Int. J. Dev. Biol. 68: 79 - 83 (2024)
TBC1D24 is likely to regulate vesicle trafficking in glia-like non-sensory epithelial cells of the cochlea
Open Access | Developmental Expression Pattern | Published: 11 June 2024
Abstract
Mutations in the gene encoding Tre2/Bub2/Cdc16 (TBC)1 domain family member 24 (TBC1D24) protein are associated with a variety of neurological disorders, ranging from non-syndromic hearing loss to drug-resistant lethal epileptic encephalopathy and DOORS syndrome [Deafness, Onychodystrophy, Osteodystrophy, intellectual disability (formerly referred to as mental Retardation), and Seizures]. TBC1D24 is a vesicle-associated protein involved in neural crest cell and neuronal migration, maturation, and neurotransmission. In the cochlea, TBC1D24 has been detected in auditory neurons, but few reliable and convergent data exist about the sensory epithelium. Here, the expression of TBC1D24 has been characterized via immunolabelling throughout the postnatal maturation of the mouse cochlear sensory epithelium. TBC1D24 was detected in glia-like non-sensory epithelial cells during early developmental stages. In contrast, TBC1D24 was virtually absent in adjacent sensory hair cells. This expression distinguishing non-sensory from sensory epithelial cells almost disappears around the onset of hearing. Until now, TBC1D24 was mainly described as a neuronal protein either in the brain or in the cochlea. The present observations suggest that TBC1D24 could also regulate vesicle trafficking in cochlear glia-like non-sensory epithelial cells. For a long time, research about epilepsy has been mainly neurocentric. However, there is now evidence proving that glial cell dysregulation contribute to pathogenesis of epilepsy and neurodevelopmental disorders. As a consequence, exploring the possibility that TBC1D24 could also have a role in glial cells of the central nervous system could help to gain insight into TBC1D24-related neurological pathogenesis.
Keywords
TBC1D24, epilepsy, cochlea, glia-like, deafness
Introduction
Pathogenic mutations in human TBC1D24 (Tre2/Bub2/Cdc16 (TBC)1 domain family member 24) are associated with several distinct more or less severe clinical phenotypes. These manifestations include mild seizures to early-infantile drug-resistant lethal epileptic encephalopathy, infantile myoclonic epilepsy, intellectual disability, non-syndromic hearing loss, and DOORS syndrome (Balestrini et al., 2016; Campeau et al., 2014). DOORS is a multisystem disorder characterized by Deafness, Onychodystrophy, Osteodystrophy, intellectual disability (formerly referred to as mental Retardation), and Seizures. Mutations in TBC1D24 responsible for focal epilepsy with intellectual disability and familiar infantile myoclonic epilepsy were initially described in 2010 (Corbett et al., 2010; Falace et al., 2010). To date, over 100 pathogenic variants in the coding sequence of TBC1D24 have been reported, including in-frame deletion, missense, nonsense, frameshift and splice site mutations (ClinVar database). Mutations in TBC1D24 do not exhibit a clear genotype-phenotype correlation, making it difficult to predict the potential clinical phenotypes based on the location and type of mutations.
The human TBC1D24 gene encodes several alternatively spliced transcripts (Rehman et al., 2014). The longest transcript encodes a protein of 559 amino acids, with an N-terminal TBC domain followed by 82 residues that link to a C-terminus TLDc domain (TBC, LysM and domain catalytic). This combination of TBC and TLDc domains is unique in the mammalian proteome. The TBC domain is shared by Rab-GTPase activating proteins (RabGAPs), and it regulates vesicle trafficking by interacting with the small Ras GTPAse ARF6 (ADP-ribosylation factor 6) (Falace et al., 2010). The TLDc domain could function as a reactive oxygen species sensor regulating vesicle trafficking rates that, when dysfunctional, causes a movement disorder in patients and in fly models (Lüthy et al., 2019). TBC1D24 is a vesicle-associated protein involved in neuritogenesis, neural crest cell and neuronal migration, maturation, and neurotransmission (Aprile et al., 2019; Falace et al., 2010, 2014; Finelli et al., 2019; Lin et al., 2020; Yoon et al., 2018). In vivo, TBC1D24 is critical for presynaptic function (Finelli et al., 2019; Fischer et al., 2016). Loss of Tbc1d24 in mice induces a deficient endocytosis, as well as an enlarged endosomal compartment in neurons with a decrease in spontaneous neurotransmission (Finelli et al., 2019). Vesicle trafficking defects induced by human TLDc mutations in flies have been rescued by antioxidant molecules. Thus, antioxidant treatments could represent a potential therapeutic strategy to TBC1D24-related disorders (Lüthy et al., 2019).
TBC1D24-related non-syndromic hearing loss can be inherited in dominant (DFNA65) or recessive (DFNB86) patterns. DFNA65 is rather associated with progressive postlingual deafness (Azaiez et al., 2014; Oziębło et al., 2021; Zhang et al., 2014), whereas DFNB86 is rather associated with profound prelingual deafness (Rehman et al., 2014). Mouse models of human TBC1D24 pathogenic variants associated with syndromic and non-syndomic forms of deafness DFNA65 and DFNB86 have been engineered. Unexpectedly, no auditory dysfunction was detected in Tbc1d24 mutant mice, although homozygosity for some of the variants caused seizures or lethality (Tona et al., 2020). In the cochlea, Tbc1d24 mRNA and TBC1D24 protein have been detected in auditory neurons (Rehman et al., 2014; Tona et al., 2020), but few reliable and convergent data exist about the sensory epithelium (Azaiez et al., 2014; Tona et al., 2020; Zhang et al., 2014). This could be due to lack of antibody specificity and potential cross-reactivity in immunolabelling. For this reason, I have characterized the expression of TBC1D24 throughout the postnatal maturation of the mouse cochlear sensory epithelium using a polyclonal antibody whose specificity has been previously definitely confirmed (Tona et al., 2019).
Results
In mammals, sounds are perceived via mechanosensory hair cells located within the sensory epithelium of the cochlea (i.e. the organ of Corti). Within the organ of Corti, sensory hair cells and several types of specialized non-sensory supporting cells are organized in a regular mosaic pattern that extends along the basal-to-apical axis of the cochlea. Throughout immature development, between the organ of Corti and the modiolus (i.e. the bony axis) of the cochlea resides the greater epithelial ridge, a transient cell population that is progressively replaced by the inner sulcus during the postnatal maturation. The edge of the organ of Corti located closest to the modiolus is composed of a single row of alternating inner hair cells and border/inner phalangeal cells. Directly adjacent to the inner hair cell row are two rows of pillar cells. Then, three or four rows of outer hair cells and Deiters’ cells are also arranged into a regular alternating mosaic. Finally, the edge of the organ of Corti located closest to the stria vascularis is formed by the interface between the most external row of outer hair cells and directly adjacent Hensen’s cells.
It has been clear for a long time that sensory hair cells play a primary role in mechanotransduction and synaptic transmission. In contrast, non-sensory supporting cells were thought to be silent and function to support adjacent sensory hair cells. A growing body of evidence now suggests that supporting cells are not silent and play important roles in sensory hair cell function and signal transduction. In this sense, non-sensory epithelial supporting cells share many molecular and physiological features with the glial cells in the central nervous system. These cells are defined as “glia-like cells” because they express typical glia markers such as PLP, GFAP, GLAST, and Aquaporin 4 and they regulate K+ buffering like astrocytes in the brain (Jang et al., 2022; Wan et al., 2013). Glia-like non-sensory epithelial supporting cells also participate in the maturation, proliferation, protection and regeneration of adjacent sensory hair cells, like glial cells in the central nervous system (Jang et al., 2022; Wan et al., 2013).
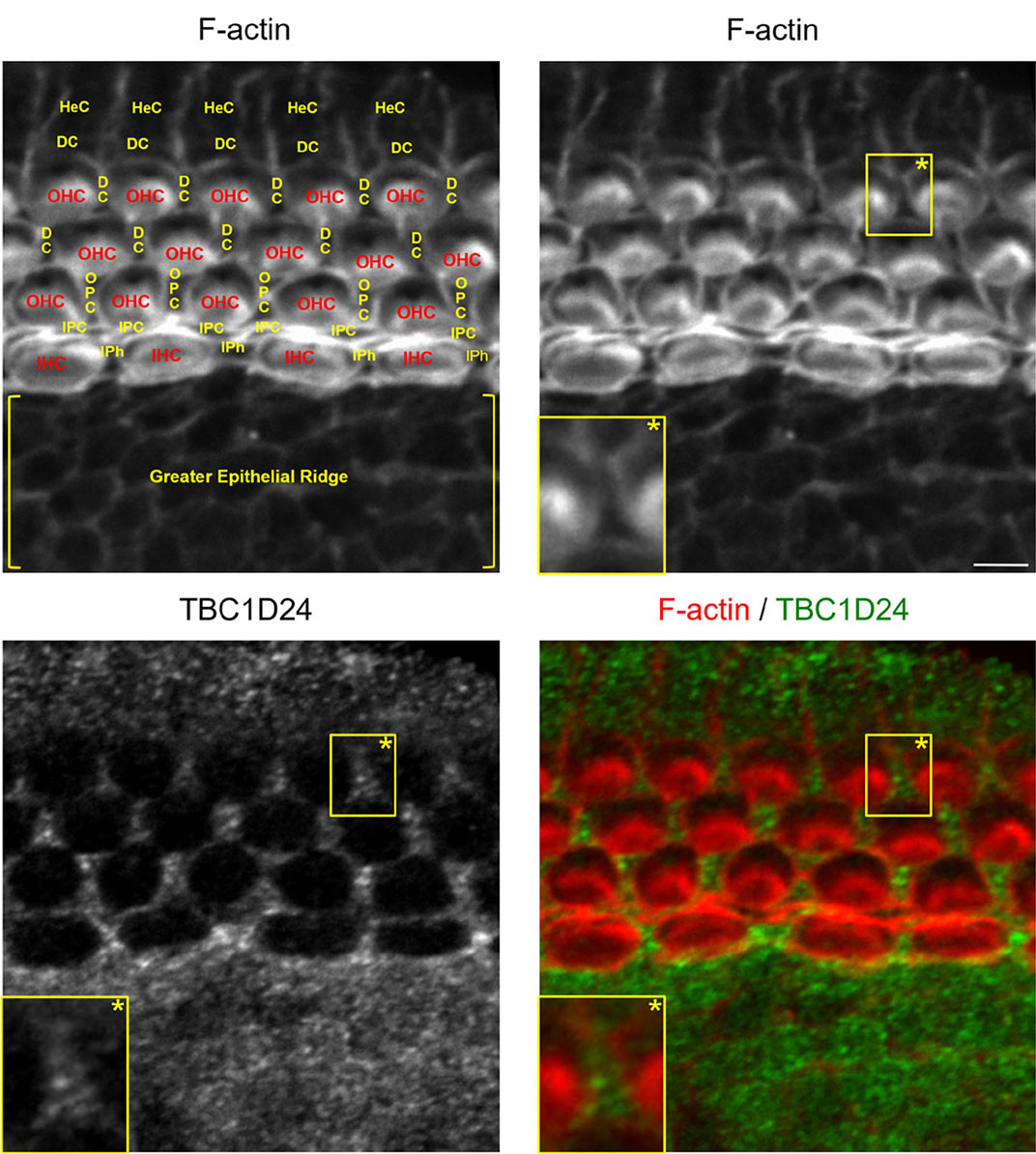
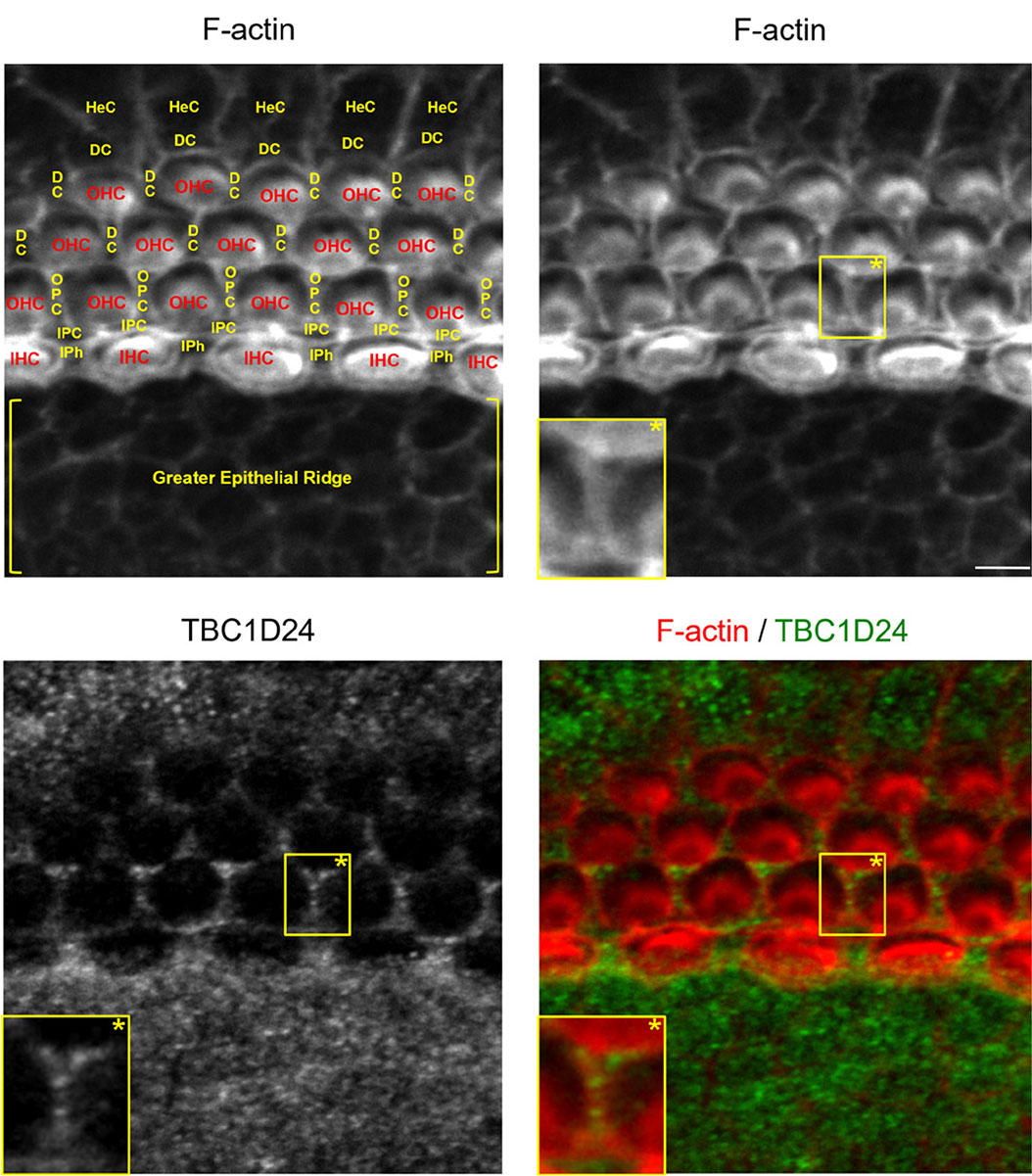
To delineate individual epithelial cells, TBC1D24 immunolabelling has been combined with F-actin staining. In the cochlear sensory epithelium of newborn (postnatal day 0, P0) and three-day-old (P3) mice, I have observed that TBC1D24 is exclusively expressed in non-sensory epithelial cells of the cochlea. Of note is that TBC1D24 immunolabelling mostly exhibits small intracellular or submembrane positive spots (Figs. 1 and 2). This type of subcellular expression profile is rather expected for a vesicle-associated protein, and it is quite similar to previous observations in auditory neurons (Tona et al., 2020). In contrast, TBC1D24 was not detected in adjacent inner and outer sensory hair cells. In six-day-old mice, I have observed a significant change in the expression pattern. At this stage, TBC1D24 expression is mostly restricted to inner and outer pillar cells (with a prominent labelling in the inner pillar cells), and is more diffuse throughout the cytoplasm. It is worth noting that a faint labelling is also present in the outer hair cells (Fig. 3). This evolution could suggest a change in TBC1D24 function. Overall, these findings somewhat differ from some previous observations in mice or humans (Azaiez et al., 2014; Tona et al., 2020; Zhang et al., 2014). However, they remain consistent with data reported in the Shared Harvard Inner Ear Laboratory Database (SHIELD) (Shen et al., 2015). These data suggest that TBC1D24 expression level is overall higher in FACS-sorted non-sensory supporting cells than in sensory hair cells throughout the embryonic and postnatal maturation of the mouse cochlear sensory epithelium (Scheffer et al., 2015).
Fig. 1. TBC1D24 is expressed in all types of glia-like non-sensory epithelial cells of a newborn mouse cochlea.
F-actin (red) staining combined with TBC1D24 (green) immunolabelling of the middle portion of a newborn mouse cochlear sensory epithelium. Abbreviations for sensory hair cells and for glia-like, non-sensory epithelial cells are written in red and in yellow, respectively. Scale bar, 5 µm. DC, Deiters’ cell; HeC, Hensen’s cell; IHC, inner hair cell; IPC, inner pillar cell; IPh, inner phalangeal cell; OHC, outer hair cell; OPC, outer pillar cell.
Fig. 2. TBC1D24 is expressed in all types of glia-like non-sensory epithelial cells of a three-day-old mouse cochlea.
F-actin (red) staining combined with TBC1D24 (green) immunolabelling of the middle portion of a three-day-old mouse cochlear sensory epithelium. Abbreviations for sensory hair cells and for glia-like non-sensory epithelial cells are written in red and in yellow, respectively. Scale bar, 5 µm. Abbreviations as in Fig. 1.
Fig. 3. TBC1D24 is mainly expressed in inner and outer pillar cells of a six-day-old mouse cochlear sensory epithelium.
F-actin (red) staining combined with TBC1D24 (green) immunolabelling of the middle portion of a six-day-old mouse cochlear sensory epithelium. Abbreviations for sensory hair cells and for glia-like non-sensory epithelial cells are written in red and in yellow, respectively. Scale bar, 5 µm. Abbreviations as in Fig. 1.
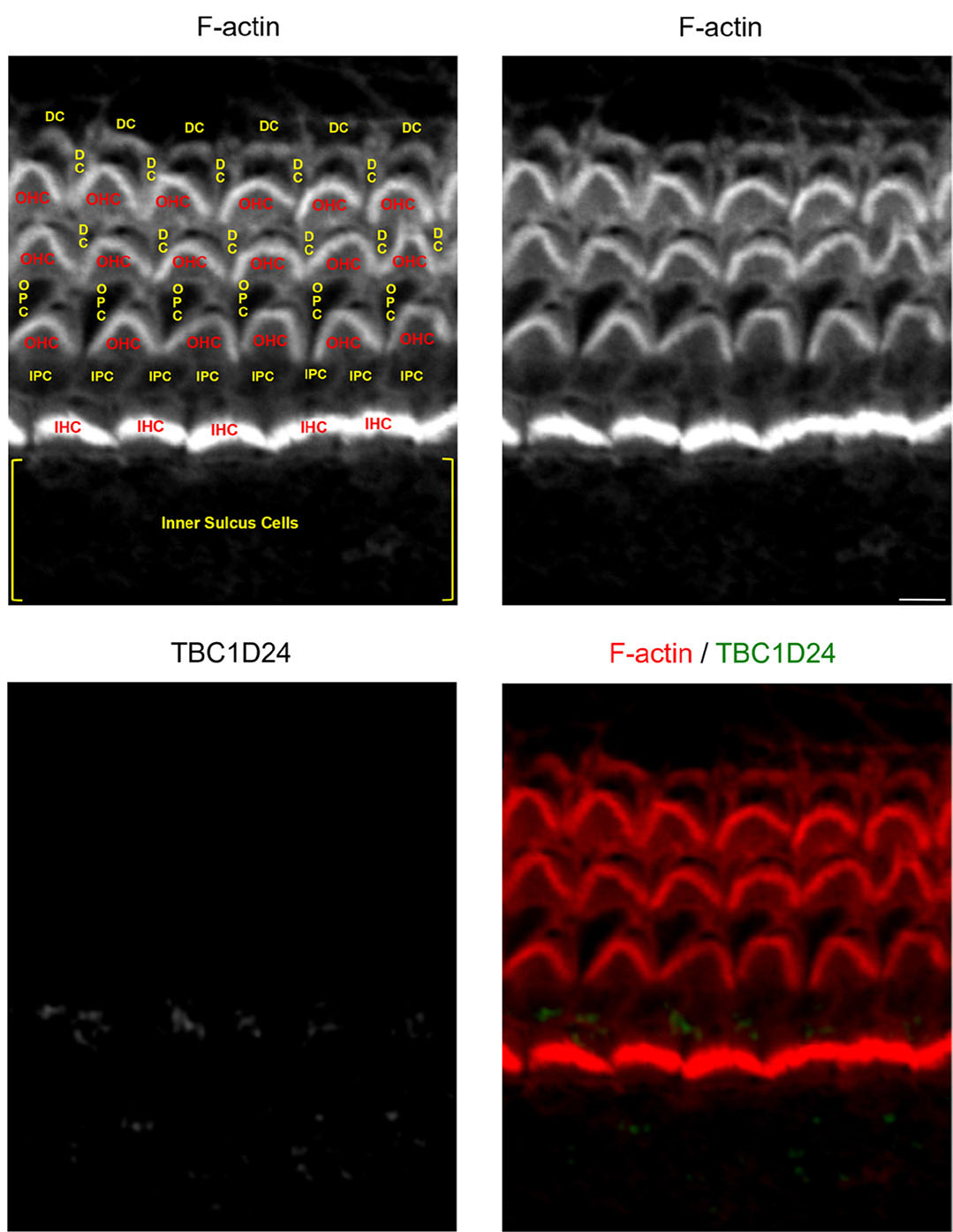
Around the onset of hearing in mice (twelve-day-old), TBC1D24 immunolabelling was no longer detectable in the cochlear sensory epithelium (Fig. 4). Still according to the SHIELD database, transcriptomic analyses revealed that Tbc1d24 does not exceed the baseline value for expression in adult cochlear sensory hair cells (Liu et al., 2014). Genes whose expression falls below the baseline are not considered to be expressed.
Fig. 4. TBC1D24 is virtually not expressed in a twelve-day-old mouse cochlear sensory epithelium.
F-actin (red) staining combined with TBC1D24 (green) immunolabelling of the middle portion of a twelve-day-old mouse cochlear sensory epithelium. Abbreviations for sensory hair cells and for glia-like non-sensory epithelial cells are written in red and in yellow, respectively. Scale bar, 5 µm. Abbreviations as in Fig. 1.
Discussion
Here, I have observed that TBC1D24 is mainly expressed in glia-like non-sensory epithelial cells of the cochlea throughout early postnatal stages. Until now, very few proteins have been found to interact with TBC1D24. Among these are ARF6 and ephrin-B2 (Falace et al., 2010; Yoon et al., 2018). Ephrin-B2 is a transmembrane protein, which belongs to the Eph/ephrin family. Eph receptors represent the largest family of receptors tyrosine kinase identified to date. Eph/ephrin interactions can trigger a wide array of cellular responses, including the control of axon guidance, intercellular junctions, cell adhesion and migration (Kania and Kein, 2016). There is now evidence proving that the Eph/ephrin system plays a leading role in the development and function of the cochlea in mammals (Defourny, 2019). In Xenopus embryos, TBC1D24 – ephrin-B2 interactions control contact inhibition of locomotion in neural crest cell migration by regulating the concentration of adherens junction protein E-cadherin at the plasma membrane (Yoon et al., 2018). Interestingly enough, the present observations suggest that TBC1D24 shares an overlapping expression profile with ephrin-B2 in glia-like non-sensory epithelial cells of the cochlea (Defourny et al., 2015). In the organ of Corti, ephrin-B2 has been shown to control the formation and the destruction of gap and adherens junctions between Deiters’ and pillar cells, respectively (Defourny et al., 2019, 2021).
Sensory and adjacent non-sensory epithelial cells express a variety of additional adherens and tight junction proteins that establish a hermetic barrier between two fluid compartments, the endolymph and the perilymph. Mechanosensory hair cells are bathed in endolymph (high electric potential and unique ion composition: high [K+] and low [Na+]) at their apical side and in perilymph (low [K+] and high [Na+]) at their basal side. Hair cells convert sound vibrations detected by stereocilia at the apical side into electric action potential and relay it to auditory neurons. Failure in barrier function in the sensory epithelium results in death of hair cells and causes deafness (Higashi and Miller, 2017). These junctional complexes require continuous endocytosis and recycling of their components (Chalmers and Whitley, 2012; Stamatovic et al., 2017). Thus, TBC1D24 could be a candidate to regulate vesicle trafficking of junction proteins between the endosomal compartment and the plasma membrane to establish and/or maintain epithelial barrier function.
It is worth noting that TBC1D24 is virtually no longer expressed around the onset of hearing. This observation suggests that the role of TBC1D24 mainly concerns the maturation and the developmental regulation of the cochlear sensory epithelium. This could indicate, to some extent, that TBC1D24 is dispensable for mechanotransduction and auditory function themselves.
For a long time, research about epilepsy has been mainly neurocentric. However, there is now evidence proving that glial cell dysregulation contribute to pathogenesis of epilepsy and neurodevelopmental disorders (Kim et al., 2020; Neniskyte and Gross, 2017; Patel et al., 2019; Vezzani et al., 2022). In the central nervous system, TBC1D24 is mainly considered as a neuronal protein. Very few data exist about the presence or the absence of TBC1D24 in glial cells in vivo. One minor exception is the virtual absence of TBC1D24 from twin astrocyte cultures in vitro (Aprile et al., 2019). However, the present observations showing that TBC1D24 is expressed in glia-like cells of the cochlear sensory epithelium raise the possibility that this protein could also have a role in glial cells of the central nervous system. Although the contribution of neuronal dysfunction in the onset of the disease is now evident, exploring this hypothesis could help to gain insight into TBC1D24-related neurological pathogenesis.
Material and methods
Animals
Mice of the BALB/c strain were grouped-housed in the animal facility of the University of Liege under standard conditions with food and water ad libitum and were maintained on a 12h light/dark cycle. All animals were taken care in accordance with the Declaration of Helsinki and following the guidelines of the Belgian ministry of agriculture in agreement with the EC laboratory animal care and use regulation (2010/63/UE, 22 September 2010).
Tissue processing and immunostaining
Cochleae of newborn, three-, six- and twelve-day-old mice were dissected and fixed for 2h in 4% formaldehyde at 4°C. If necessary, tissues were decalcified for two or three days (0.1 M in EDTA acid in phosphate buffered saline (PBS)). Permeabilization and blockage unspecific binding sites were performed by 30 minutes of incubation at room temperature in blocking solution (0.25% gelatin and 0.1% Triton X-100 in PBS). Dissected cochleae were incubated overnight at 4°C with a primary antibody directed against TBC1D24 (rabbit polyclonal IgG, 1:250, NBP1-82925, RRID: AB_11061868, Novus Biologicals, Littleton, CO, USA). The epitope used to raise this antibody was 77 residues (51-127) of human TBC1D24. The amino acid sequence of this epitope is 94.8% identical to the same region of mouse TBC1D24. As mentioned above, the specificity of this antibody has been confirmed. It shows little or no cross-reactivity with other proteins in the mouse brain (Tona et al., 2019). After several washings in PBS, tissues were incubated for 1h at room temperature in blocking solution containing fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG secondary antibody (1:1000, Jackson Immunoresearch Laboratories, Suffolk, UK) and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin (1:1000, Sigma Aldrich, St Louis, MO, USA) as F-actin marker. Finally, tissues were washed in PBS, mounted and coverslipped using VectaShield Hard Set mounting medium (Vector Laboratories, Burlingame, CA, USA).
Confocal microscopy and imaging
Fluorescence images were acquired using the Olympus Fluoview FV1000 confocal system (Olympus Europa GmbH) with objective LUCPLFLN 40X numerical aperture 0.60, working distance 2.7-4 mm, oil immersion. Images are representative of three different animals.
Acknowledgements
This work was supported by the Belgian Fonds de la Recherche Scientifique – FNRS.
References
Aprile D., Fruscione F., Baldassari S., Fadda M., Ferrante D., Falace A., Buhler E., Sartorelli J., Represa A., Baldelli P., Benfenati F., Zara F., Fassio A. (2019). TBC1D24 regulates axonal outgrowth and membrane trafficking at the growth cone in rodent and human neurons. Cell Death & Differentiation 26: 2464-2478.
Azaiez H., Booth K. T., Bu F., Huygen P., Shibata S. B., Shearer A. E., Kolbe D., Meyer N., Black-Ziegelbein E. A., Smith R. J.H. (2014). TBC1D24 Mutation Causes Autosomal-Dominant Nonsyndromic Hearing Loss . Human Mutation 35: 819-823.
Balestrini S., Milh M., Castiglioni C., Lüthy K., Finelli M. J., Verstreken P., Cardon A., Stražišar B. G., Holder J. L., Lesca G., Mancardi M. M., Poulat A. L., Repetto G. M., Banka S., Bilo L., Birkeland L. E., Bosch F., Brockmann K., Cross J. H., Doummar D., Félix T. M., Giuliano F., Hori M., Hüning I., Kayserili H., Kini U., Lees M. M., Meenakshi G., Mewasingh L., Pagnamenta A. T., Peluso S., Mey A., Rice G. M., Rosenfeld J. A., Taylor J. C., Troester M. M., Stanley C. M., Ville D., Walkiewicz M., Falace A., Fassio A., Lemke J. R., Biskup S., Tardif J., Ajeawung N. F., Tolun A., Corbett M., Gecz J., Afawi Z., Howell K. B., Oliver K. L., Berkovic S. F., Scheffer I. E., de Falco F. A., Oliver P. L., Striano P., Zara F., Campeau P. M., Sisodiya S.M. (2016). TBC1D24 genotype–phenotype correlation . Neurology 87: 77-85.
Campeau P. M., Kasperaviciute D., Lu J. T., Burrage L. C., Kim C., Hori M., Powell B. R., Stewart F., Félix T. M., van den Ende J., Wisniewska M., Kayserili H., Rump P., Nampoothiri S., Aftimos S., Mey A., Nair L. D. V., Begleiter M. L., De Bie I., Meenakshi G., Murray M. L., Repetto G. M., Golabi M., Blair E., Male A., Giuliano F., Kariminejad A., Newman W. G., Bhaskar S. S., Dickerson J. E., Kerr B., Banka S., Giltay J. C., Wieczorek D., Tostevin A., Wiszniewska J., Cheung S. W., Hennekam R. C., Gibbs R. A., Lee B. H., Sisodiya S. M. (2014). The genetic basis of DOORS syndrome: an exome-sequencing study. The Lancet Neurology 13: 44-58.
Chalmers A. D., Whitley P. (2012). Continuous endocytic recycling of tight junction proteins: how and why?. Essays in Biochemistry 53: 41-54.
Corbett M. A., Bahlo M., Jolly L., Afawi Z., Gardner A. E., Oliver K. L., Tan S., Coffey A., Mulley J. C., Dibbens L. M., Simri W., Shalata A., Kivity S., Jackson G. D., Berkovic S. F., Gecz J. (2010). A Focal Epilepsy and Intellectual Disability Syndrome Is Due to a Mutation in TBC1D24. The American Journal of Human Genetics 87: 371-375.
Defourny J. (2019). Eph/ephrin signalling in the development and function of the mammalian cochlea. Developmental Biology 449: 35-40.
Defourny J., Audouard C., Davy A., Thiry M. (2021). Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Research Bulletin 174: 153-160.
Defourny J., Mateo Sánchez S., Schoonaert L., Robberecht W., Davy A., Nguyen L., Malgrange B. (2015). Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nature Communications 6: 7017.
Defourny J., Peuckert C., Kullander K., Malgrange B. (2019). EphA4-ADAM10 Interplay Patterns the Cochlear Sensory Epithelium through Local Disruption of Adherens Junctions. iScience 11: 246-257.
Falace A., Buhler E., Fadda M., Watrin F., Lippiello P., Pallesi-Pocachard E., Baldelli P., Benfenati F., Zara F., Represa A., Fassio A., Cardoso C. (2014). TBC1D24 regulates neuronal migration and maturation through modulation of the ARF6-dependent pathway. Proceedings of the National Academy of Sciences 111: 2337-2342.
Falace A., Filipello F., La Padula V., Vanni N., Madia F., De Pietri Tonelli D., de Falco F. A., Striano P., Dagna Bricarelli F., Minetti C., Benfenati F., Fassio A., Zara F. (2010). TBC1D24, an ARF6-Interacting Protein, Is Mutated in Familial Infantile Myoclonic Epilepsy. The American Journal of Human Genetics 87: 365-370.
Finelli M. J., Aprile D., Castroflorio E., Jeans A., Moschetta M., Chessum L., Degiacomi M. T., Grasegger J., Lupien-Meilleur A., Bassett A., Rossignol E., Campeau P. M., Bowl M. R., Benfenati F., Fassio A., Oliver P. L. (2019). The epilepsy-associated protein TBC1D24 is required for normal development, survival and vesicle trafficking in mammalian neurons. Human Molecular Genetics 28: 584-597.
Fischer B., Lüthy K., Paesmans J., De Koninck C., Maes I., Swerts J., Kuenen S., Uytterhoeven V., Verstreken P., Versées W. (2016). Skywalker-TBC1D24 has a lipid-binding pocket mutated in epilepsy and required for synaptic function. Nature Structural & Molecular Biology 23: 965-973.
Higashi T., Miller A. L. (2017). Tricellular junctions: how to build junctions at the TRICkiest points of epithelial cells. Molecular Biology of the Cell 28: 2023-2034.
Jang M. W., Lim J., Park M. G., Lee J.H., Lee C. J. (2022). Active role of glia‐like supporting cells in the organ of Corti: Membrane proteins and their roles in hearing. Glia 70: 1799-1825.
Kania A., Klein R. (2016). Mechanisms of ephrin–Eph signalling in development, physiology and disease. Nature Reviews Molecular Cell Biology 17: 240-256.
Kim Y. S., Choi J., Yoon B.E. (2020). Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 9: 2176.
Lin L., Lyu Q., Kwan P.Y., Zhao J., Fan R., Chai A., Lai C. S. W., Chan Y.S., Shen X., Lai K.O. (2020). The epilepsy and intellectual disability-associated protein TBC1D24 regulates the maintenance of excitatory synapses and animal behaviors. PLOS Genetics 16: e1008587.
Liu H., Pecka J. L., Zhang Q., Soukup G. A., Beisel K. W., He D. Z. Z. (2014). Characterization of Transcriptomes of Cochlear Inner and Outer Hair Cells. Journal of Neuroscience 34: 11085-11095.
Lüthy K., Mei D., Fischer B., De Fusco M., Swerts J., Paesmans J., Parrini E., Lubarr N., Meijer I. A., Mackenzie K. M., Lee W.T., Cittaro D., Aridon P., Schoovaerts N., Versées W., Verstreken P., Casari G., Guerrini R. (2019). TBC1D24-TLDc-related epilepsy exercise-induced dystonia: rescue by antioxidants in a disease model. Brain 142: 2319-2335.
Neniskyte U., Gross C. T. (2017). Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nature Reviews Neuroscience 18: 658-670.
Oziębło D., Leja M. L., Lazniewski M., Sarosiak A., Tacikowska G., Kochanek K., Plewczynski D., Skarżyński H., Ołdak M. (2021). TBC1D24 emerges as an important contributor to progressive postlingual dominant hearing loss. Scientific Reports 11: 10300.
Patel D. C., Tewari B. P., Chaunsali L., Sontheimer H. (2019). Neuron–glia interactions in the pathophysiology of epilepsy. Nature Reviews Neuroscience 20: 282-297.
Rehman A. U., Santos-Cortez R. L. P., Morell R. J., Drummond M. C., Ito T., Lee K., Khan A. A., Basra M. A. R., Wasif N., Ayub M., Ali R. A., Raza S. I., Nickerson D. A., Shendure J., Bamshad M., Riazuddin S., Billington N., Khan S. N., Friedman P. L., Griffith A. J., Ahmad W., Riazuddin S., Leal S. M., Friedman T. B. (2014). Mutations in TBC1D24, a Gene Associated With Epilepsy, Also Cause Nonsyndromic Deafness DFNB86. The American Journal of Human Genetics 94: 144-152.
Scheffer D. I., Shen J., Corey D. P., Chen Z.Y. (2015). Gene Expression by Mouse Inner Ear Hair Cells during Development. The Journal of Neuroscience 35: 6366-6380.
Shen J., Scheffer D. I., Kwan K. Y., Corey D. P. (2015). SHIELD: an integrative gene expression database for inner ear research. Database 2015: bav071.
Stamatovic S. M., Johnson A. M., Sladojevic N., Keep R. F., Andjelkovic A. V. (2017). Endocytosis of tight junction proteins and the regulation of degradation and recycling. Annals of the New York Academy of Sciences 1397: 54-65.
Tona R., Chen W., Nakano Y., Reyes L. D., Petralia R. S., Wang Y.X., Starost M. F., Wafa T. T., Morell R. J., Cravedi K. D., du Hoffmann J., Miyoshi T., Munasinghe J. P., Fitzgerald T. S., Chudasama Y., Omori K., Pierpaoli C., Banfi B., Dong L., Belyantseva I. A., Friedman T. B. (2019). The phenotypic landscape of a Tbc1d24 mutant mouse includes convulsive seizures resembling human early infantile epileptic encephalopathy. Human Molecular Genetics 28: 1530-1547.
Tona R., Lopez I. A., Fenollar-Ferrer C., Faridi R., Anselmi C., Khan A. A., Shahzad M., Morell R. J., Gu S., Hoa M., Dong L., Ishiyama A., Belyantseva I. A., Riazuddin S., Friedman T. B. (2020). Mouse Models of Human Pathogenic Variants of TBC1D24 Associated with Non-Syndromic Deafness DFNB86 and DFNA65 and Syndromes Involving Deafness. Genes 11: 1122.
Vezzani A., Ravizza T., Bedner P., Aronica E., Steinhäuser C., Boison D. (2022). Astrocytes in the initiation and progression of epilepsy. Nature Reviews Neurology 18: 707-722.
Wan G., Corfas G., Stone J. S. (2013). Inner ear supporting cells: Rethinking the silent majority. Seminars in Cell & Developmental Biology 24: 448-459.
Yoon J., Hwang Y.S., Lee M., Sun J., Cho H. J., Knapik L., Daar I. O. (2018). TBC1d24-ephrinB2 interaction regulates contact inhibition of locomotion in neural crest cell migration. Nature Communications 9: 3491.
Zhang L., Hu L., Chai Y., Pang X., Yang T., Wu H. (2014). A Dominant Mutation in the Stereocilia-Expressing Gene TBC1D24 is a Probable Cause for Nonsyndromic Hearing Impairment . Human Mutation 35: 814-818.