Int. J. Dev. Biol. 68: 189 - 198 (2024)
Special Issue: Developmental Biology in Nordic Countries
Genetic targeting of lymphatic endothelial cells in mice: current strategies and future perspectives
Open Access | Review | Published: 12 August 2024
Abstract
Lymphatic vessels within different organs have diverse developmental origins, depend on different growth factor signaling pathways for their development and maintenance, and display notable tissue-specific adaptations that contribute to their roles in normal physiology and in various diseases. Functional studies on the lymphatic vasculature rely extensively on the use of mouse models that allow selective gene targeting of lymphatic endothelial cells (LECs). Here, we discuss LEC diversity and provide an overview of some of the commonly used LEC-specific inducible Cre lines and induction protocols, outlining essential experimental parameters and their implications. We describe optimized treatment regimens for embryonic, postnatal and adult LECs, efficiently targeting organs that are commonly studied in lymphatic vascular research, such as the mesentery and skin. We further highlight the anticipated outcomes and limitations associated with each induction scheme and mouse line. The proposed protocols serve as recommendations for laboratories initiating studies involving targeting of the lymphatic vasculature, and aim to promote uniformity in lineage tracing and functional studies within the lymphatic vascular field.
Keywords
Cre/loxP, endothelium, lineage tracing, lymphatic vasculature, tamoxifen
Introduction
The expanding functions of lymphatic vasculature in tissue growth and homeostasis
Lymphatic vessels play an important role in draining excess interstitial fluid, macromolecules and immune cells from peripheral tissues, facilitating their transport to lymph nodes and the systemic circulation. These vessels are distributed throughout the body with the exception of avascular tissues and the brain parenchyma. The lymphatic system is organized into a unidirectional hierarchical tree-like structure composed of blunt-ended lymphatic capillaries, also known as initial lymphatic vessels, and collecting lymphatic vessels (Petrova and Koh, 2020). Fluid uptake and immune cell entry occur at the level of lymphatic capillaries, and is facilitated by discontinuous button-like cell-cell junctions between capillary lymphatic endothelial cells (LECs) (Baluk and McDonald, 2022). The collected fluid, now called lymph, is further drained through a network of collecting lymphatic vessels, passing through a chain of lymph nodes, to large central lymphatic ducts that drain to the venous circulation. Collecting vessels and ducts are characterized by tighter continuous zipper-like junctions, coverage by smooth muscle cells and the presence of luminal valves, all of which ensure efficient unidirectional propulsion of fluid (Petrova and Koh, 2020).
While traditional lymphatic functions involve the clearance of interstitial fluid and the transport of immune cells to lymph nodes, recent discoveries have unveiled additional roles for lymphatic vessels and LECs. These newly described functions include their direct influence on adaptive immunity and ability to regulate organ growth and regeneration through paracrine signalling in multiple organs (reviewed in Petrova and Koh, 2020; Stritt et al., 2021). For example, in the skin, lymphatic capillaries closely associate with hair follicle stem cell nice. They undergo dynamic remodelling during hair follicle regeneration and shape the niche to regulate hair cycling (Gur-Cohen et al., 2019; Peña-Jimenez et al., 2019; Yoon et al., 2022). LECs may also co-regulate broader physiological functions on a whole-body level. For example, recent research suggests a crosstalk between adipose tissues and lymphatic vessels within them, whereby paracrine, so called lymphangiocrine, signals originating from LECs negatively regulate thermogenesis in brown adipose tissue (Li et al., 2021). One of the most intricate examples of paracrine communication is the crosstalk between the lymphatic vasculature and the immune system. Specifically, within lymph nodes, LECs directly interact with T cells to regulate adaptive immune responses that are essential for establishing self-tolerance (reviewed in Arroz-Madeira et al., 2023). In addition to the extensive interactions between LECs and various cell types, lymphatic vessels serve distinct and specialized functions in various organs. For example, lacteal lymphatic vessels located within the intestinal villi play an essential role in the absorption of dietary fats and fat-soluble vitamins (Petrova and Koh, 2020).
Molecular heterogeneity within lymphatic endothelium
While the concepts of angiocrine and immunomodulatory functions of blood ECs have been established (reviewed in Amersfoort et al., 2022; Rafii et al., 2016), we have only recently begun to appreciate and explore the multifaceted roles of the lymphatic vasculature beyond their drainage function. This has been stimulated, in part, by the advent of single cell transcriptomics, which has made the distinct molecular identities of LEC subtypes increasingly evident. For example, single cell RNA sequencing of dermal and mesenteric LECs identified molecularly distinct populations of valve, collecting vessel and capillary LECs (González-Loyola et al., 2021; Petkova et al., 2023). These studies have not only revealed novel markers for subtypes of lymphatic vessels and their compartments, but also identified previously unknown LEC subtypes. The complexity of intra-organ heterogeneity of LECs is illustrated within the lymph node, where transcriptionally distinct subtypes are found in subcapsular, cortical and medullary sinuses (Takeda et al., 2019; Xiang et al., 2020). These distinctive identities mirror the diverse and compartmentalized roles of LECs and lymphatic vessels in immune surveillance processes involving antigen presentation and immune cell trafficking within lymph nodes. Moreover, transcriptome analysis of LECs isolated from the mouse ear dermis revealed zonation along the collecting vessel-capillary axis and identified a previously unknown subpopulation of LECs in capillary terminals, marked by the expression of Ptx3 (Petkova et al., 2023). Notably, this cluster was characterized by the expression of genes involved in immune cell interaction and regulation, similar to the distinct Ptx3+ subpopulation of lymph node LECs within medullary sinuses (Xiang et al., 2020). This raises interesting questions about potential immunomodulatory functions of both peripheral and lymph node lymphatic vessels.
Heterogeneity within lymphatic endothelium can also be found across different organs (Kalucka et al., 2020). The increasing availability of single cell transcriptomic datasets at single organ level provides a valuable resource for future efforts to integrate individual studies on a multi-organ scale. This integration can help identify organotypic gene signatures that may provide valuable insights into the organ-specific roles of lymphatic vessels in tissue homeostasis and immune function.
Diversity in the developmental origins of lymphatic endothelial cells
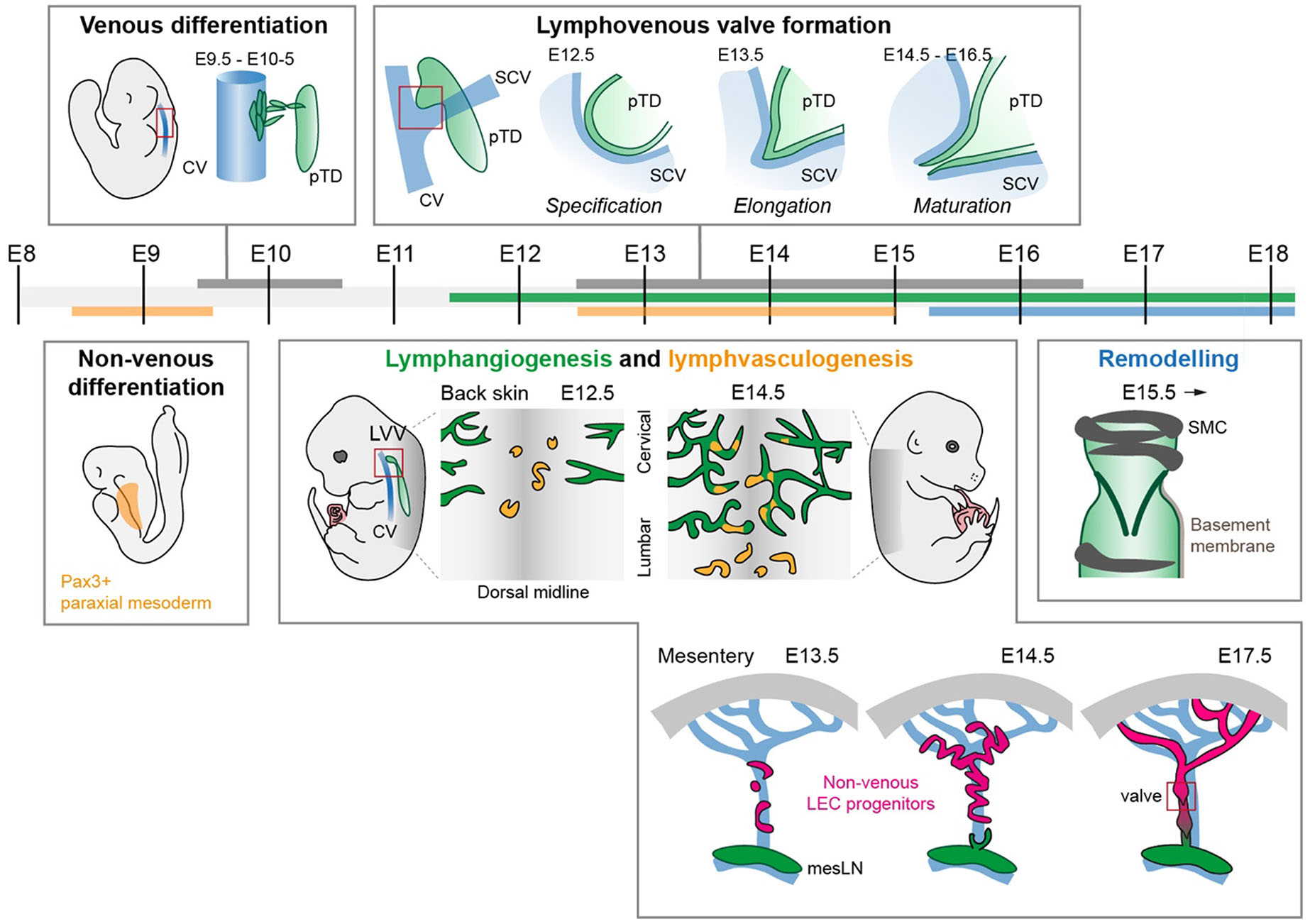
In mouse, the early populations of LECs emerge within the cardinal veins (CV) at around embryonic day (E)9.5-E10 (Fig. 1). During this period, LEC progenitors initiate the expression of the lymphatic fate-determining PROX1 homeobox transcription factor and migrate out of the CV to form the primordial thoracic ducts (pTD), which are also referred to as lymph sacs (Hägerling et al., 2013; Wigle and Oliver, 1999; Yang et al., 2012) (Fig. 1). Further expansion of the vasculature occurs by lymphangiogenic sprouting of vessels from these lymph sacs. Genetic lineage-tracing using a constitutive pan-endothelial Tie2-Cre, as well as the inducible Prox1-CreERT2 and Vegfr3-CreERT2, have provided evidence for the venous origin of lymphatic vessels (Martinez-Corral et al., 2015; Srinivasan et al., 2007). However, recent research has additionally uncovered the existence of other non-venous sources of LECs in certain organs (reviewed in Jafree et al., 2021). For example, the developing dermal lymphatic vasculature forms in part by the assembly of LEC progenitors through a process termed lymphvasculogenesis into isolated cell clusters that later incorporate into the growing vessels (Martinez-Corral et al., 2015)(Fig. 1). The origin of LECs within these clusters was assigned to the local blood vessel network (Pichol-Thievend et al., 2018). However, evidence from tracing Tie2-lineage ECs (Martinez-Corral et al., 2015; reviewed in Arroz-Madeira et al., 2023) and more recent findings from temporally restricted tracing of Cdh5-lineage ECs showing negligible contribution to the LEC (Zhang et al., 2022) clusters suggests a predominant non-endothelial origin.
Fig. 1. Developmental timeline of lymphatic vessel morphogenesis in commonly studied organ systems.
The contribution of venous and non-venous sources of lymphatic endothelial cells (LECs) to different developmental processes in different organs are depicted. Early LEC progenitors emerge from Pax3-positive paraxial mesoderm and from the endothelium of cardinal veins (CV). Initial lymphatic networks expand by lymphangiogenic sprouting and lymphvasculogenic assembly of vessels from progenitors, which is followed by maturation of vessels through formation of lymphatic valves, deposition of a basement membrane and recruitment of smooth muscle cells (SMC). Abbreviations: E, embryonic day; CV, cardinal vein; LVV, lymphovenous valve; mesLN, mesenteric lymph node; pTD, primordial thoracic duct; SCV, subclavian vein; SMC, smooth muscle cell.
While most ECs are derived from the lateral plate mesoderm, genetic lineage tracing has indicated Pax3-positive paraxial mesoderm as a major source of lymphatic vessels in several organs, including the skin (Stone and Stainier, 2019). Although initially Pax3-lineage LECs were thought to acquire their fate by transitioning through a venous EC intermediate (Stone and Stainier, 2019), latest findings suggest direct de novo differentiation of non-venous paraxial mesoderm-derived ETV2+PROX1+ progenitors into LECs (Lupu et al., 2022, pre-print). Pax3-positive mesoderm lineage has been also shown to contribute to the cardiac lymphatic vasculature, as well as LECs of inguinal, axillary, brachial and popliteal lymph nodes (Lenti et al., 2022). LECs of mesenteric lymph nodes instead originate from a distinct Hoxb6-lineage lateral plate mesoderm (Lenti et al., 2022). Interestingly, LECs within mesenteric lymphatic vessels are similarly unique in that, unlike in other organs, they are from Pax3-lineage independent origin, yet they form by lymphvasculogenic assembly of PROX1-positive LEC progenitors (Stanczuk et al., 2015). Their origin was traced to early embryonic arterial and cKit-lineage positive cells, distinct from the venous-derived LECs in the mesenteric lymph sac, suggesting a progenitor source from hemogenic EC-derived progenitor source (Stanczuk et al., 2015) (Fig. 1).
These recent findings indicate multiple developmental origins of LECs in different organs. However, their relative contributions to the mature, established lymphatic vasculature remain unknown. It is also unclear if LECs of different origins are molecularly distinct and serve different functions. Development of specific tools targeting the different progenitor populations will help address these questions in the future.
Genetic tools for targeting of lymphatic endothelial cells
The Cre/loxP technology offers a powerful tool for targeting of specific cell types, including ECs, in genetically modified mice. Over the past decades, significant advancements have been made in the vascular biology field, enabling temporally controlled targeting of either all ECs or specific subpopulations of ECs in a vessel-type or organ-specific manner. For example, various Cre lines have been developed by utilizing pan-endothelial or EC-type-specific gene promoters to selectively target veins, arteries or tip cells at the sprouting front of the blood vasculature (Ehling et al., 2013; Lee et al., 2021; Xu et al., 2014). This has facilitated detailed studies of angiogenic processes, particularly in the context of retina. In addition, Cre-lines allowing targeting of blood vessels in specific organs such as the brain or liver have been successfully developed (Pu et al., 2016, 2018). Combinatorial targeting using Cre and Dre recombinases, each recognizing their respective Lox or Rox recognition sites, has further expanded the genetic toolbox (Han et al., 2021). In such intersectional genetic approaches, sequential action of the two recombinases, each under the control of their distinct promotors, enables a more precise and controlled targeting of EC populations. This is achieved by restricting recombination by the second recombinase to a specific cell lineage defined by the activity of the first recombinase.
Pan-endothelial Cre lines, such as Cdh5-CreERT2 and Pdgfb-CreERT2, have been used to efficiently target LECs as well (Bazigou et al., 2009; Stanczuk et al., 2015; Wang et al., 2017; Zhang et al., 2022). However, specific studies of the lymphatic vasculature have become possible only after tamoxifen-inducible Cre lines utilizing LEC-specific gene promoters including Prox1, Flt4/Vegfr3 and Lyve1 were established (Bazigou et al., 2011; Connor et al., 2016; Martinez-Corral et al., 2016; Srinivasan et al., 2007) (Table 1). Yet, there is a notable lag in the development of mouse lines targeting specific LEC subpopulations compared to those targeting blood ECs. To date, only a few examples exist, such as the Cldn11-CreERT2 line, which selectively targets Cldn11-expressing valve LECs (Ortsäter et al., 2021), and Pax3-Cre (Engleka et al., 2005), which targets paraxial mesoderm-derived LECs in the skin and heart, but not in the mesentery (Stone and Stainier, 2019). A recently developed approach for sequential recombination utilizing both Cdh5-Dre and Prox1-RSR-CreER drivers provides a valuable method for a precise and specific targeting of LECs without affecting other Prox1-expressing tissues by limiting Prox1-driven recombination expression to Cdh5-expressing ECs (Han et al., 2021). As our understanding of LEC heterogeneity continues to increase, we can anticipate the development of more refined genetic tools that allow detailed studies of the diverse subpopulations of LECs of different developmental origins, organs and vessel types. Such advanced tools will facilitate functional studies aimed at understanding developmental disorders with organ-specific manifestations of lymphatic disease.
Table 1
Commonly used inducible Cre/Dre lines for temporally controlled targeting of lymphatic endothelial cells (LECs)
| Mouse line | MGI ID | LEC targets | Major non-LEC targets (reported/expected) | Genetic modification | Ref | |
|---|---|---|---|---|---|---|
| LEC | Lyve1-CreERT2 | 6758737 | All LECs | CD11b+ macrophages, certain BECs (e.g. yolk sac, liver sinusoids, spleen BECs)) | Transgene (BAC) | (Connor et al., 2016) |
| Lyve1-CreERT2 | n.a. | All LECs | Certain BECs (e.g. yolk sac), macrophages | Knockin | (Chen et al., 2023) | |
| Prox1-CreERT2 | 5616256 | All LECs | Cardiomyocytes, myocytes, hepatocytes, neurons, lens epithelial cells | Knockin | ||
| Prox1-CreERT2 | 5617984 | All LECs | Venous valve EC (with low recombination in major veins), Schlemm’s canal, cardiomyocytes, myocytes, hepatocytes, neurons, lens epithelial cells | Transgene (BAC) | (Bazigou et al., 2011) | |
| Prox1-CreERT2 | 7537333 | All LECs | Cardiomyocytes, myocytes, hepatocytes, neurons, lens epithelial cells | Transgene (BAC) | (Iyer et al., 2023) | |
| Prox1-RSR-2A-CreER | n.a. | All LEC | Intersectional Cre/Dre line; specific expression in LECs when crossed to Cdh5-Dre | Knockin | (Han et al., 2021) | |
| Prox1-2A-DreER | n.a. | All LECs | Cardiomyocytes, myocytes, hepatocytes, neurons, fiber cells | Knockin | (Han et al., 2021) | |
| Vegfr3-CreERT2 | 5750213 | All LECs | Certain BECs (e.g. embryonic, angiogenic, sinusoidal) | Knockin | (Martinez-Corral et al., 2016) | |
| Vegfr3-CreERT2 | n.a. | All LECs | Sinusoidal EC in the bone marrow | Transgene (BAC) | (Poulos et al., 2024) | |
| Cldn11-CreERT2 | 6727043 | Valve LECs | hair follicles, oligodendrocytes, arachnoid barrier cells, Sertoli cells | Transgene (BAC) | (Ortsäter et al., 2021) | |
| Pan-EC | Cdh5-CreERT2 | 3848982 | All LECs | All BECs, hematopoietic cells if early embryonic induction targeting hemogenic EC | Transgene (PAC) | (Wang et al., 2010) |
| Pdgfb-CreERT2 | 3793852 | Mainly collecting vessel LECs | All BECs, especially angiogenic, hematopoietic cells if early embryonic induction targeting hemogenic EC | Transgene (BAC) | (Claxton et al., 2008; Wang et al., 2017) |
Abbreviations: BAC, bacterial artificial chromosome; BEC, blood endothelial cell; PAC, P1-derived artificial chromosome.
Protocols for functional analysis of lymphatic vessels by conditional gene targeting
Here, we discuss a selection of Cre lines (Table 1) and optimized protocols (Table 2) that we have employed and developed in our research to efficiently target LECs at different developmental stages and tissues that are commonly used for lymphatic vessel analysis by us and others. For additional protocols involving alternative Cre lines, tissues, or developmental stages, we refer the reader to other published studies and description of Cre lines, some of which are summarized in Table 1.
Table 2
Protocols for efficient targeting of lymphatic endothelial cells (LECs)
| Tissue and/or stage | Cre line | Induction scheme | Comment |
|---|---|---|---|
| Venous-derived LEC progenitors | Prox1-CreERT2 | E9.5 + E10.5: 1 mg 4-OHT (intraperitoneal) | Inefficient targeting |
| Vegfr3-CreERT2 | E9.5: 3 mg 4-OHT (intraperitoneal) | Efficient targeting of BECs in CV and venous-derived LECs | |
| Lymphovenous valves | Prox1-CreERT2 | E10.5 + E11.5 (+ E12.5): 1 mg 4-OHT (intraperitoneal) | Efficient targeting of LVV, mosaic targeting of pTD + JLS |
| Embryonic skin | Prox1-CreERT2 | E12.5 + E13.5 + E14.5: 1 mg 4-OHT (intraperitoneal) | Poor targeting prior to E12.5, poor targeting of lymphvasculogenic clusters and vessel sprouts at the dorsal midline |
| Vegfr3-CreERT2 | E12.5 + E13.5 + E14.5: 1 mg 4-OHT (intraperitoneal) | Targeting of both the vessels and lymphvasculogenic clusters, also BECs | |
| Embryonic mesentery | Prox1-CreERT2 | E15.5 + E16.5 (+ E17.5): 1 mg 4-OHT (intraperitoneal) | Poor targeting prior to E15.5, no targeting of lymphvasculogenic LEC progenitors |
| Vegfr3-CreERT2 | E11.5: 1-2 mg 4-OHT (i.p.) E11.5 + E12.5: 1 mg 4-OHT (intraperitoneal) |
Mosaic targeting of LEC progenitors Efficient targeting of LEC progenitors |
|
| Neonatal vessels | Prox1-CreERT2 | Between P0-P2, a single or two consecutive administrations: 25-50 µg 4-OHT (intragastric) | Efficient targeting |
| After P0 (abdominal skin) or P7 (ear skin): 150 µg Tam every 2 days (topical treatment) | Suited for long-term induction protocols | ||
| Vegfr3-CreERT2 | Between P0-P2, a single or two consecutive administrations: 25-50 µg 4-OHT (intragastric) | Less efficient compared to Prox1-CreERT2 | |
| After P0 (abdominal skin) or P7 (ear skin): 150 µg Tam every 2 days (topical treatment) | Suited for long term induction protocols | ||
| Adult vessels | Prox1-CreERT2 | Systemic: 2-3 x 1 mg Tam on consecutive days (oral gavage) | Full systemic targeting |
| Local: 50 µg 4-OHT (topical application to dorsal skin of each ear) | Localized targeting with limited systemic recombination | ||
| Vegfr3-CreERT2 | Local: 50 µg 4-OHT (topical application to dorsal skin of each ear) | Less efficient compared to Prox1-CreERT2 |
|
| Valves | Cldn11-CreERT2 | Systemic: 2-3 x 1 mg Tam on consecutive days (oral gavage) | Efficient targeting of lymphatic valves, mosaic targeting of venous valves |
Abbreviations: 4-OHT, 4-hydroxy-tamoxifen; BEC, blood endothelial cell; CV, cardinal vein; E, embryonic day; JLS, jugular lymph sac; LVV, lymphovenous valve; P, postnatal day; pTD, primordial thoracic duct; Tam, tamoxifen.
General considerations
The use of tamoxifen vs 4-OHT
Tamoxifen is metabolized in the liver to its bio-active metabolites, 4-hydroxy-tamoxifen (4-OHT) and N-desmethyl-4-hydroxytamoxifen (endoxifen) (Borgna and Rochefort, 1981). These metabolites can bind the CreER fusion protein with a 100-fold higher affinity compared to tamoxifen, thereby driving its efficient nuclear localization and recombination process (Hayashi and McMahon, 2002; Katzenellenbogen et al., 1984). Acute induction and short <24 h time-window of Cre activity (Martinez-Corral et al., 2016), which is often desirable for developmental studies and lineage tracing experiments, can be achieved by direct administration of 4-OHT. In contrast, the kinetics of tamoxifen-induced Cre-activity is slower, and leads to an extended Cre activity period of up to 48-72h (Martinez-Corral et al., 2015). Depending on the tissue as well as tamoxifen dosage and administration route used, Cre activity may be preserved significantly longer (Ye et al., 2015).
Cre-recombination: efficiency, specificity and reporting
Efficient CreER-mediated recombination of LoxP sites is influenced by multiple parameters, which include genomic distance between the individual LoxP sites, accessibility of the genomic locus, abundance of the CreERT protein, and the effective tamoxifen dose. Recombination efficiency of a given Cre line is commonly assessed using reporter alleles, which are activated after tamoxifen administration to drive the expression of reporter genes such as fluorescent proteins. However, it is important to note that recombination of common reporter lines does not necessarily correlate with the recombination of other alleles, including conditional knockout alleles (Fernández-Chacón et al., 2019). Moreover, recombination can occur even in the absence of tamoxifen (Álvarez-Aznar et al., 2020), especially when promoters of highly expressed genes are used to drive Cre expression (Kristianto et al., 2017). Strategies aimed at enhancing accurate reporting of recombination events have been developed (reviewed in Garcia-Gonzalez et al., 2020; Tian and Zhou, 2021), but it remains important to experimentally validate and optimize the optimal conditions for each CreER line and floxed allele, tamoxifen dose and developmental stage.
Cre toxicity and adverse effects of tamoxifen
Cre expression can lead to adverse cellular effects on organ development and physiology, including the vasculature (reviewed in Rashbrook et al., 2022). Toxicity has been attributed to the non-specific endonuclease activity, caused by Cre binding to endogenous DNA sequences, which results in DNA breaks (Rashbrook et al., 2022). Mice expressing the pan-endothelial Cdh5-CreERT2 transgene showed a delayed development of the retinal blood vasculature upon tamoxifen administration (Brash et al., 2020). In addition, the ubiquitous Rosa26-CreERT2 led to Cre toxicity in hematopoietic cells (Higashi et al., 2009; Rossi et al., 2023), suggesting that different cell types may be differentially sensitive to Cre. So far, no specific phenotypes associated with Cre toxicity in LECs have been reported. CreER exhibits toxicity only once tamoxifen is administered, as the rapid induction of nuclear translocation of the CreER protein, particularly when 4-OHT is administered, results in acute presence of high Cre protein levels within the nucleus. Constitutive Cre lines, on the other hand, must maintain a more moderate expression level of the protein to allow normal development. Tamoxifen-treated littermate mice carrying the CreER transgene are therefore important controls for conditional knockout studies.
Tamoxifen itself can also cause various side effects due to its ability to act as an estrogen receptor antagonist. High doses of tamoxifen can be lethal to developing embryos and affect the ability of pregnant females to give birth naturally (Lizen et al., 2015). In addition, tamoxifen treatment has been reported to result in adverse effects on the reproductive system (Smith, 2011) and bone turnover (Zhong et al., 2015) in juvenile males. Tamoxifen has also been shown to promote transient lipoatrophy, followed by de novo adipogenesis (Ye et al., 2015).
Inducible Cre lines for targeting of LECs
A number of inducible Cre lines targeting LECs have been generated (Table 1). Here we discuss a selection of lines that have been characterized and used by us and others in a number of studies.
Prox1-CreERT2 (Tg(Prox1-cre/ERT2)1Tmak, MGI:5617984)
The transcription factor Prospero Homeobox PROX1 is the master regulator of LEC fate (Wigle and Oliver, 1999). It is continuously expressed in all LECs from early development through adulthood, and is crucial for establishing and maintaining LEC identity (Johnson et al., 2008). However, it must be noted that PROX1 is expressed in multiple tissues and cell types, that are thus also targeted by Prox1-CreERT2, including skeletal and heart myocytes, cells in the lens placode, liver hepatocytes, kidney epithelial cells as well as neurons in the spinal cord, hippocampus and cerebellum (Lavado and Oliver, 2007; Risebro et al., 2009; Sosa-Pineda et al., 2000; Wigle et al., 1999). Within the vasculature, Prox1 is additionally expressed in ECs of venous valves, certain large veins and hybrid vessels (Aspelund et al., 2014; Bazigou et al., 2011; Park et al., 2014). When assessing phenotypes in Prox1-CreERT2-driven genetic knockout models of genes with potential non-endothelial functions, it is therefore important to exclude any potential defects in vital tissues, particularly the heart or liver. Below we discuss protocols optimized for the line generated through BAC transgenesis, but it should be noted that several independent Prox1-CreERT2 lines have been established and successfully used for LEC targeting (Table 1) (Han et al., 2021; Iyer et al., 2023; Srinivasan et al., 2007). Although the efficiency may vary, similar patterns of recombination have been reported in all lines.
Vegfr3-CreERT2 (Flt4tm2.1(cre/ERT2)Sgo, MGI:5750213)
Vascular endothelial growth factor receptor 3 (VEGFR3), encoded by Flt4, is the principal receptor tyrosine kinase for the lymphangiogenic VEGF-C growth factor and is expressed in all LECs across embryonic and adult tissues. VEGF-C - VEGFR3 signalling plays a critical role in both developmental and pathological lymphatic vessel growth (reviewed in Grimm and Hogan, 2021). However, VEGFR3 is also expressed in angiogenic blood vessels, and regulates embryonic development of the blood vasculature and neo-angiogenesis in adult tissues (Dumont Daniel J. et al., 1998; Laakkonen et al., 2007). The Vegfr3-CreERT2 line was generated by inserting IRES-CreERT2-cassette into the 3’-UTR of the mouse Flt4 gene, to preserve endogenous regulatory elements and ensuring correct expression of the endogenous VEGFR3 protein (Martinez-Corral et al., 2016). Validation of the line confirmed that Vegfr3-CreERT2-driven recombination faithfully recapitulated endogenous Vegfr3 expression preferentially restricted to the lymphatic endothelium. Additionally, recombination was observed in certain blood vessel beds, including angiogenic and sinusoidal vessels (Martinez-Corral et al., 2016). The latter was exploited for the targeting of sinusoidal vasculature in the bone marrow, using another Vegfr3-CreERT2 line generated through BAC transgenesis (Poulos et al., 2024). Mice carrying the Vegfr3-CreERT2 knockin allele at homozygosity do not exhibit any apparent phenotypes. This provides a strategy for increasing Cre expression and activity in ECs, including blood ECs, that naturally express low levels of Vegfr3.
Cldn11-CreERT2 (Tg(Cldn11-cre/ERT2)151Tmak, MGI:6727043)
Transcriptomic analysis of LECs has identified Cldn11 as a specific marker of lymphatic valves (Ortsäter et al., 2021; Takeda et al., 2019). CLDN11 belongs to the claudin family of tight junction proteins, which play an important role in the formation of cell-cell junctions. Despite its expression in lymphatic valves, CLDN11 does not have an apparent function in valve development (Ortsäter et al., 2021). Within the vasculature, Cldn11-CreERT2 exhibited selective targeting of mature lymphatic valve LECs, with lower levels of recombination also observed in venous valves (Ortsäter et al., 2021). Notably, developing valves in embryonic lymphatic vessels were not efficiently targeted (Ortsäter et al., 2021). Previous research has shown that CLDN11 is also expressed in Sertoli cells and oligodendrocytes, and contributes to the formation of the myelin sheath (Bronstein et al., 2000; Gow et al., 1999; Mazaud-Guittot et al., 2010). In line with this, additional non-vascular cells targeted by the Cldn11-CreERT2 transgene include oligodendrocytes, as well as hair follicles (Ortsäter et al., 2021) and arachnoid barrier cells (Pietilä et al., 2023)
Targeting of embryonic lymphatic vessels
Venous LEC progenitors
Venous LEC progenitors that differentiate within the embryonic cardinal veins after E9.5 (Fig. 1) can be efficiently targeted using constitutive pan-EC lines such as Tie2-Cre. Attempts to efficiently target these progenitors using the Prox1-CreERT2 BAC transgenic line (Bazigou et al., 2011) and 4-OHT administration between E9.5-E10.5 were not successful (Martinez-Corral et al., 2015). Possible explanations for this include the absence of critical enhancers driving Prox1 expression in early LEC progenitors within the transgene construct, and insufficient levels of CreERT2 protein accumulated in the differentiating LECs at the time of induction. The use of tamoxifen to provide a broader window of Cre activation, and the use of the Prox1-CreERT2 knock-in line have been reported to result in more efficient targeting of venous LEC progenitors and vessels derived thereof (Martinez-Corral et al., 2015; Srinivasan et al., 2007). Vegfr3-CreERT2 has been reported to target venous LEC progenitors after administration of 3 mg of 4-OHT to the pregnant dam at E9.5, but it should be noted that BECs within the cardinal vein are also targeted (Martinez-Corral et al., 2016). In addition, LECs within jugular lymph sacs can be efficiently targeted by administration of 3 mg of 4-OHT at E10.5 (Martinez-Corral et al., 2016).
Lymphovenous valves (LVVs)
LVVs, located between the pTD and the junction of the jugular and subclavian veins, form the connection through which lymph is returned to the venous circulation. In mice, LVV morphogenesis is initiated at E12.5 and completed by E14.5 (Geng et al., 2016) (Fig. 1). Efficient targeting of the PROX1-expressing ECs forming the LVV can be achieved using the Prox1-CreERT2 line when 4-OHT is administered before and during the entire period of LVV formation from early EC specification until first morphogenetic processes. Optimal results are achieved by consecutive administrations of 1 mg of 4-OHT to the pregnant dam at E10.5, E11.5 and E12.5 (Martin-Almedina et al., 2016).
Embryonic back skin
Embryonic back skin serves as a well-established model for studying sprouting lymphangiogenesis. It is relatively easy to dissect and can be flat-mounted for whole-mount analysis. The first dermal lymphatic vessels appear at around E12.5-E13.5 primarily on the lateral sides of the thoracic region of the back skin as sprouts originating from the venous-derived lymph sacs (Fig. 1). New lateral sprouts form in a thoracic to lumbar fashion and continue to sprout towards the dorsal midline, where the vessel networks ultimately connect by E17.5 (Martinez-Corral et al., 2015). Additionally, at approximately E12.5 an additional source of dermal LECs, distinct from the venous origin, emerges at the midline and later at E14.5 also in the lumbar region of the skin (Fig. 1). These cells form lymphvasculogenic clusters that locally proliferate and give rise to isolated vessels, which later incorporate into the sprouting vessel network (Martinez-Corral et al., 2015; Pichol-Thievend et al., 2018). The lymphatic vasculature of the embryonic skin can be efficiently targeted using both Prox1-CreERT2 and Vegfr3-CreERT2 lines. Repeated administration of 1 mg of 4-OHT at E12.5, E13.5 and E14.5 to pregnant dams was reported to result in a nearly complete recombination within LECs of the lateral lymphatic vessel network when using Prox1-CreERT2. However, this regimen failed to efficiently recombine the lymphvasculogenic clusters at the dorsal midline (Martinez-Corral et al., 2015). Moreover, 4-OHT administration prior to E12.5 resulted in minimal recombination in dermal LECs in this line (Martinez-Corral et al., 2015). For early developmental studies, optimal targeting of both the lymphvasculogenic clusters and lymphangiogenic vessels can instead be achieved using the Vegfr3-CreERT2 line and repeated administration of 1 mg of 4-OHT at E12.5, E13.5 and E14.5. However, Vegfr3-CreERT2 also exhibits low-frequency recombination within the embryonic dermal blood vasculature.
Embryonic mesentery
Embryonic mesentery provides a favorable model for studying many aspects of lymphatic vessel biology, including collecting vessel remodeling and valve morphogenesis (Bazigou et al., 2009; Sabine et al., 2012). Formation of the mesenteric lymphatic network occurs from two distinct sources: lymphangiogenic sprouting from the mesenteric lymph sac and lymphvasculogenesis from non-venous progenitors (Stanczuk et al., 2015)(Fig. 1). Between E13.5-E14.5, a highly branched initial network forms, which is extensively remodeled into collecting lymphatic vessels with valves by E17.5-E18.5 (Fig. 1). Efficient targeting of developing mesenteric vessels can be achieved using Prox1-CreERT2 and repeated administration of 1 mg of 4-OHT at E15.5 and E16.5. Administration prior to E15 results in limited targeting, but an additional injection at E17.5 can be considered to ensure complete recombination (Tatin et al., 2013). Lymphvasculogenic clusters, which develop between E12.5-E13.5, can be targeted using Vegfr3-CreERT2 and 4-OHT administration as early as at E11.5 (Stanczuk et al., 2015). Only incomplete mosaic recombination can be obtained with this early induction regimen, and for efficient targeting repeated administration of 1 mg of 4-OHT at E11.5 and E12.5 is recommended.
Targeting of neonatal lymphatic vessels
Efficient recombination can be achieved in lymphatic endothelium of all neonatal organs using the Prox1-CreERT2 line (Frye et al., 2020; Zhang et al., 2018). Although the Vegfr3-CreERT2 line also drives a high level of recombination of a single reporter allele in LECs, our experience from studies involving various floxed knockout alleles suggests incomplete recombination, especially when two or more floxed alleles are combined. When recombination in other Prox1-expressing tissues is not a concern, the Prox1-CreERT2 line is thus a preferred choice for gene knockout studies.
In both lines, different routes of 4-OHT and tamoxifen administration have been used successfully in previous research. We routinely use either a single or two consecutive intra-gastric injections of 25-50 µg of 4-OHT, dissolved in ethanol, between P0 and P3 into the milk spot of neonates (Frye et al., 2020) (Fig. 2). If a longer time-window of Cre activity is required, for example due to active selection of non-recombined ECs to contribute to the studied developmental process (Zhang et al., 2018), administration can be done during the entire postnatal period by topical application of a higher dose (150 µg) of tamoxifen, dissolved in acetone, to the abdominal skin of the pups every second day. Since tamoxifen provides a long activity window of up to 72 hours, such regimen will ensure continuously high levels of 4-OHT during the entire postnatal period without the need for repetitive invasive injections (Zhang et al., 2018).
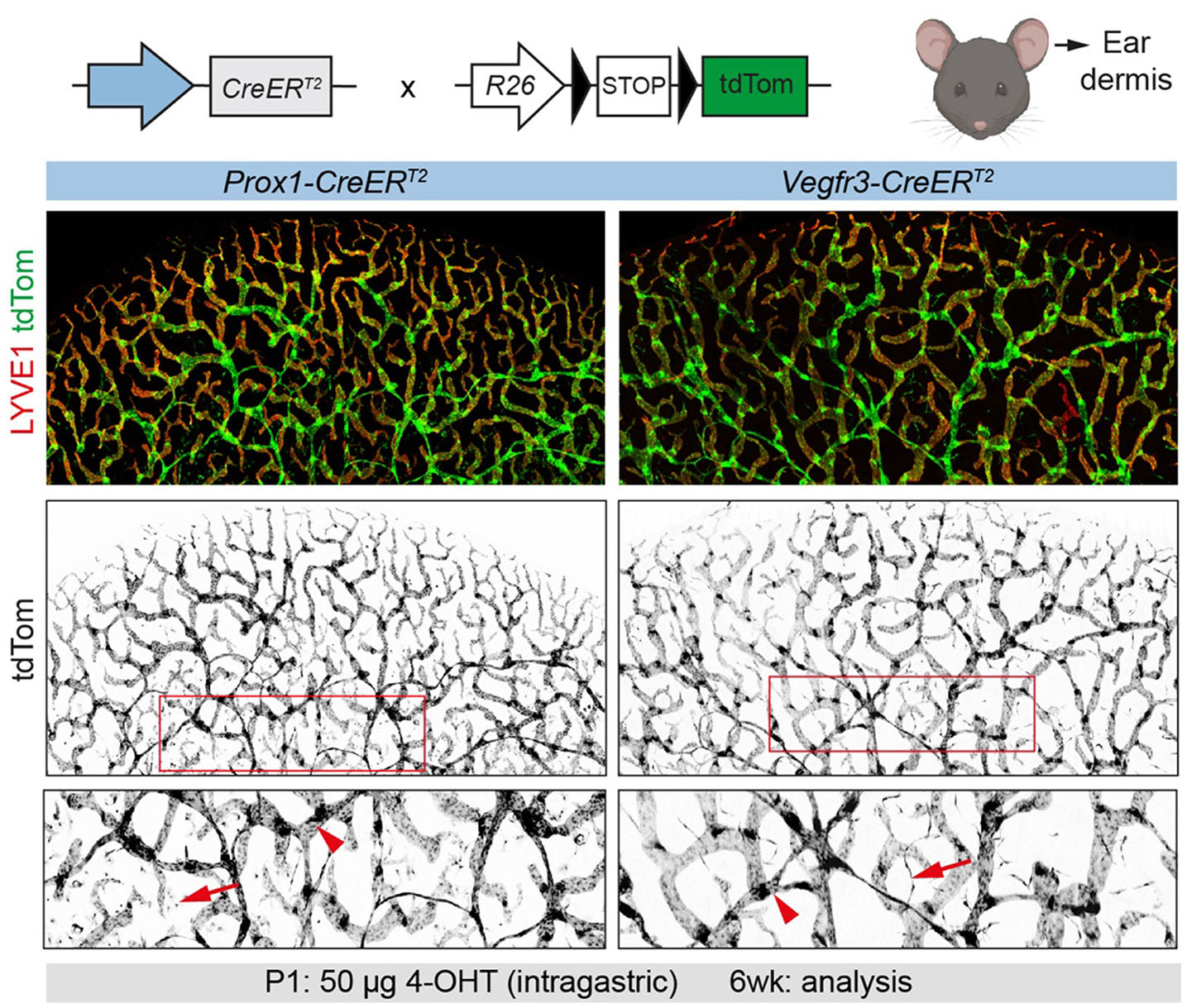
Fig. 2. Anticipated outcomes of postnatal targeting of lymphatic endothelial cells (LECs) using Prox1-CreERT2 or Vegfr3-CreERT2 lines.
Assessment of R26-tdTom reporter recombination after intragastric administration of 50 µg of 4-OHT at postnatal day 1 (P1) by whole mount immunofluorescence analysis of the dorsal ear skin in 6-week-old mice. Efficient recombination, visualized by tdTom expression, is detected in LYVE1-positive LECs (arrowheads), but also in LYVE1-negative non-LEC interstitial cells in Prox1-CreERT2 mice and in blood capillary endothelium in Vegfr3-CreERT2 mice (arrows). Abbreviations: 4-OHT, 4-hydroxy-tamoxifen; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1.
The above strategies have been successfully used for studying the lymphatic network in the ear skin (pinna), which develops postnatally and is fully established at weaning age of 3 weeks (Fig. 2). The dermal vasculature of the ear can be separated into two layers separated by the ear cartilage: the thicker dorsal layer is comprised of a two-layered network of lymphatic vessels: a deeper network of LYVE1- collecting vessels harboring valves and a superficial network of LYVE1+ lymphatic capillaries. Development of the superficial capillary plexus is initiated postnatally around P3 when lymphatics from the deeper plexus start sprouting towards the dermis (Mäkinen et al., 2005). The ventral ear skin on the other hand is comprised of a single-layered network of lymphatic capillaries, which develops in two phases. An initial sprouting phase results in a non-optimal primitive network, and a second phase of side branching from the initial network, which ensures optimal coverage (Uçar et al., 2023). Similarly, the above strategies efficiently target lymphatic vessels in the neonatal mesentery, which, in comparison to adult mice, is not associated with adipose tissue at this stage and thus allows high-resolution imaging of collecting vessels and valves, the majority of which are fully developed at birth.
Targeting of adult lymphatic vessels
The mature lymphatic vasculature in post-weaning aged mice, including the skin (Zhang et al., 2018), intestine (Sabine et al., 2015) and lymph node (Rouhani et al., 2015), can be effectively targeted using the Prox1-CreERT2 transgene. For most alleles, complete recombination can be induced with three consecutive administrations of 1 mg of tamoxifen. The commonly recommended protocols involve intraperitoneal injection of tamoxifen dissolved in oil (www.Jax.Org/Research-and-Faculty/Resources/Cre-Repository/Tamoxifen). However, oil has been shown to promote local inflammation and associated vascular expansion within the omentum and mesenteric membranes (Alsina-Sanchis et al., 2021), affecting both peritoneal tissues and potentially causing systemic effects. Oral gavage provides therefore a preferred administration route as it is without such a side effect (Alsina-Sanchis et al., 2021), and is also less invasive while inducing comparable recombination efficiency at routine tamoxifen doses (Donocoff et al., 2020). If targeting of other Prox1-expressing tissues is a concern, Vegfr3-CreERT2 can be used if sufficiently high doses of tamoxifen are administered (Tan et al., 2023).
Targeting of specific organs and vessel compartments
Systemic Cre-mediated gene deletion or activation may lead severe adverse effects and prevent long-term studies of advanced or severe phenotypes. To achieve local targeting of the dermal lymphatic vasculature, topical application of 4-OHT dissolved in acetone directly to the ear skin can be effectively used. Successful induction of recombination of Cre-activated transgenes, including reporters, which only require recombination of a single allele, has been achieved with doses of 5-50 µg of 4-OHT in volumes of up to 10 µl (Martinez-Corral et al., 2020; Petkova et al., 2023). Notably, despite local administration of 4-OHT, systemic recombination may occur due to grooming and subsequent ingestion of 4-OHT as well as direct absorption into the blood circulation. The extent of systemic recombination depends on the dose of 4-OHT applied and the specific CreER line used, potentially making the complete deletion of alleles requiring two recombination events in the same cell while limiting systemic recombination a challenging task.
Specific targeting of lymphatic valves can be achieved using the Cldn11-CreERT2 line (Ortsäter et al., 2021). Notably, however, efficient recombination occurs only in mature valves at postnatal stages but not in developmental stages. Neonatal targeting of valves can be achieved by a single or two consecutive intra-gastric administrations of 25-50 µg of 4-OHT dissolved in ethanol (Ortsäter et al., 2021). In juvenile and adult mice, three consecutive administrations of 1 mg of tamoxifen by oral gavage has been shown to result in efficient targeting, as assessed by reporter gene expression (Ortsäter et al., 2021).
Acknowledgements
Research in the authors’ laboratory is supported by grants from Knut and Alice Wallenberg Foundation (2018.0218 and 2020.0057; TM), the Swedish Research Council (2020-02692; TM), the Swedish Cancer Society (22 2025 Pj; TM), Göran Gustafsson foundation (TM) and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 814316 (TM, HS).
References
Alsina-Sanchis E., Mülfarth R., Moll I., Mogler C., Rodriguez-Vita J., Fischer A. (2021). Intraperitoneal Oil Application Causes Local Inflammation with Depletion of Resident Peritoneal Macrophages. Molecular Cancer Research 19: 288-300.
Álvarez-Aznar A., Martínez-Corral I., Daubel N., Betsholtz C., Mäkinen T., Gaengel K. (2020). Tamoxifen-independent recombination of reporter genes limits lineage tracing and mosaic analysis using CreERT2 lines. Transgenic Research 29: 53-68.
Amersfoort J., Eelen G., Carmeliet P. (2022). Immunomodulation by endothelial cells — partnering up with the immune system?. Nature Reviews Immunology 22: 576-588.
Arroz-Madeira S., Bekkhus T., Ulvmar M. H., Petrova T. V. (2023). Lessons of Vascular Specialization From Secondary Lymphoid Organ Lymphatic Endothelial Cells. Circulation Research 132: 1203-1225.
Aspelund A., Tammela T., Antila S., Nurmi H., Leppänen V.M., Zarkada G., Stanczuk L., Francois M., Mäkinen T., Saharinen P., Immonen I., Alitalo K. (2014). The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. Journal of Clinical Investigation 124: 3975-3986.
Baluk P., McDonald D. M. (2022). Buttons and Zippers: Endothelial Junctions in Lymphatic Vessels. Cold Spring Harbor Perspectives in Medicine 12: a041178.
Bazigou E., Lyons O. T.A., Smith A., Venn G. E., Cope C., Brown N. A., Makinen T. (2011). Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. Journal of Clinical Investigation 121: 2984-2992.
Bazigou E., Xie S., Chen C., Weston A., Miura N., Sorokin L., Adams R., Muro A. F., Sheppard D., Makinen T. (2009). Integrin-α9 Is Required for Fibronectin Matrix Assembly during Lymphatic Valve Morphogenesis. Developmental Cell 17: 175-186.
Borgna J.L., Rochefort H. (1981). Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues.. Journal of Biological Chemistry 256: 859-868.
Brash J. T., Bolton R. L., Rashbrook V. S., Denti L., Kubota Y., Ruhrberg C. (2020). Tamoxifen-Activated CreERT Impairs Retinal Angiogenesis Independently of Gene Deletion. Circulation Research 127: 849-850.
Bronstein J. M., Tiwari-Woodruff S., Buznikov A. G., Stevens D. B. (2000). Involvement of OSP/claudin-11 in oligodendrocyte membrane interactions: Role in biology and disease. Journal of Neuroscience Research 59: 706-711.
Chen K., Mou R., Zhu P., Xu X., Wang H., Jiang L., Hu Y., Hu X., Ma L., Xiao Q., Xu Q. (2023). The Effect of Lymphangiogenesis in Transplant Arteriosclerosis. Circulation 147: 482-497.
Claxton S., Kostourou V., Jadeja S., Chambon P., Hodivala‐Dilke K., Fruttiger M. (2008). Efficient, inducible Cre-recombinase activation in vascular endothelium. genesis 46: 74-80.
Connor A. L., Kelley P. M., Tempero R. M. (2016). Lymphatic endothelial lineage assemblage during corneal lymphangiogenesis. Laboratory Investigation 96: 270-282.
Donocoff R. S., Teteloshvili N., Chung H., Shoulson R., Creusot R. J. (2020). Optimization of tamoxifen-induced Cre activity and its effect on immune cell populations. Scientific Reports 10: 15244.
Dumont D. J., Jussila L., Taipale J., Lymboussaki A., Mustonen T., Pajusola K., Breitman M., Alitalo K. (1998). Cardiovascular Failure in Mouse Embryos Deficient in VEGF Receptor-3. Science 282: 946-949.
Ehling M., Adams S., Benedito R., Adams R. H. (2013). Notch controls retinal blood vessel maturation and quiescence. Development 140: 3051-3061.
Engleka K. A., Gitler A. D., Zhang M., Zhou D. D., High F. A., Epstein J. A. (2005). Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Developmental Biology 280: 396-406.
Fernández-Chacón M., Casquero-García V., Luo W., Francesca Lunella F., Ferreira Rocha S., Del Olmo-Cabrera S., Benedito R. (2019). iSuRe-Cre is a genetic tool to reliably induce and report Cre-dependent genetic modifications. Nature Communications 10: 2262.
Frye M., Stritt S., Ortsäter H., Hernandez Vasquez M., Kaakinen M., Vicente A., Wiseman J., Eklund L., Martínez-Torrecuadrada J. L., Vestweber D., Mäkinen T. (2020). EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity. eLife 9: e57732.
Garcia-Gonzalez I., Mühleder S., Fernández-Chacón M., Benedito R. (2020). Genetic Tools to Study Cardiovascular Biology. Frontiers in Physiology 11: 1084.
Geng X., Cha B., Mahamud M. R., Lim K.C., Silasi-Mansat R., Uddin M. K.M., Miura N., Xia L., Simon A. M., Engel J. D., Chen H., Lupu F., Srinivasan R. S. (2016). Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Developmental Biology 409: 218-233.
González-Loyola A., Bovay E., Kim J., Lozano T. W., Sabine A., Renevey F., Arroz-Madeira S., Rapin A., Wypych T. P., Rota G., Durot S., Velin D., Marsland B., Guarda G., Delorenzi M., Zamboni N., Luther S. A., Petrova T. V. (2021). FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure. Science Advances 7: eabf4335.
Gow A., Southwood C. M., Li J. S., Pariali M., Riordan G. P., Brodie S. E., Danias J., Bronstein J. M., Kachar B., Lazzarini R. A., (1999). CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99: 649-659.
Grimm L., Hogan B. M. (2021). Network patterning, morphogenesis and growth in lymphatic vascular development. In Cellular Networks in Development. Elsevier.
Gur-Cohen S., Yang H., Baksh S. C., Miao Y., Levorse J., Kataru R. P., Liu X., de la Cruz-Racelis J., Mehrara B. J., Fuchs E. (2019). Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science 366: 1218-1225.
Hägerling R., Pollmann C., Andreas M., Schmidt C., Nurmi H., Adams R. H., Alitalo K., Andresen V., Schulte-Merker S., Kiefer F. (2013). A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. The EMBO Journal 32: 629-644.
Han X., Zhang Z., He L., Zhu H., Li Y., Pu W., Han M., Zhao H., Liu K., Li Y., Huang X., Zhang M., Jin H., Lv Z., Tang J., Wang J., Sun R., Fei J., Tian X., Duan S., Wang Q.D., Wang L., He B., Zhou B. (2021). A suite of new Dre recombinase drivers markedly expands the ability to perform intersectional genetic targeting. Cell Stem Cell 28: 1160-1176.e7.
Hayashi S., McMahon A. P. (2002). Efficient Recombination in Diverse Tissues by a Tamoxifen-Inducible Form of Cre: A Tool for Temporally Regulated Gene Activation/Inactivation in the Mouse. Developmental Biology 244: 305-318.
Higashi A. Y., Ikawa T., Muramatsu M., Economides A. N., Niwa A., Okuda T., Murphy A. J., Rojas J., Heike T., Nakahata T., Kawamoto H., Kita T., Yanagita M. (2009). Direct Hematological Toxicity and Illegitimate Chromosomal Recombination Caused by the Systemic Activation of CreERT2. The Journal of Immunology 182: 5633-5640.
The Jackson Laboratory (2011). Intraperitoneal Injection of Tamoxifen for Inducible Cre-Driver Lines. https://www.jax.org/research-and-faculty/resources/cre-repository/tamoxifen
Iyer D., Mastrogiacomo D. M., Li K., Banerjee R., Yang Y., Scallan J. P. (2023). eNOS Regulates Lymphatic Valve Specification by Controlling β-Catenin Signaling During Embryogenesis in Mice. Arteriosclerosis, Thrombosis, and Vascular Biology 43: 2197-2212.
Jafree D. J., Long D. A., Scambler P. J., Ruhrberg C. (2021). Mechanisms and cell lineages in lymphatic vascular development. Angiogenesis 24: 271-288.
Johnson N. C., Dillard M. E., Baluk P., McDonald D. M., Harvey N. L., Frase S. L., Oliver G. (2008). Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes & Development 22: 3282-3291.
Kalucka J., de Rooij L. P.M.H., Goveia J., Rohlenova K., Dumas S. J., Meta E., Conchinha N. V., Taverna F., Teuwen L.A., Veys K., García-Caballero M., Khan S., Geldhof V., Sokol L., Chen R., Treps L., Borri M., de Zeeuw P., Dubois C., Karakach T. K., Falkenberg K. D., Parys M., Yin X., Vinckier S., Du Y., Fenton R. A., Schoonjans L., Dewerchin M., Eelen G., Thienpont B., Lin L., Bolund L., Li X., Luo Y., Carmeliet P. (2020). Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 180: 764-779.e20.
Katzenellenbogen B. S., Norman M. J., Eckert R. L., Peltz S. W., Mangel W. F., (1984). Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer research 44: 112-119.
Kristianto J., Johnson M. G., Zastrow R. K., Radcliff A. B., Blank R. D. (2017). Spontaneous recombinase activity of Cre–ERT2 in vivo. Transgenic Research 26: 411-417.
Laakkonen P., Waltari M., Holopainen T., Takahashi T., Pytowski B., Steiner P., Hicklin D., Persaud K., Tonra J. R., Witte L., Alitalo K. (2007). Vascular Endothelial Growth Factor Receptor 3 Is Involved in Tumor Angiogenesis and Growth. Cancer Research 67: 593-599.
Lavado A., Oliver G. (2007). Prox1 expression patterns in the developing and adult murine brain. Developmental Dynamics 236: 518-524.
Lee H.W., Xu Y., He L., Choi W., Gonzalez D., Jin S.W., Simons M. (2021). Role of Venous Endothelial Cells in Developmental and Pathologic Angiogenesis. Circulation 144: 1308-1322.
Lenti E., Genovese L., Bianchessi S., Maurizio A., Sain S. B., di Lillo A., Mattavelli G., Harel I., Bernassola F., Hehlgans T., Pfeffer K., Crosti M., Abrignani S., Evans S. M., Sitia G., Guimarães-Camboa N., Russo V., van de Pavert S. A., Garcia-Manteiga J. M., Brendolan A. (2022). Fate mapping and scRNA sequencing reveal origin and diversity of lymph node stromal precursors. Immunity 55: 606-622.e6.
Li J., Li E., Czepielewski R. S., Chi J., Guo X., Han Y.H., Wang D., Wang L., Hu B., Dawes B., Jacobs C., Tenen D., Lin S. J., Lee B., Morris D., Tobias A., Randolph G. J., Cohen P., Tsai L., Rosen E. D. (2021). Neurotensin is an anti-thermogenic peptide produced by lymphatic endothelial cells. Cell Metabolism 33: 1449-1465.e6.
Lizen B., Claus M., Jeannotte L., Rijli F. M., Gofflot F. (2015). Perinatal induction of Cre recombination with tamoxifen. Transgenic Research 24: 1065-1077.
Lupu I. E., Kirschnick N., Weischer S., Martinez-Corral I., Forrow A., Lahmann I., Riley P. R., Zobel T., Makinen T., Kiefer F., Stone O. A., (2022). Direct specification of lymphatic endothelium from non-venous angioblasts. bioRxiv Preprint: 2022.05.11.491403.
Mäkinen T., Adams R. H., Bailey J., Lu Q., Ziemiecki A., Alitalo K., Klein R., Wilkinson G. A. (2005). PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes & Development 19: 397-410.
Martin-Almedina S., Martinez-Corral I., Holdhus R., Vicente A., Fotiou E., Lin S., Petersen K., Simpson M. A., Hoischen A., Gilissen C., Jeffery H., Atton G., Karapouliou C., Brice G., Gordon K., Wiseman J. W., Wedin M., Rockson S. G., Jeffery S., Mortimer P. S., Snyder M. P., Berland S., Mansour S., Makinen T., Ostergaard P. (2016). EPHB4 kinase–inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. Journal of Clinical Investigation 126: 3080-3088.
Martinez-Corral I., Stanczuk L., Frye M., Ulvmar M. H., Diéguez-Hurtado R., Olmeda D., Makinen T., Ortega S. (2016). Vegfr3-CreER T2 mouse, a new genetic tool for targeting the lymphatic system. Angiogenesis 19: 433-445.
Martinez-Corral I., Ulvmar M. H., Stanczuk L., Tatin F., Kizhatil K., John S. W.M., Alitalo K., Ortega S., Makinen T. (2015). Nonvenous Origin of Dermal Lymphatic Vasculature. Circulation Research 116: 1649-1654.
Martinez-Corral I., Zhang Y., Petkova M., Ortsäter H., Sjöberg S., Castillo S. D., Brouillard P., Libbrecht L., Saur D., Graupera M., Alitalo K., Boon L., Vikkula M., Mäkinen T. (2020). Blockade of VEGF-C signaling inhibits lymphatic malformations driven by oncogenic PIK3CA mutation. Nature Communications 11: 2869.
Mazaud-Guittot S., Meugnier E., Pesenti S., Wu X., Vidal H., Gow A., Le Magueresse-Battistoni B. (2010). Claudin 11 Deficiency in Mice Results in Loss of the Sertoli Cell Epithelial Phenotype in the Testis1. Biology of Reproduction 82: 202-213.
Ortsäter H., Hernández-Vásquez M. N., Ulvmar M. H., Gow A., Mäkinen T. (2021). An inducible Cldn11-CreER T2 mouse line for selective targeting of lymphatic valves. Genesis 59: e23439.
Park D.Y., Lee J., Park I., Choi D., Lee S., Song S., Hwang Y., Hong K. Y., Nakaoka Y., Makinen T., Kim P., Alitalo K., Hong Y.K., Koh G. Y. (2014). Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. Journal of Clinical Investigation 124: 3960-3974.
Peña-Jimenez D., Fontenete S., Megias D., Fustero-Torre C., Graña-Castro O., Castellana D., Loewe R., Perez-Moreno M. (2019). Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. The EMBO Journal 38: e101688.
Petkova M., Kraft M., Stritt S., Martinez-Corral I., Ortsäter H., Vanlandewijck M., Jakic B., Baselga E., Castillo S. D., Graupera M., Betsholtz C., Mäkinen T. (2023). Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation. Journal of Experimental Medicine 220: e20220741.
Petrova T. V., Koh G. Y. (2020). Biological functions of lymphatic vessels. Science 369: eaax4063.
Pichol-Thievend C., Betterman K. L., Liu X., Ma W., Skoczylas R., Lesieur E., Bos F. L., Schulte D., Schulte-Merker S., Hogan B. M., Oliver G., Harvey N. L., Francois M. (2018). A blood capillary plexus-derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development 145: dev160184.
Pietilä R., Del Gaudio F., He L., Vázquez-Liébanas E., Vanlandewijck M., Muhl L., Mocci G., Bjørnholm K. D., Lindblad C., Fletcher-Sandersjöö A., Svensson M., Thelin E. P., Liu J., van Voorden A. J., Torres M., Antila S., Xin L., Karlström H., Storm-Mathisen J., Bergersen L. H., Moggio A., Hansson E. M., Ulvmar M. H., Nilsson P., Mäkinen T., Andaloussi Mäe M., Alitalo K., Proulx S. T., Engelhardt B., McDonald D. M., Lendahl U., Andrae J., Betsholtz C. (2023). Molecular anatomy of adult mouse leptomeninges. Neuron 111: 3745-3764.e7.
Poulos M. G., Ramalingam P., Winiarski A., Gutkin M. C., Katsnelson L., Carter C., Pibouin-Fragner L., Eichmann A., Thomas J.L., Miquerol L., Butler J. M. (2024). Complementary and Inducible creERT2 Mouse Models for Functional Evaluation of Endothelial Cell Subtypes in the Bone Marrow. Stem Cell Reviews and Reports 20: 1135-1149.
Pu W., He L., Han X., Tian X., Li Y., Zhang H., Liu Q., Huang X., Zhang L., Wang Q.D., Yu Z., Yang X., Smart N., Zhou B. (2018). Genetic Targeting of Organ-Specific Blood Vessels. Circulation Research 123: 86-99.
Pu W., Zhang H., Huang X., Tian X., He L., Wang Y., Zhang L., Liu Q., Li Y., Li Y., Zhao H., Liu K., Lu J., Zhou Y., Huang P., Nie Y., Yan Y., Hui L., Lui K. O., Zhou B. (2016). Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nature Communications 7: 13369.
Rafii S., Butler J. M., Ding B.S. (2016). Angiocrine functions of organ-specific endothelial cells. Nature 529: 316-325.
Rashbrook V. S., Brash J. T., Ruhrberg C. (2022). Cre toxicity in mouse models of cardiovascular physiology and disease. Nature Cardiovascular Research 1: 806-816.
Risebro C. A., Searles R. G., Melville A. A. D., Ehler E., Jina N., Shah S., Pallas J., Hubank M., Dillard M., Harvey N. L., Schwartz R. J., Chien K. R., Oliver G., Riley P. R. (2009). Prox1 maintains muscle structure and growth in the developing heart. Development 136: 495-505.
Rossi M., Salomon A., Chaumontel N., Molet J., Bailly S., Tillet E., Bouvard C. (2023). Warning regarding hematological toxicity of tamoxifen activated CreERT2 in young Rosa26CreERT2 mice. Scientific Reports 13: 5976.
Rouhani S. J., Eccles J. D., Riccardi P., Peske J. D., Tewalt E. F., Cohen J. N., Liblau R., Mäkinen T., Engelhard V. H. (2015). Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nature Communications 6: 6771.
Sabine A., Agalarov Y., Maby-El Hajjami H., Jaquet M., Hägerling R., Pollmann C., Bebber D., Pfenniger A., Miura N., Dormond O., Calmes J.M., Adams R. H., Mäkinen T., Kiefer F., Kwak B. R., Petrova T. V. (2012). Mechanotransduction, PROX1, and FOXC2 Cooperate to Control Connexin37 and Calcineurin during Lymphatic-Valve Formation. Developmental Cell 22: 430-445.
Sabine A., Bovay E., Demir C. S., Kimura W., Jaquet M., Agalarov Y., Zangger N., Scallan J. P., Graber W., Gulpinar E., Kwak B. R., Mäkinen T., Martinez-Corral I., Ortega S., Delorenzi M., Kiefer F., Davis M. J., Djonov V., Miura N., Petrova T. V. (2015). FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. Journal of Clinical Investigation 125: 3861-3877.
Smith L. (2011). Good planning and serendipity: exploiting the Cre/Lox system in the testis. REPRODUCTION 141: 151-161.
Sosa-Pineda B., Wigle J. T., Oliver G. (2000). Hepatocyte migration during liver development requires Prox1. Nature Genetics 25: 254-255.
Srinivasan R. S., Dillard M. E., Lagutin O. V., Lin F.J., Tsai S., Tsai M.J., Samokhvalov I. M., Oliver G. (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes & Development 21: 2422-2432.
Stanczuk L., Martinez-Corral I., Ulvmar M. H., Zhang Y., Laviña B., Fruttiger M., Adams R. H., Saur D., Betsholtz C., Ortega S., Alitalo K., Graupera M., Mäkinen T. (2015). cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Reports 10: 1708-1721.
Stone O. A., Stainier D. Y.R. (2019). Paraxial Mesoderm Is the Major Source of Lymphatic Endothelium. Developmental Cell 50: 247-255.e3.
Stritt S., Koltowska K., Mäkinen T. (2021). Homeostatic maintenance of the lymphatic vasculature. Trends in Molecular Medicine 27: 955-970.
Takeda A., Hollmén M., Dermadi D., Pan J., Brulois K. F., Kaukonen R., Lönnberg T., Boström P., Koskivuo I., Irjala H., Miyasaka M., Salmi M., Butcher E. C., Jalkanen S. (2019). Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 51: 561-572.e5.
Tan C., Norden P. R., Yu W., Liu T., Ujiie N., Lee S. K., Yan X., Dyakiv Y., Aoto K., Ortega S., De Plaen I. G., Sampath V., Kume T. (2023). Endothelial FOXC1 and FOXC2 promote intestinal regeneration after ischemia–reperfusion injury. EMBO reports 24: e56030.
Tatin F., Taddei A., Weston A., Fuchs E., Devenport D., Tissir F., Makinen T. (2013). Planar Cell Polarity Protein Celsr1 Regulates Endothelial Adherens Junctions and Directed Cell Rearrangements during Valve Morphogenesis. Developmental Cell 26: 31-44.
Tian X., Zhou B. (2021). Strategies for site-specific recombination with high efficiency and precise spatiotemporal resolution. Journal of Biological Chemistry 296: 100509.
Uçar M. C., Hannezo E., Tiilikainen E., Liaqat I., Jakobsson E., Nurmi H., Vaahtomeri K. (2023). Self-organized and directed branching results in optimal coverage in developing dermal lymphatic networks. Nature Communications 14: 5878.
Wang Y., Jin Y., Mäe M. A., Zhang Y., Ortsäter H., Betsholtz C., Mäkinen T., Jakobsson L. (2017). Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity. Development 144: 3590-3691.
Wang Y., Nakayama M., Pitulescu M. E., Schmidt T. S., Bochenek M. L., Sakakibara A., Adams S., Davy A., Deutsch U., Lüthi U., Barberis A., Benjamin L. E., Mäkinen T., Nobes C. D., Adams R. H. (2010). Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465: 483-486.
Wigle J. T., Chowdhury K., Gruss P., Oliver G. (1999). Prox1 function is crucial for mouse lens-fibre elongation. Nature Genetics 21: 318-322.
Wigle J. T., Oliver G. (1999). Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 98: 769-778.
Xiang M., Grosso R. A., Takeda A., Pan J., Bekkhus T., Brulois K., Dermadi D., Nordling S., Vanlandewijck M., Jalkanen S., Ulvmar M. H., Butcher E. C. (2020). A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature. Frontiers in Cardiovascular Medicine 7: 52.
Xu C., Hasan S. S., Schmidt I., Rocha S. F., Pitulescu M. E., Bussmann J., Meyen D., Raz E., Adams R. H., Siekmann A. F. (2014). Arteries are formed by vein-derived endothelial tip cells. Nature Communications 5: 5758.
Yang Y., García-Verdugo J. M., Soriano-Navarro M., Srinivasan R. S., Scallan J. P., Singh M. K., Epstein J. A., Oliver G. (2012). Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120: 2340-2348.
Ye R., Wang Q. A., Tao C., Vishvanath L., Shao M., McDonald J. G., Gupta R. K., Scherer P. E. (2015). Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular Metabolism 4: 771-778.
Yoon S.Y., Detmar M. (2022). Sostdc1 Secreted from Cutaneous Lymphatic Vessels Acts as a Paracrine Factor for Hair Follicle Growth. Current Issues in Molecular Biology 44: 2167-2174.
Zhang Y., Ortsäter H., Martinez-Corral I., Mäkinen T. (2022). Cdh5 -lineage–independent origin of dermal lymphatics shown by temporally restricted lineage tracing. Life Science Alliance 5: e202201561.
Zhang Y., Ulvmar M. H., Stanczuk L., Martinez-Corral I., Frye M., Alitalo K., Mäkinen T. (2018). Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nature Communications 9: 1296.
Zhong Z. A., Sun W., Chen H., Zhang H., Lay Y. E., Lane N. E., Yao W. (2015). Optimizing tamoxifen-inducible Cre/loxp system to reduce tamoxifen effect on bone turnover in long bones of young mice. Bone 81: 614-619.