2000). Genetics of gametophyte biogenesis in Arabidopsis. Current Opinion in Plant Biology 3: 53-57.
Yelina N. E., Smith L. M., Jones A. M. E., Patel K., Kelly K. A., Baulcombe D. C. (2010). Putative Arabidopsis THO/TREX mRNA export complex is involved in transgene and endogenous siRNA biosynthesis . Proceedings of the National Academy of Sciences 107: 13948-13953.
Zhao H., Guo M., Yan M., Cheng H., Liu Y., She Z., Lai L., Shi C., Zhang M., Li Y., Lin D., Qin Y. (2020). Comparative Expression Profiling Reveals Genes Involved in Megasporogenesis. Plant Physiology 182: 2006-2024.
Zhao L., Liu L., Liu Y., Dou X., Cai H., Aslam M., Hou Z., Jin X., Li Y., Wang L., Zhao H., Wang X., Sicard A., Qin Y. (2021). Characterization of germline development and identification of genes associated with germline specification in pineapple. Horticulture Research 8: 239.
Zhao X., De Palma J., Oane R., Gamuyao R., Luo M., Chaudhury A., Hervé P., Xue Q., Bennett J. (2008). OsTDL1A binds to the LRR domain of rice receptor kinase MSP1, and is required to limit sporocyte numbers. The Plant Journal 54: 375-387.
Zhao L., He J., Cai H., Lin H., Li Y., Liu R., Yang Z., Qin Y. (2014). Comparative expression profiling reveals gene functions in female meiosis and gametophyte development in Arabidopsis. The Plant Journal 80: 615-628.
Zhao X.A., Bramsiepe J., Van Durme M., Komaki S., Prusicki M. A., Maruyama D., Forner J., Medzihradszky A., Wijnker E., Harashima H., Lu Y., Schmidt A., Guthörl D., Logroño R. S., Guan Y., Pochon G., Grossniklaus U., Laux T., Higashiyama T., Lohmann J. U., Nowack M. K., Schnittger A. (2017). RETINOBLASTOMA RELATED1 mediates germline entry in Arabidopsis . Science 356: eaaf6532.
Zhong X., Du J., Hale C. J., Gallego-Bartolome J., Feng S., Vashisht A. A., Chory J., Wohlschlegel J. A., Patel D. J., Jacobsen S. E. (2014). Molecular Mechanism of Action of Plant DRM De Novo DNA Methyltransferases. Cell 157: 1050-1060.