Int. J. Dev. Biol. 68: 127 - 133 (2024)
Cell number regulation occurs during the pre-gastrulation period of postimplantation development in double chimeric mouse embryos
Open Access | Original Article | Published: 12 December 2024
Abstract
Aggregates of two mouse embryos produce viable offspring of normal size, indicating that there are mechanisms in the embryo that can downregulate their size to the size of the corresponding normal (single) embryos. Very little is known about the mechanisms controlling compensation for increased preimplantation size. Also, it is still elusive when exactly during development chimeric embryos regulate their size. Here, we determined the exact period of size regulation in chimeras. Using a chimeric embryo produced by aggregating two 8-cell stage embryos, we revealed that size regulation initiates shortly after implantation (E5.5) and ends with the start of gastrulation (E7.5). Importantly, processes that regulate cell number in chimeric embryos do not disturb morphogenesis, so that the formation of the proamniotic cavity occurs in parallel with size regulation.
Keywords
early mammalian development, chimeric embryo, size regulation, regulative development
Introduction
Chimeras are organisms composed of cells originating from two (or more) zygotes and thus differing genetically. Mouse chimeras were obtained for the first time by Tarkowski by aggregation of two cleaving embryos (Tarkowski, 1961). Since then the chimeras have been obtained by several other methods (review: Tarkowski, 1998), and they have become a part of such important experimental techniques, as gene targeting, tetraploid complementation, or cell lineage tracing. One of the most striking characteristic demonstrated by chimeric embryos is their ability to regulate their body size. Even though at the time of implantation the chimeric blastocysts (constructed by aggregation of two 8-cell stage embryos) have approximately twice the number of cells than normal embryos, the offspring born after transfer of chimeric embryos to foster mothers are of normal size (Tarkowski, 1961; Tarkowski, 1963). Subsequently, it has been demonstrated that aggregates of three (Markert and Petters, 1978), four (Petters and Markert, 1980) or even nine (Petters and Mettus, 1984) mouse embryos develop to normal size offspring after transfer of giant chimeric blastocysts to the recipient mouse females. What is important, half-embryos, obtained from single blastomeres of mouse 2-cell embryos also develop properly and, although initially smaller in size, ultimately give rise to normal size pups (Tarkowski, 1959). However, the mechanisms controlling downregulation of the size of chimeric embryos and upregulation of the size of half-embryos during development still remain vague.
It is still elusive when exactly during development the chimeric embryos regulate their size. It seems not to happen before implantation because it has been shown that the chimeric blastocysts constructed by aggregation of two 8-cell stage mouse embryos contain twice the number of cells of normal blastocysts (Buehr and McLaren, 1974). According to the early studies exploring indirect methods for the evaluation of the degree of regulation, the adjustment of size of chimeric embryos occurs between the time of the formation of the egg cylinder (E5.0) and the time of gastrulation (E7.0) (Buehr and McLaren, 1974) (Lewis and Rossant, 1982). Thus, the goal of our current study was to directly determine the exact period of the size regulation in the chimeras composed by aggregation of two mouse 8-cell embryos. To this end, we analyzed control and chimeric blastocysts and egg cylinders during pre- and perigastrulation period of development using confocal microscopy in order to calculate the cell number digitally on 3D reconstructions. This allowed us to detect the exact period of the regulation of the size of chimeras.
Results
To check when during the development of chimeric mouse embryos the size is downregulated, their cell number was examined and compared with the cell number of control single embryos at the blastocyst stage, and at E5.5, E6.5 and E7.5 day of pregnancy of recipient females.
Blastocysts
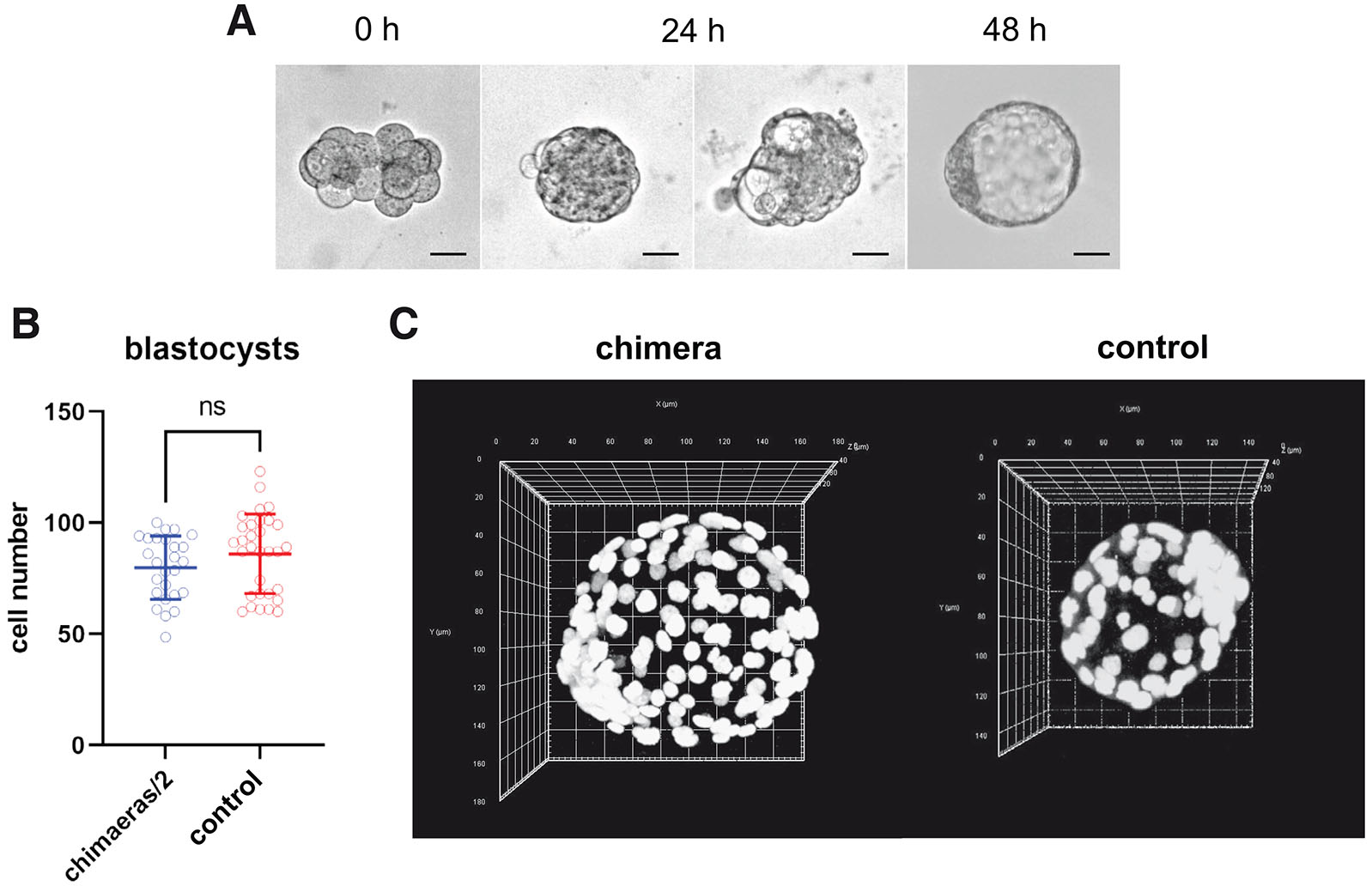
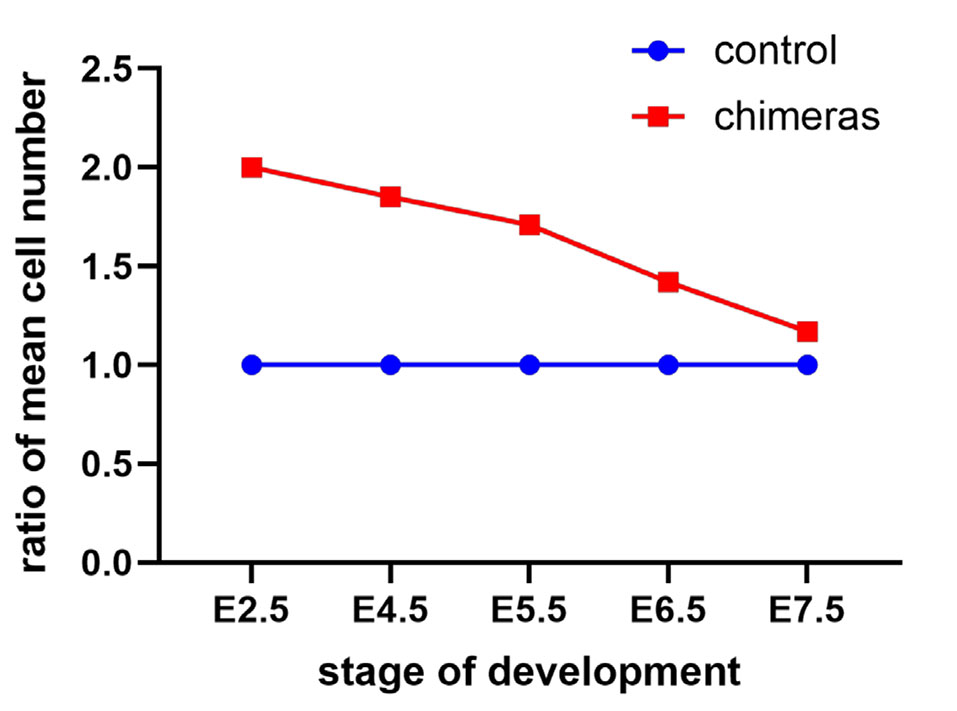
Chimeras were created by aggregation of two embryos at 8-cell stage and cell number of chimeric and control, single blastocysts was counted after 48 h of in vitro culture. At this stage of development chimeric blastocysts (n=26) were significantly larger than control blastocyst (n=31; Fig. 1 A,B). Mean cell number in experimental blastocyst was 159.6±28.5, when in control blastocysts it was 86.1±17.9. The proportion of the cell number of chimeric blastocyst to the cell number of control blastocysts was 1.85 (Fig. 5). To statistically analyze if the cell regulation is observed at this stage of development of chimeric embryos, for each chimeric blastocyst its cell number was divided by two, and the mean value was compared by Student’s t-test with the mean cell number in control blastocysts (Fig. 1A). The p value for this analysis was equal 0.1543, what indicates that processes that regulate the cell number in chimeric embryos are not active during formation of the blastocyst.
Fig. 1. Cell number regulation does not occur during the preimplantation development of chimeric embryos.
(A) Representative brightfield images showing a chimeric embryo developed from an aggregate after 24 and 48 h of in vitro culture. Within 24 hours after aggregation, most of the embryos reached the morula stage. Less commonly, the embryos displayed multiple incipient cavities. After the next 24 h, the embryos developed to the late blastocyst stage. Scale bar: 20 µm. (B) The average number of cells in chimeric (n=26) and control (n=31) E4.5 blastocysts. The graph presents means and standard deviations. ns, not significant. (C) Representative confocal images of chimeric and control E4.5 blastocysts. Chromatin (white) was labeled with chromomycin A3.
E5.5 egg cylinders
To verify if the regulation of cell number in chimeric embryos occurs at the E5.5 day of pregnancy, chimeric egg cylinders (n=18) at this stage of development were compared with the control egg cylinders at the same stage (n=22). We observed that during the early stages of postimplantation development a significant developmental asynchrony is observed between egg cylinders of the same litter. Thus, only the embryos in which proamniotic cavity was already formed, were selected for the examination of the cell number (Christodoulou et al., 2018). We scored the embryos according to the five distinct stages of proamniotic cavity formation (Christodoulou et al., 2018). At E5.5, most of the single and double embryos had opened a central lumen (stage III). Less commonly, we noted control and chimeric embryos with an incipient luminal cavity (stage II). The rarest were embryos with an expanded cavity extending toward the extra-embryonic ectoderm compartment (stage IV). We did not observe any differences in the percentage of such embryos between control and experimental groups. Embryos without proamniotic cavity were not selected for the examination of the cell number. At E6.5 and 7.5 stages, the two cavities fused into a unified single cavity spanning the whole embryo in both control and experimental groups (stage V).
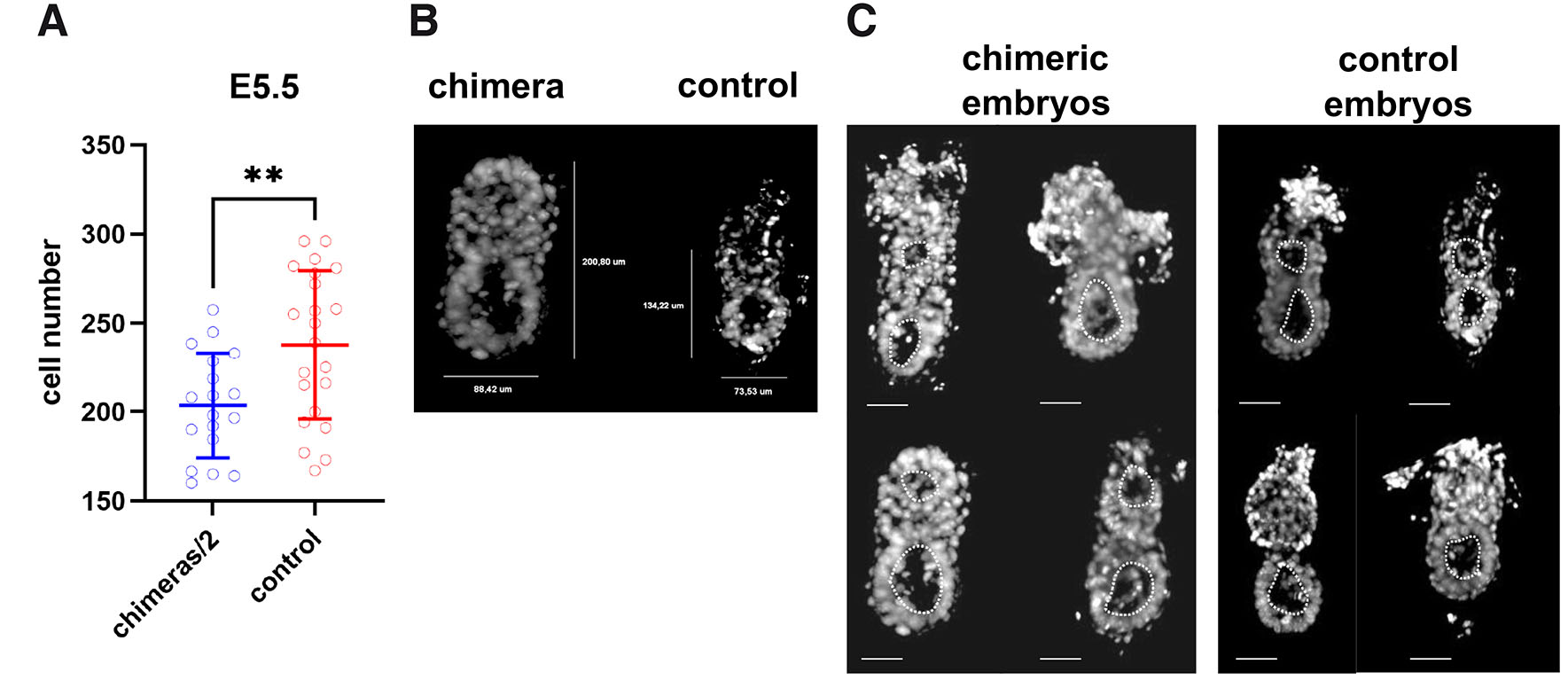
Experimental chimeric egg cylinders were considerably larger than control egg cylinders (Fig. 2 A,B). Mean cell number of chimeric egg cylinders at E5.5 was 407.2±58.9, while for control egg cylinders this value was 237.7±41.9. The cell number of each chimeric egg cylinder was divided by two, and the mean value for this group was compared by the Student’s t-test with the mean cell number of control E5.5 cylinders (Fig. 2A). The p-value for this test was 0.0059, which indicates that at this stage of development the regulative mechanisms already significantly reduced the cell number of chimeric embryos. However, the proportion of the mean cell number of chimeric egg cylinders to the mean cell number of control egg cylinders was 1.71 at this stage (Fig. 5). This means that at E5.5 the regulation of the cell number in chimeric embryos is still undergoing.
Fig. 2. Processes that regulate cell number in chimeric embryos are active at E5.5 and do not delay proamniotic cavity formation.
(A) Cell numbers of chimeric (n=18) and control (n=22) embryos recovered at E5.5. Data are represented as mean±standard deviation. **p<0.01. (B) Representative confocal images showing chimeric and control E5.5 cylinders. Chromatin (white) was labeled with Hoechst 33342. (C) Proamniotic cavity formation in chimeric and control E5.5 cylinders. Scale bar: 50 µm.
There was no delay in formation of proamniotic cavity in chimeric egg cylinders in comparison with control embryos. In both groups at E5.5, early proamniotic cavity was observed both in embryonic and extraembryonic region of the egg cylinder (Fig. 2C). These two cavities were still separated at this stage, which is in agreement with the previously described normal morphology of the mouse embryos at E5.5 (Christodoulou et al., 2018). This indicates that in chimeras the formation of proamniotic cavity of the egg cylinder occurs in parallel with the size regulation, and that higher than normal number of cells does not disturb morphogenesis.
E6.5 egg cylinders
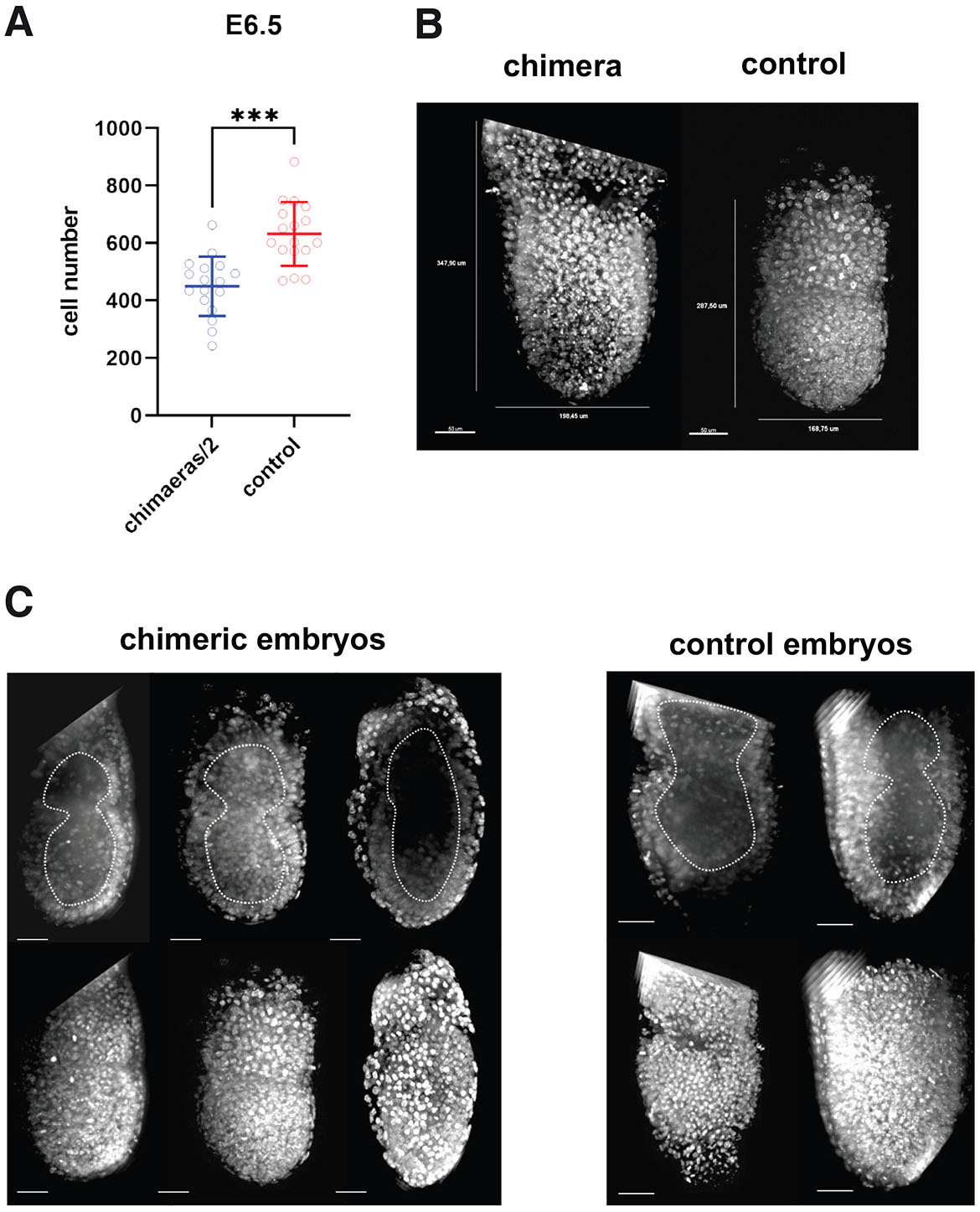
Chimeric egg cylinders at this stage were still larger than corresponding control embryos (Fig. 3 A,B). The mean number of cells in chimeric egg cylinders (n=17) was 899.2±207.0, meanwhile control egg cylinders had on average 632±110.5 cells (n=17). When analysis of statistical significance by the Student’s t-test was performed for the mean value of the cell number of “half” E6.5 chimeric egg cylinders and control egg cylinders at the same stage of development, the p value was 0.000022, what indicates that intensive regulation of size occurred in chimeras between E5.5 and E6.5 (Fig. 3A). The proportion of the mean cell number of chimeric egg cylinders to the mean cell number of control egg cylinders at E6.5 was 1.42 (Fig. 5). This suggests that at E6.5 the regulation of the cell number in chimeric embryos is still not fully completed.
Fig. 3. At E6.5, the regulation of cell number in chimeric embryos is still not fully completed.
(A) The average number of cells in chimeric (n=17) and control (n=17) embryos recovered at E6.5. The graph presents means and standard deviations. ***p<0.001. (B) Representative confocal images of chimeric and control E6.5 cylinders. Chromatin (white) was labeled with Hoechst 33342. (C) Proamniotic cavity in chimeric and control E6.5 cylinders. Scale bar: 50 µm.
The morphology of chimeric E6.5 egg cylinders was unchanged when compared with the control egg cylinders of the same age. In cylinders from both groups the proamniotic cavity extended in the full length of the embryo and was fully formed (Fig. 3C). This observation indicates that the regulation of the size of chimeric embryos occurs also after the completion of the proamniotic cavity formation.
E7.5 egg cylinders
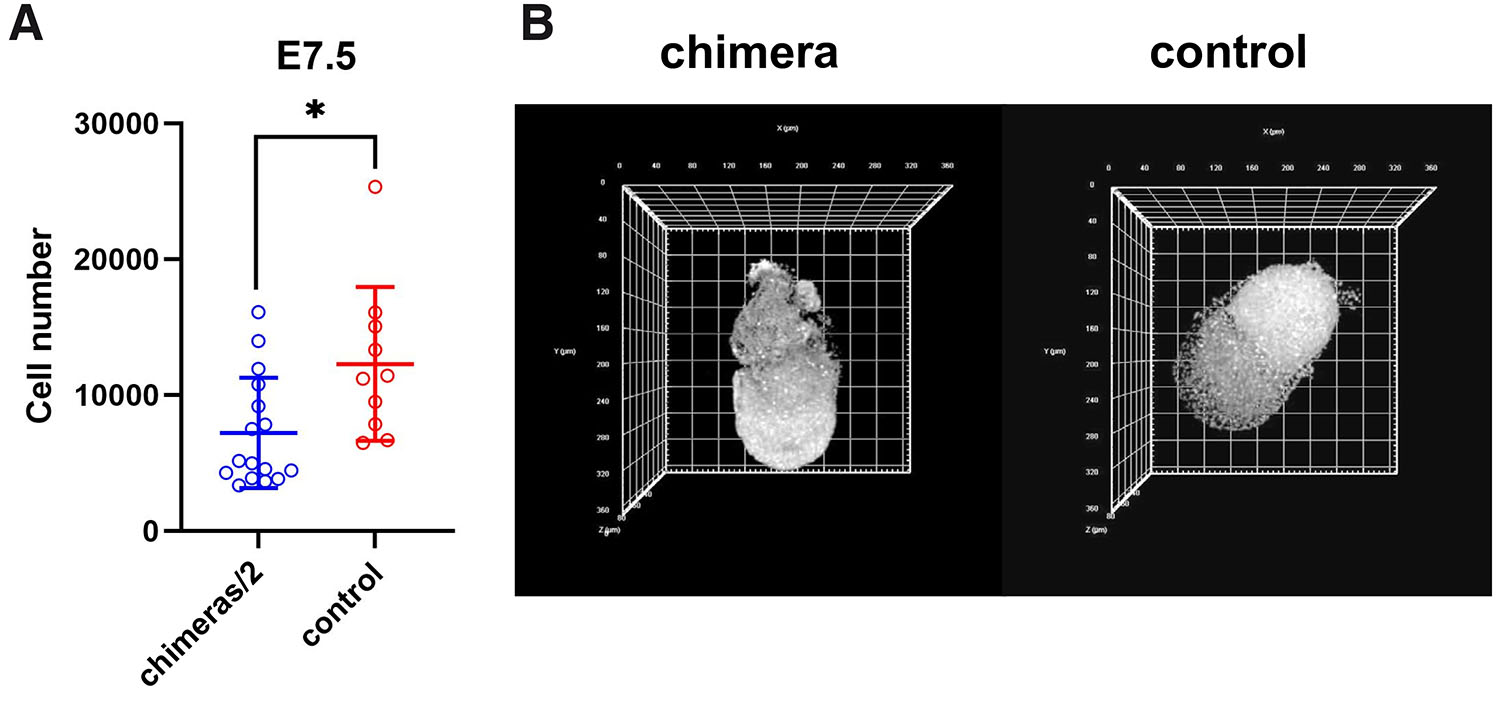
At E7.5, the chimeric egg cylinders displayed a similar size compared to the controls (Fig. 4 A,B). Chimeric embryos had an average of 14447.6±8111.1 cells (n=16), while the total number of cells in the control egg cylinders was 12298.3±5654.4 (n=10). For further analysis, the cell number of each chimeric embryo was divided by 2, and the mean values for chimeric and control egg cylinders were analyzed with the Student’s t-test (Fig. 4A). The p value was 0.02, what indicates that the rate of embryo size regulation slowed down. The ratio of the mean cell numbers of chimeric egg and control egg cylinders at E7.5 was 1.17, demonstrating that size regulation was completed at this stage (Fig. 5).
Fig. 4. Regulation of cell number is finished at stage E7.5.
(A) Cell numbers of chimeric (n=16) and control (n=10) embryos recovered at E7.5. Data are represented as mean±standard deviation. *p<0.05. (B) Representative confocal images showing chimeric and control E7.5 cylinders. Chromatin (white) was labeled with DRAQ5.
Discussion
First, in this work we confirmed the early observation reported by Buehr and McLaren (Buehr and McLaren, 1974) that the regulation of the cell number in double embryos created by the aggregation of two 8-cell embryos does not occur during formation of the chimeric blastocysts. We found that the ratio of the mean cell number of chimeric and control blastocysts, which developed after 48 h of culture in vitro of double and single embryos (E4.5) was lower than 2, nonetheless this difference was not statistically significant. However, we cannot definitively exclude that regulative mechanisms are already active in chimeric blastocysts or even earlier. Indeed, Boiani et al., (Boiani et al., 2003) have demonstrated that cell number regulation in mouse aggregation chimeras could begin already at the blastocyst stage. They showed that double chimeric aggregates of fertilized embryos had 1.75 times and triple chimeric aggregates had 1.9 times the number of cells of single controls. It is also possible that the timing and speed of the downregulation depends on the number of the components that make up the chimera. Our statistical analysis of the cell number of control and chimeric E5.5 egg cylinders indicates that regulation of the size of chimeras is already ongoing at this stage. Thus, we can suspect that the difference observed among chimeric and control preimplantation blastocysts are not accidental, and are a sign of the beginning of regulation already in chimeric blastocysts.
We observed that regulation of the cell number was carried on the following day of development, at E6.5, and at this stage, chimeric egg cylinders were still significantly larger than their single control counterparts. Size regulation in chimeric embryos was completed by E7.5, so that chimeric and control embryos were no longer substantially different.
Data available on the size regulation in mouse chimeric embryos indicate that this process, at least predominantly, occurs during early period of in utero development, however, the exact period of which during postimplantation development the downregulation of the size of chimeric embryos takes place remains a subject of controversy. According to the initial studies of this topic, the adjustment of size of chimeric embryos occurs between the time of the formation of the egg cylinder (E5.0) and the time of gastrulation (E7.0). Buehr and McLaren calculated the mean volume of egg cylinders and found that a fully formed chimeric egg cylinder at E5.5 is significantly larger than control one (Buehr and McLaren, 1974). However, these authors reported that already at the beginning of the formation of proamniotic cavity (E5.8 - E6.0) egg cylinders of control and chimeric embryos have the same volume (Buehr and McLaren, 1974). Other authors (Lewis and Rossant, 1982), in contrast, indicate that the formation of the proamniotic cavity occurs before size regulation, and that size regulation lasts longer than it has been suggested by Buehr and McLaren (1974). They assessed the number of cells in egg cylinders on various stages of pregastrulation period of development by counting the number of nuclei in a small area of histological sections and by multiplying that number by the total tissue area in the embryo. They found that up to the day 5 and 16 hours of embryonic development chimeric embryos have two times more cells than control embryos. After next 24 h, they observed a shift toward 1:1 ratio of the cell number in chimeric and control egg cylinders. The same time of regulation was observed in all cell lines of the egg cylinders (Lewis and Rossant, 1982). In quadruple embryos created by aggregation of four 8-cell embryos, the time of regulation was longer (Rands, 1986). In this work, the volume of egg cylinders was calculated based on the measurements made of histological slides. Some reduction of the original 4:1 cell number ratio was observed already at the blastocyst stage. Further reduction of the size of quadruple embryos occurred until the E7.5, when mean size ratio of quadrupled and control embryos reached 1:1(Rands, 1986).
Direct approach to the study of the reduction of the size of chimeric egg cylinders was applied recently by Orietti et al., (2021) who counted cells in embryos under confocal microscope, and concluded that regulation begins at E5.25 and is completed at E5.5 (Orietti et al., 2021). These results differ significantly from these, which we present in current work. There can be numerous reasons for this discrepancy, the main difference between Orietti et al., (2021) work and our current study is that in our experiments the cell number was counted on 3D reconstructions of the whole egg cylinders. Meanwhile in Orietti et al., (2021) the analysis of the progress of regulation was carried on only by comparison of the cell number of the epiblast of chimeric and control egg cylinders. Dynamics of cell number within individual cell lineages (e.g. trophectoderm and epiblast) would help to reconcile these differences, or confirm previous data. It would be beneficial to know how the cell number is regulated for each developmental lineage separately and whether the dynamics of changes within each lineage is similar or is one lineage regulated more precisely than the other. In at least one work, it was demonstrated that there are significant differences in the time of regulation between descendants of trophectoderm and inner cell mass of the blastocyst in chimeric egg cylinders (Rands, 1986). The other source of abovementioned inconsistency between our results and those of Orietti et al., (2021) may be the difference in the number and type of embryo transferred. It is not clear how many embryos these authors transplanted into one female, and whether they transferred control embryos to one oviduct and the chimeric embryos to the opposite oviduct of each recipient female, as it was performed in our study. If number of transplanted embryos may have affected the timeline of regulation remains to be elucidated. The last but not least, this discrepancy may also result from the small sample sizes used by Orietti et al., (2021).
We observed that the regulation of cell number in chimeras proceeds concomitantly with the formation of proamniotic cavity and lasts up to the gastrulation. We did not notice any delay in development of proamniotic cavity in double chimeric embryos, which was reported by others (Orietti et al., 2021). However, this difference can be explained by the lower uniformity of egg cylinders. In our experiments, embryos were collected at the given time-point and classified as a one experimental group only according to their age. Meanwhile Orietti et al., (2021) grouped embryos according to developmental stages proposed by Christodoulou et al., (Christodoulou et al., 2018). Thus, basing on our results, we cannot exclude that indeed there is some delay in morphogenesis of chimeric egg cylinders in relation to control, single embryos.
The studies of the mechanisms regulating the size of chimeric mouse embryos are very limited. Lewis and Rossant (1982) using [3H]thymidine to label and analyze histological slides of egg cylinders demonstrated that the number of dividing cells in control and chimeric embryos was the same. However, colcemid treatment, which blocked cells in M-phase and allowed better comparison of the mitotic activity of double and single embryos, indicated that in the control embryos the burst of mitotic activity occurred between day 5 and 16 hours, and day 6 and 16 hours of embryonic development and mitotic activity was the highest in embryonic ectoderm at day 6 and 8 hours. Such high mitotic activity was not observed in chimeras. These authors suggest that increased cell cycle length in chimeras may be responsible for the size adjustment (Lewis and Rossant, 1982). Thus, it is possible that other mechanisms, such as a different frequency of apoptosis in control and chimeric embryos are also involved in the regulation of embryo size. Accordingly, it has been demonstrated that the levels of apoptosis in chimeric embryos are increased compared with controls prior to proamniotic cavity formation (Orietti et al., 2021). However, the mechanism by which cell cycle is altered in the chimeric embryos remains unknown. Recently, it has been shown that male embryos tend to develop faster than female embryos during early stage of preimplantation in mice (Kawase et al., 2021). Therefore, it will be tempting to speculate that the mechanisms of cell number regulation in aggregation chimeras are potentially dependent on chromosomal sex of the embryos.
Taken together, in this study, chimeric embryos produced by aggregating 8-cell mouse embryos were shown to remain twice the size of single embryos until at least 5 days. Size regulation occurred rapidly over the next 72 h; there was no difference in cell number between chimeric and control embryos by E7.5. Our study also shows that proamniotic cavity formation was observed to occur at the same times in chimeric and control embryos. These findings may extend our knowledge of the control of body and organ size during mammalian development. However, future studies will be needed to determine the cellular and molecular nature of these processes. Further research directions should include investigating molecular mechanisms behind cell cycle alterations in chimeras, or apoptosis analysis. One of the strategies is to suppress p53 protein functions using the inhibitor α-piphitrin so as to reduce apoptosis, or to pulse the embryos with DNA precursors so as to estimate the duration of the cell cycle. However, these methods are probably difficult to implement in vivo, therefore it will be necessary to use in vitro models such as those developed by Bedzhov (Bedzhov et al., 2014), which allow to obtain egg cylinder stages in vitro. Similar assessment of the developing embryo halves in which the regulation of cell number takes place too, albeit in the opposite direction, would be also interesting and could provide important information.
Materials and Methods
Animals
The study was approved by the Local Ethical Committee No.1, Warsaw, Poland (No. of the permit: 942/2019, 1384/2022, 1521/2023). F1(C57BL/6/Tar × CBA/Tar) females and Tg(CAG-DsRed*MST)1Nagy/J, C57BL/6-Tg(UBC-GFP)30Scha/J or F1(C57BL/6/Tar × CBA/Tar) males were used in this study. For embryo transfer F1 females were used, which were mated with vasectomized F1 males to induce pseudopregnancy. Animals were kept under 14 h light/10 h dark lighting regime.
Embryos
Eight-cell embryos were obtained from 7 to 11 weeks old F1 females spontaneously ovulated or superovulated with 10 IU of pregnant mare serum gonadotropin (PMSG; Folligon, Intervet), followed after 47 – 49 h by 10 IU of human chorionic gonadotropin (hCG; Chorulon, Intervet), and mated with F1, Tg(CAG-DsRed*MST)1Nagy/J or C57BL/6-Tg(UBC-GFP)30Scha/J males. Females with vaginal plugs were culled by cervical dislocation 62 h after mating or the hCG injection. Embryos were flushed out from the oviducts with M2 medium supplemented with 4mg/ml bovine serum albumin (BSA; Sigma-Aldrich) (Fulton and Whittingham, 1978). Embryos were kept in M2+BSA medium under mineral oil (Sigma-Aldrich, Poland) at 37.5 °C in 5% CO2 in air until aggregation.
Construction of chimeric embryos
Before the formation of chimeric embryos the zonae pellucidae were removed with acid Tyrode solution (Nicolson et al., 1975). Next embryos were divided into control and experimental group. Aggregation was performed according to the method of Tarkowski (Tarkowski, 1961) and Mintz (Mintz, 1962). Pairs of experimental embryos were placed in a solution of phytohemagglutinin (300 µg/ml; Sigma-Aldrich, Poland) in BSA-free M2 medium and were brought into contact at 37 °C using a glass needle. After the embryos had stuck firmly together, the chimeric aggregates were washed in M2+BSA and then cultured in KSOM medium (Erbach et al., 1994) (Speciality Media, USA) under mineral oil at 37.5 °C in 5% CO2 in air for 24 or 48 h. Control embryos were treated in the same way but without aggregation. After 24 h some control and chimeric morulas/early blastocysts were transferred into oviducts of pseudopregnant F1 females. The remaining embryos, after 48 h of the culture, reached the late blastocyst stage and were subsequently fixed and used for the examination of the cell number at this stage.
Counting of the cell number in blastocysts
Blastocysts were fixed 20 min in 4% paraformaldehyde (PFA; Thermo Fisher Scientific, Poland) in PBS. To stain cell nuclei blastocysts were placed separately in drops of PBS containing chromomycin A3 (0.01 mg/ml; Sigma-Aldrich, Poland) under oil in glass bottom dishes (MatTek Corporation; Ashland, MA, USA) and were incubated for 30 minutes at 37.5 °C. Subsequently blastocysts were analyzed under LSM 510 Zeiss laser scanning confocal microscope. Z-stacks of ~100 optical sections per blastocyst were collected. Imaris software was used to count cells.
Transfer of embryos
Before transfer, the morphology of chimeric and control embryos was evaluated under the inverted microscope and only properly developed morulas/blastocysts were transferred to the oviduct of mice on the first day of pseudopregnancy. Experimental chimeric embryos were transferred into the right oviduct and control embryos into the left oviduct of the same mice. Total number of embryos transferred into the one oviduct did not exceed 10. Day of transplantation was regarded as a first day of the pregnancy (E0.5).
Recovery of egg cylinders
Pregnant females were autopsied and egg cylinders were isolated into PBS containing 0.3% polyvinylopirolidon (PVP; Sigma-Aldrich, Poland), at middle of the 6th (E5.5), 7th (E6.5) and 8th (E7.5) day of the pregnancy. Recovered egg cylinders were fixed for 20 min in 4% paraformaldehyde (PFA; Thermo Fisher Scientific, Poland) in PBS, and next were kept in drops of PBS with PVP containing 0.01% of sodium azide (Fluka, Milwaukee, WI,USA) in plastic dish (35x10 Tissue Culture Dishes; Falcon, Becton Dickinson, Thermo Fisher Scientific, Poland) under mineral oil.
Cell number counting in E5.5, E6.5 and E7.5 egg cylinders
To visualize cell nuclei egg cylinders were incubated in PBS containing Hoechst 33342 (1 µg/ml; Sigma-Aldrich, Poland) or DRAQ5TM (5mM, 1:400, Thermo Fisher Scientific, Poland) for 20 min in 37.5 °C in plastic dish under mineral oil. Subsequently E5.5 and E6.5 egg cylinders were placed separately in drops of PBS under mineral oil in glass bottom dishes (MatTek Corporation; Ashland, MA, USA). To enhance the visualization of cells in E7.5 egg cylinders we used a clearing method, named ClearT, which requires immersion in a graded series of formamide solutions (Kuwajima et al., 2013). E5.5 and E6.5 egg cylinders were analyzed in Axio Observer Z1 Zeiss fluorescent microscope. The fluorescence images of the E7.5 egg cylinders were acquired with a Nikon Ti2-U microscope with focus motor assembly (Prior Scientific Instruments) using the rescan confocal microscopy module RCM1 (Confocal.nl) with an ORCA-Flash4.0 LT + camera (Hamamatsu) and iChrome CLE 50 laser engine (Toptica), all controlled by μManager software. The confocal fluorescence images were captured with a CFI Plan Apochromat Lambda S 25XC Sil objective (Nikon). Z-stacks of 120 optical sections per egg cylinder were collected. Imaris software was used to count cells.
Statistical analysis
To assess if the regulation of the cell number of chimeric embryos has already been occurring before given stage of development, the cell number of each chimeric blastocyst or egg cylinder isolated at this stage was divided by 2. Next, the mean cell number of “half” chimeric embryos was compared with the mean number of cells of control embryos in corresponding age, in Student’s t-test. If the calculated p-value was equal or lower 0.05, then the difference in the cell number between the given group of “half” chimeric embryos and the group of equivalent control embryos was considered as significant. Accordingly, in such a case it was assumed that proportion of cell number of chimeric double embryos and single control embryos, which at the time of aggregation equaled two, significantly departed from this value. Such result was considered as proof of regulation occurring in chimeric embryos at this stage of development.
References
Bedzhov I., Leung C. Y., Bialecka M., Zernicka-Goetz M. (2014). In vitro culture of mouse blastocysts beyond the implantation stages. Nature Protocols 9: 2732-2739.
Boiani M. (2003). Pluripotency deficit in clones overcome by clone-clone aggregation: epigenetic complementation?. The EMBO Journal 22: 5304-5312.
Buehr M., McLaren A. (1974). Size regulation in chimaeric mouse embryos. Development 31: 229-234.
Christodoulou N., Kyprianou C., Weberling A., Wang R., Cui G., Peng G., Jing N., Zernicka-Goetz M. (2018). Sequential formation and resolution of multiple rosettes drive embryo remodelling after implantation. Nature Cell Biology 20: 1278-1289.
Erbach G. T., Lawitts J. A., Papaioannou V. E., Biggers J. D. (1994). Differential Growth of the Mouse Preimplantation Embryo in Chemically Defined Media1. Biology of Reproduction 50: 1027-1033.
Fulton B. P., Whittingham D. G. (1978). Activation of mammalian oocytes by intracellular injection of calcium. Nature 273: 149-151.
Kawase Y., Tachibe T., Kamada N., Jishage K., Watanabe H., Suzuki H. (2021). Male advantage observed for in vitro fertilization mouse embryos exhibiting early cleavage. Reproductive Medicine and Biology 20: 83-87.
Kuwajima T., Sitko A. A., Bhansali P., Jurgens C., Guido W., Mason C. (2013). ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development 140: 1364-1368.
Lewis N. E., Rossant J. (1982). Mechanism of size regulation in mouse embryo aggregates. Development 72: 169-181.
Markert C. L., Petters R. M. (1978). Manufactured Hexaparental Mice Show That Adults Are Derived from Three Embryonic Cells. Science 202: 56-58.
Mintz B. (1962). Experimental Study of the Developing Mammalian Egg: Removal of the Zona Pellucida. Science 138: 594-595.
Nicolson G. L., Yanagimachi R., Yanagimachi H. (1975). Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. The Journal of cell biology 66: 263-274.
Orietti L. C., Rosa V. S., Antonica F., Kyprianou C., Mansfield W., Marques-Souza H., Shahbazi M. N., Zernicka-Goetz M. (2021). Embryo size regulates the timing and mechanism of pluripotent tissue morphogenesis. Stem Cell Reports 16: 1182-1196.
Petters R. M., MARKERT C. L. (1980). Production and reproductive performance of hexaparental and octaparental mice. Journal of Heredity 71: 71-74.
Petters R.M., Mettus R.V. (1984). Survival rate to term of chimeric morulae produced by aggregation of five to nine embryos in the mouse,. Theriogenology 22: 167-174.
Rands G. F. (1986). Size regulation in the mouse embryo. Development 94: 139-148.
Tarkowski A. K. (1959). Experiments on the Development of Isolated Blastomeres of Mouse Eggs. Nature 184: 1286-1287.
Tarkowski A. K. (1961). Mouse Chimeras Developed from Fused Eggs. Nature 190: 857-860.
Tarkowski A. K., (1998). Mouse chimaeras revisited: recollections and reflections. The International Journal of Developmental Biology 42: 903-908.
Tarkowski A. K., (1963). Studies on mouse chimeras developed from eggs fused in vitro. National Cancer Institute Monograph 11: 51-71.