Int. J. Dev. Biol. 69: 51 - 59 (2025)
Placental transcriptome reveals the placental brain axis genes and pathways of gestational diabetes mellitus (GDM) affecting offspring neurodevelopment
Open Access | Original Article | Published: 15 April 2025
Abstract
This study aims to analyze the pathways and the placental brain axis genes of gestational diabetes mellitus (GDM) affecting offspring neurodevelopment. Differentially expressed genes (DEGs) were identified through transcriptome sequencing of placental tissues. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed on DEGs. A protein-protein interaction (PPI) network was constructed and annotated using the STRING online software. The expression of neurodevelopment-related genes was analyzed by qPCR. Hubgenes were analyzed using Cytoscape 3.7.1 software. The correlation between Hubgenes and placental brain axis genes was analyzed through literatures alignment. The pathways of GDM affecting offspring neural development were predicted using the KEGG database. The placental transcriptome revealed that there were 404 DEGs between GDM and Normal groups. Among these DEGs, 125 were upregulated and 279 were downregulated. GO analysis indicated that DEGs were mainly involved in intracellular calcium activated chloride channel activity, anion channel activity, G protein-coupled peptide receptors, etc. Additionally, KEGG analysis revealed that DEGs were predominantly involved in neuroactive ligand receptor interaction pathways. STRING online software analysis revealed that the DLGAP1, NXNL2, SCG2, SLC18A2, LYNX1, GRM1, DLGAP1, BIRC7 genes were associated with neurodevelopment. PCR validation of these 8 genes was consistent with transcriptome results (P<0.05). Literatures alignment showed that DLGAP1, GRM1 and SLC18A2 are placental brain axis genes that influence offspring neurodevelopment. The placental brain axis genes DLGAP1, GRM1, SLC18A2 have been found to influence GDM offspring neurodevelopment through the regulation of the Gq/PLC/PKC pathway.
Keywords
gestational diabetes mellitus, placenta brain axis, transcriptome analysis, neurodevelopment, Gq/PLC/PKC pathway
Introduction
Gestational diabetes mellitus (GDM) is a common disease specific to pregnancy, affecting approximately 14% of pregnant women. It has a complex pathogenesis and poses a significant risk to the short-term and long-term health of both mothers and infants. GDM has been found to have adverse effects on the neurodevelopment of offspring, including fetal neuro-inflammation, iron deficiency, epigenetic changes, lipid metabolism disorders, and abnormalities in brain structure (Rosenfeld 2020).
In addition to its role in nutrient and gas transfer, the placenta also plays a crucial role in understanding the intrauterine regulatory mechanisms of neurodevelopmental outcomes(Rosenfeld 2020). The placenta brain axis was introduced in 2022, which can influence the development of the fetal brain through the abnormal regulation of numerous genes (Shallie and Naicker 2019; Behura et al., 2019). Maternal GDM affects the fatty acid profile of the fetal brain both by downregulating the Wnt3/β-catenin/MFSD2a pathway of the placental-fetal barrier and by affecting maternal fatty acid metabolism(Yu et al., 2024). Exposure to phthalates during pregnancy affects the neurodevelopment of children by fetal brain axis (Ren et al., 2024). Besides, study has also indicated that extracellular vesicles from mouse trophoblast cells affect in neural progenitor cells through placenta-brain axis (Kinkade et al., 2024). However, the exact mechanism by which GDM affects offspring neurodevelopment through placental-brain axis genes remains unclear.
Results
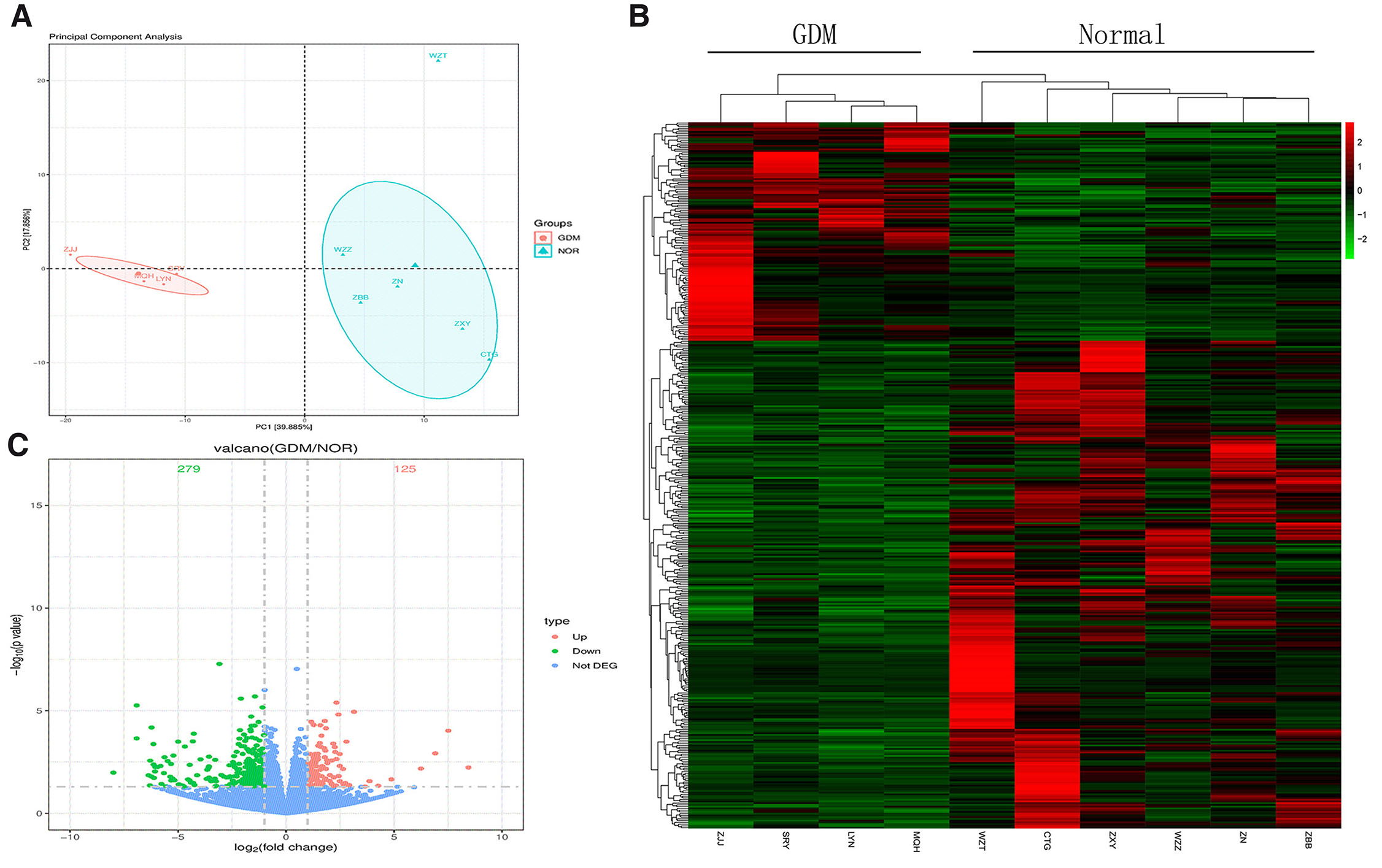
Analysis of differentially expressed genes (DEGs) in the placental transcriptome
The research programme was depicted in the flowchart (Supplementary Fig. 1).By conducting transcriptome analysis, a total of 404 DEGs were screened between 4 placental tissues from cases and 6 placental tissues from normal pregnancies. Among these DEGs, 279 were determined to be down-regulated, while 125 were up-regulated (Fig. 1).
Analysis of DEGs through gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment
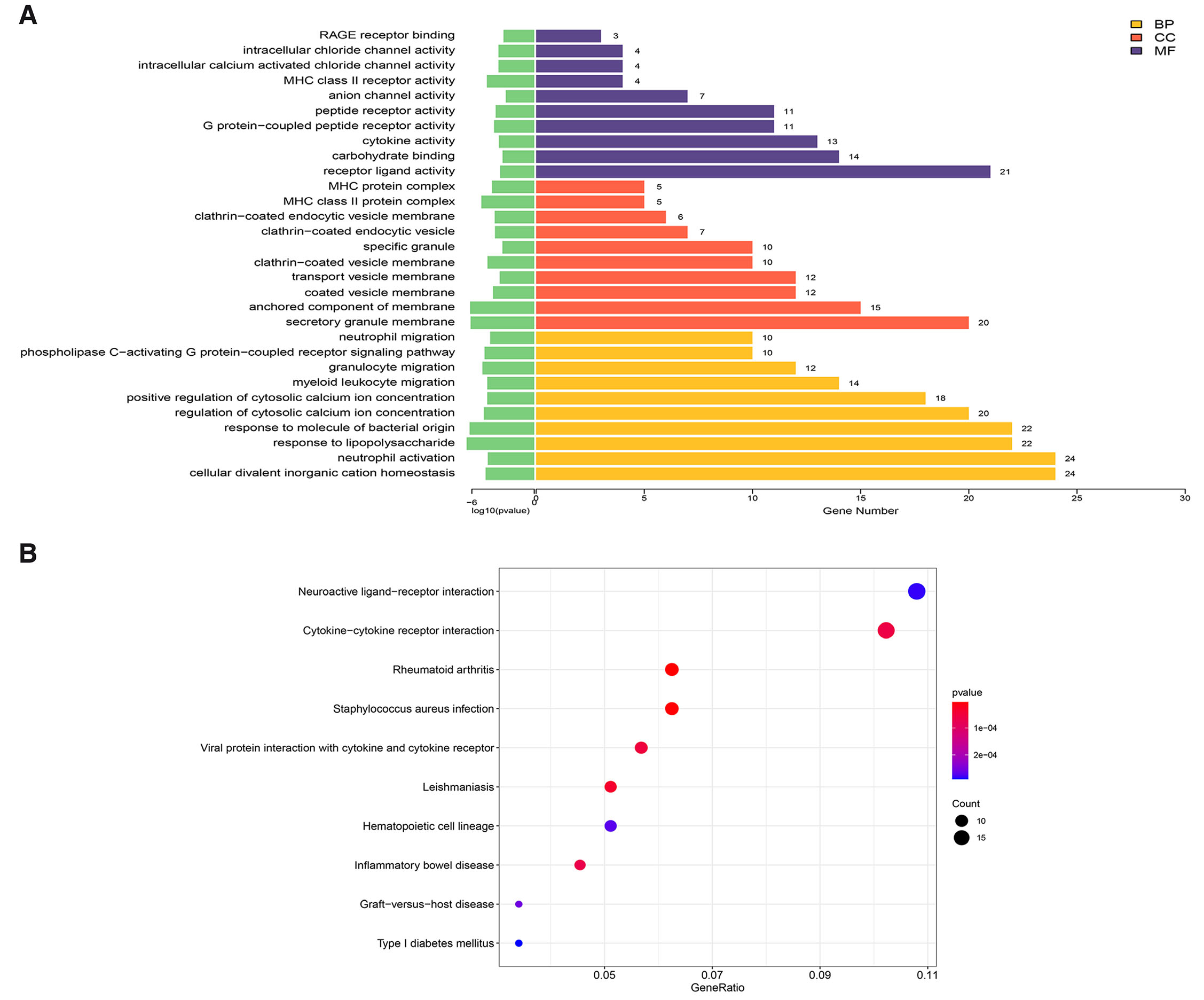
The DEGs between the GDM group and the Normal group were subjected to GO annotation analysis. The functional enrichment categories were classified into three main categories: biological process, cellular component, and molecular function. For each category, GO terms with more than two DEGs were selected, resulting in a total of 10 terms chosen based on their -log10 P values. The top 10 enriched GO terms encompassed RAGE receptor binding, intracellular chloride channel activity, MHC class II receptor activity, anion channel activity, peptide receptor activity, etc. Regarding cellular components, the main findings included MHC protein complex, MHC class II protein complex, clathrin-coated endocytic vesicle membrane, clathrin-coated endocytic vesicle membrane, etc. The key biological processes identified included neutrophil migration, phospholipase C-activating G protein-coupled receptor signaling pathway, granulocyte migration, myeloid leukocyte migration, positive regulation of cytosolic calcium ion concentration, etc. (Fig. 2A).
Fig. 2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of DEGs between GDM and Normal groups.
(A) GO terms between GDM and Normal groups based on their functions. Top 10 significantly enriched GO terms. The x-axis is the enriched GO terms, the y-axis is the -log10 P value. Orange terms represent biological processes, red terms represent cellular components, and purple terms represent molecular functions. (B) The top 10 enriched Kyoto Encyclopedia of KEGG pathway terms of differentially expressed genes between GDM and Normal groups. The x-axis is the GeneRatio, and the y-axis is the enriched KEGG pathway terms. The size of the bubbles indicates the number of genes; the color represents the P Value.
The KEGG pathway enrichment analysis was conducted to explore the significantly enriched pathways for DEGs. Among the top 10 KEGG pathways identified between the GDM group and the Normal group, the most enriched pathways included neuroactive ligand-receptor interaction, cytokine-cytokine receptor interaction, rheumatoid arthritis, staphylococcus auresu infection, viral protein interaction with cytokine and cytokine receptor, etc. Notably, the neuroactive ligand-receptor interaction pathway was discovered to be associated with the neurodevelopment (Fig. 2B).
Construction of protein-protein interaction (PPI) network and analysis of nodes, as well as mRNA expression verification of the top 50 DEGs involved in neurodevelopment
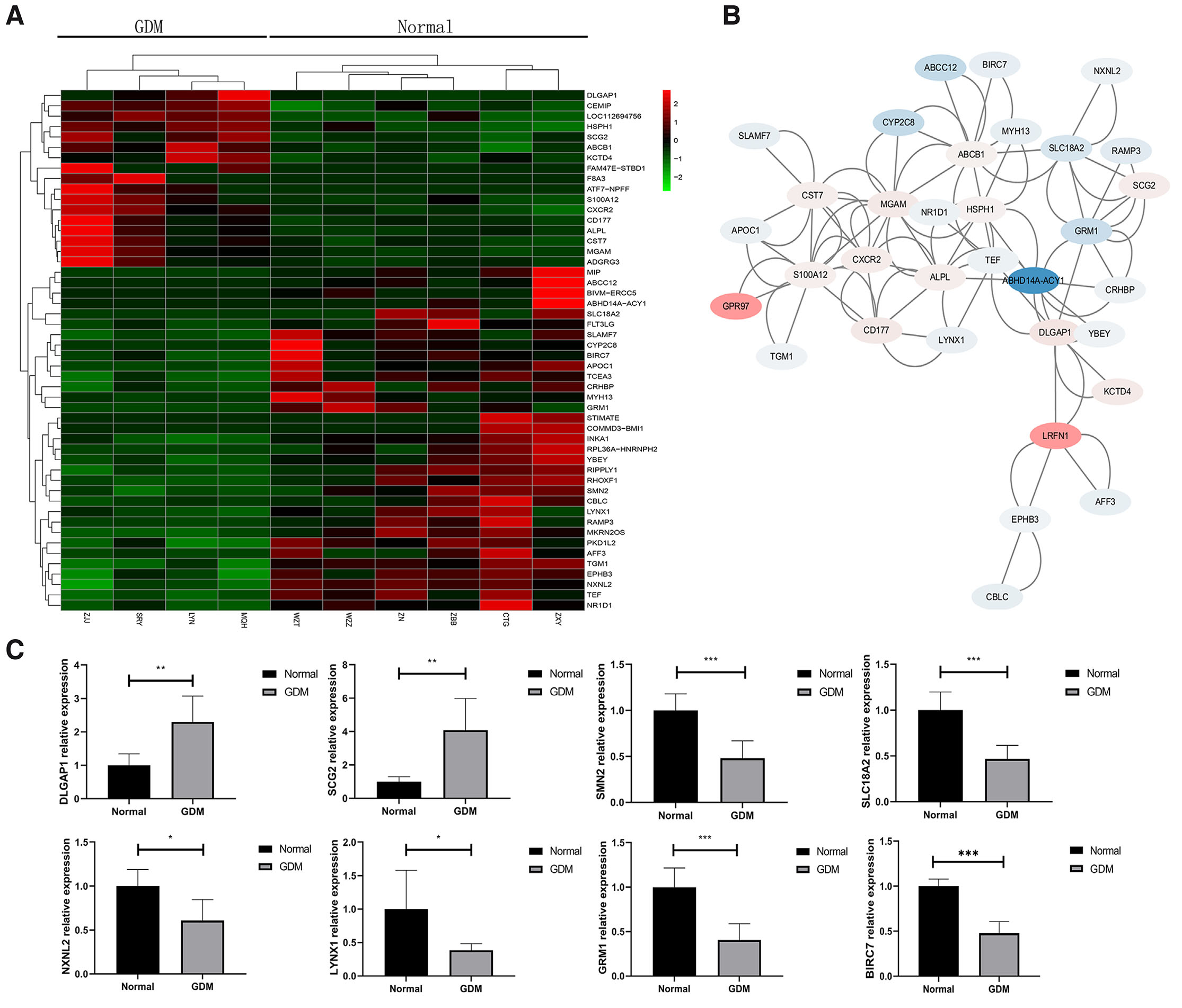
The top 50 DEGs were analyzed by implementing cluster heat-map, and the results revealed that DLGAP1, CEMIP, LOC112694756, HSPH1, SCG2, ABCB1, and other genes were upregulated in the GDM group, while MIP, ABCC12, BIVM-ERCC5, ABHD14A-ACY1, SLC18A2, FLT3LG, SLAMF7, etc. were downregulated in the Normal group (Fig. 3A).
Fig. 3. Protein-protein interaction (PPI) network and nodes analysis and mRNA expression verification of the top 50 DEGs involved in neurodevelopment.
(A) Heatmap of top 50 DEGs between the GDM and Normal groups. The x-axis is sample code, and the y-axis is gene terms. (B) The PPI network of DEGs between the GDM and Normal groups. (C) Verification of mRNA levels of 8 genes related to neuronal maturation, apoptosis and signal transduction in the top 50 DEGs of the GDM and Normal groups. Levels of statistical significance: * P<0.05, **P<0.01, ***P<0.001.
A PPI network was constructed for the top 50 DEGs using STRING online software (Fig. 3B). The nodes were annotated and 8 DEGs related to neuronal maturation, neuronal signal transduction, and neuronal apoptosis were screened. DLGAP1 and SCG2 were upregulated, while SMN2, SLC18A2, NXNL2, LYNX1, GRM1, and BIRC7 were downregulated. DLGAP1 and NXNL2 primarily participated in neuronal maturation, whereas SCG2, SLC18A2, LYNX1, and GRM1 were involved in neuronal signal transduction. SMN2 and BIRC7 were associated with neuronal apoptosis (Table 1).
Table 1
8 Genes involved in neural cell biological function in top50 DEGs of GDM placenta
| Gene name | Classification | Functions |
|---|---|---|
| DLGAP1 | Up | Part of the postsynaptic scaffold in neuronal cells |
| SCG2 | Up | Secretogranin-2 is a neuroendocrine secretory granule protein, being the precursor for biologically active peptides. |
| SMN2 | Down | Survival motor neuron protein plays a catalyst role in the assembly of small nuclear ribonucleoproteins (snRNPs), the building blocks of the spliceosome. |
| SLC18A2 | Down | Synaptic vesicular amine transporter involves in the ATP-dependent vesicular transport of biogenic amine neurotransmitters. Pumps cytosolic monoamines including dopamine, norepinephrine, serotonin, and histamine into synaptic vesicles. Requisite for vesicular amine storage prior to secretion via exocytosis. |
| NXNL2 | Down | Nucleoredoxin-like protein may be involved in the maintenance of both the function and the viability of sensory neurons, including photoreceptors and olfactory neurons. |
| LYNX1 | Down | It acts in different tissues through interaction to nicotinic acetylcholine receptors (nAChRs), and involves an effect on nAChR trafficking and its cell surface expression, and on single channel properties of the nAChR inserted in the plasma membrane, and modulates functional properties of nicotinic acetylcholine receptors (nAChRs) to prevent excessive excitation, and hence neurodegeneration. |
| GRM1 | Down | Metabotropic glutamate receptor 1; G-protein coupled receptor for glutamate. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors. Signaling activates a phosphatidylinositol-calcium second messenger system. May participate in the central action of glutamate in the CNS, such as long-term potentiation in the hippocampus and long-term depression in the cerebellum. |
| BIRC7 | Down | Baculoviral IAP repeat-containing protein 7; Apoptotic regulator capable of exerting proapoptotic and anti-apoptotic activities and plays crucial roles in apoptosis, cell proliferation, and cell cycle Normal. Its anti-apoptotic activity is mediated through the inhibition of CASP3, CASP7 and CASP9, as well as by its E3 ubiquitin-protein ligase activity. As it is a weak caspase inhibitor, its anti-apoptotic activity is thought to be due to its ability to ubiquitinate DIABLO/SMAC targeting it for degradation thereby promoting cell survival. |
The mRNA expression of these 8 DEGs was confirmed through qPCR, as they played a role in neuronal maturation, neuronal signal transduction, and neuronal apoptosis. Sequences were retrieved from the NCBI database, and qPCR primers were designed using Primer Premier 5.0 software (Table 2).
Table 2
8 DEGs qPCR primer sequences
| Gene | Function | primer sequences(5'→3') |
|---|---|---|
| DLGAP1 | neuronal maturation | F’-gCTCTCAgCTgCACTATACCA R’-TCCACTgTCTTCATCCCCCA |
| NXNL2 | F’-ACTTCTATACggCgCTggTg R’-TACCTCTTCCTCAgCTCATg |
|
| SCG2 | neuronal signal transduction | F’-TgAAgCgAgTTCCTggTCA R’-ATgCTCTTTgATggCCTgCT |
| SLC18A2 | F’-ACATgCTgCTCACTgTCgT R’-CTggAAgCTgTCTgAgATggA |
|
| LYNX1 | F’-ACCTCTgCACCAACCATCC R’-AggATCCAACTCAgggTg |
|
| GRM1 | F’-TgAAACggACAAggCAAC R’-ACCggTAgTgCATCAAggT |
|
| SMN2 | neuronal apoptosis | F’-ACTgCAgCTTCCTTACAAC R’-TCCATggAgCAgATTTgggC |
| BIRC7 | F’-gCCAggAgCCAgggATg R’-gCACAAAgACgATggACACg |
The qPCR results demonstrated that NXNL2, SLC18A2, LYNX1, GRM1, SMN2, and BIRC7 were downregulated, while DLGAP1 and SCG2 were upregulated in the GDM group, exhibiting a significant difference (P<0.05). The verification results were consistent with the expression trends observed in the transcriptome sequencing data (Fig. 3C).
Analysis of the correlation between Hub genes in 8 DEGs related to neurodevelopment and placental brain axis genes
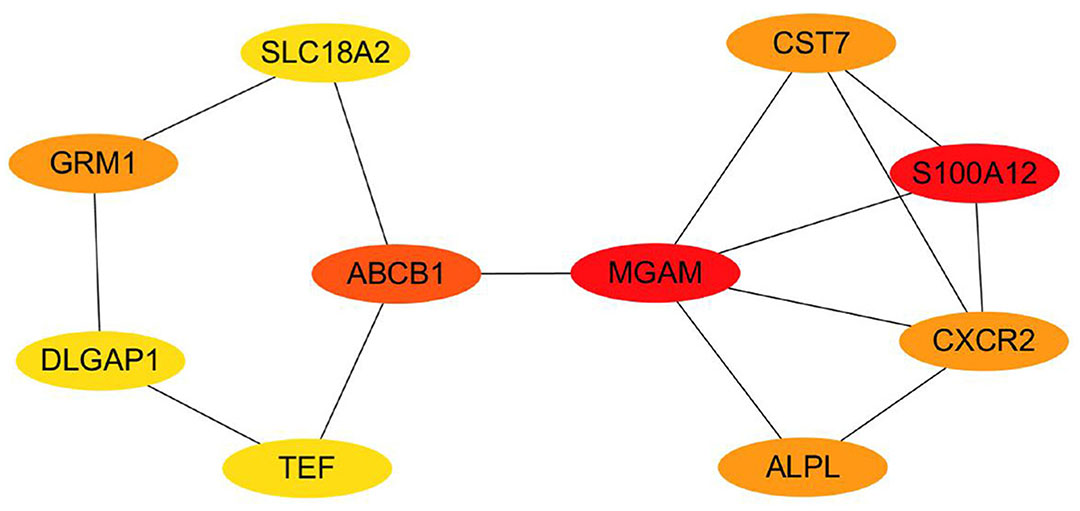
The PPI analysis was conducted on the top 50 DEGs, 8 DEGs of DLGAP1, SCG2, SMN2, SLC18A2, NXNL2, LYNX1, GRM1 and BIRC7 were related to neurodevelopment (Table 1). Ten Hub genes were screened from the top 50 genes using Cytoscape software (Fig. 4), revealing that DLGAP1, SLC18A2, and GRM1 play pivotal roles in neurodevelopment, but TEF, MGAM, CXCR2, ABCB1, ALPL, CST7 and S100A12 were not associated with neurodevelopment. Specifically, DLGAP1 primarily contributes to the formation of neuronal synapses, while SLC18A2 is responsible for the transportation of cytoplasmic monoamine substances, including dopamine, norepinephrine, serotonin, and histamine, into synaptic vesicles. Moreover, SLC18A2 is involved in the ATP dependent vesicular transport of biogenic amine neurotransmitters. On the other hand, GRM1 is a glutamate G protein coupled receptor that activates the phosphatidylinositol calcium second messenger system through G protein triggered signaling. It is also involved in the central role of glutamate in the central nervous system, such as long-term enhancement of the hippocampus and long-term inhibition of the cerebellum. TEF is the thyrotroph embryonic factor, and it binds to and transactivates the TSHB promoter. MGAM is maltase-glucoamylase, and it serves as an alternate pathway for starch digestion when luminal alpha-amylase activity is reduced because of immaturity or malnutrition. It plays a unique role in the digestion of malted dietary oligosaccharides used in food manufacturing. CXCR2 is C-X-C chemokine receptor type 2, and is receptor for interleukin-8, which is a powerful neutrophil chemotactic factor. Binding of IL-8 to the receptor causes activation of neutrophils. ABCB1 is multidrug resistance protein 1, and energy-dependent efflux pump responsible for decreased drug accumulation in multidrug-resistant cells. ALPL is alkaline phosphatase, and is tissue-nonspecific isozyme, which may play a role in skeletal mineralization. CST7 is cystatin-F; it inhibits papain and cathepsin L, and it may play a role in immune regulation through inhibition of a unique target in the hematopoietic system. S100A12 is protein S100-A12, and is a calcium-, zinc- and copper-binding protein which plays a prominent role in the regulation of inflammatory processes and immune response. Its pro-inflammatory activity involves recruitment of leukocytes, promotion of cytokine and chemokine production, and regulation of leukocyte adhesion and migration.
Through literatures search, the results revealed that DLGAP1, SLC18A2, and GRM1 are categorized as placental brain axis genes. DLGAP1 is associated with the transmission of dopamine neurotransmitter, specifically involving the Neuroscience pathway. SLC18A2, on the other hand, is associated with 5-HT neurotransmitters, particularly involving the selective serotonin reuptake inhibitor pathway. Finally, GRM1 is associated with Glu neurotransmitters, specifically involving the neurophysiological process of glutamate regulation of the dopamine D1A receptor signaling pathway (Table 3).
Table 3
Placenta-brain axis genes and pathways
| Gene | Associated neurotransmitter | Pathway | Literatures |
|---|---|---|---|
| DLGAP1 | Dopamine | Neuroscience | (Baier et al., 2012; Zhu et al., 2023) |
| SLC18A2 | 5-HT | Selective Serotonin Reuptake Inhibitor Pathway, Pharmacodynamics | (Bonnin et al., 2011; Zhu et al., 2023; Muller et al., 2016; Bonnin and Levitt 2011) |
| GRM1 | Glu | Neurophysiological process Glutamate regulation of Dopamine D1A receptor signaling | (Baier et al., 2012; Zhu et al., 2023) |
Impact of the placental brain axis genes DLGAP1, SLC18A2 and GRM1 on the neurodevelopment of GDM offspring via Gq/PLC/PKC pathway
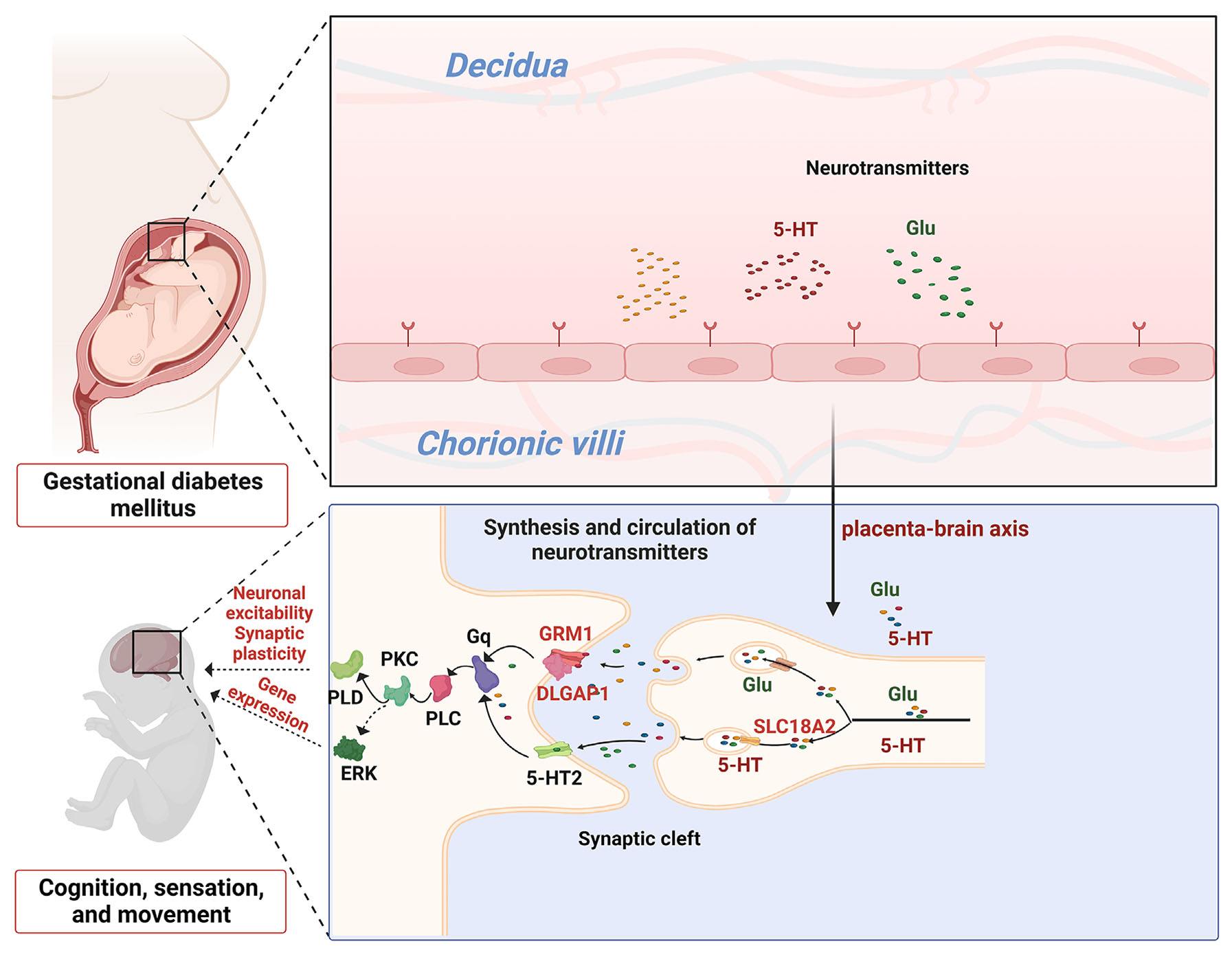
DLGAP1, SLC18A2, and GRM1 were analyzed using the KEGG database (https://www.kegg.jp/kegg), and the results indicated that Glu triggers the Gq/PLC/PKC pathway through the DLGAP1-GRM1 receptor complex, leading to neuronal excitability and synaptic plasticity (Supplementary Fig. 2). SLC18A2 activates the Gq/PLC/PKC pathway by facilitating the transport of 5-HT to regulate gene expression (Supplementary Fig. 3). Therefore, these results indicated that the placental brain axis genes DLGAP1, SLC18A2, and GRM1 modulate the Gq/PLC/PKC signaling pathway via Glu and 5-HT neurotransmitters, thereby affecting the neuronal excitability, synaptic plasticity, and gene expression of GDM offspring (Fig. 5).
Fig. 5. Pattern diagram of GDM affecting offspring cognition, sensation and movement through the Gq/PLC/PKC pathway.
The placental brain axis genes DLGAP1, GRM1, and SLC18A2 affecting GDM offspring neurodevelopment. Glu and 5-HT neurotransmitters produced by the placenta are encapsulated and transported within synaptic vesicles, and Glu actives the GRM1 and DLGAP1 receptor complex to regulate GDM offspring neuronal excitability and synaptic plasticity through the Gq/PLC/PKC/PLD pathway; 5-HT, in contrast, actives the 5-HT2 receptor to regulate GDM offspring gene expression through the Gq/PLC/PKC/ERK pathway. DLGAP1,GRM1 and SLC18A2 belong to the placental brain axis.
Discussion
Exploring the placental-mediated changes in the transfer of maternal fetal substances and information is crucial for understanding the congenital development of offspring. This study revealed that GDM modulates the Gq/PLC/PKC pathway through the placental brain axis genes DLGAP1, GRM1 and SLC18A2, thereby influencing the neurodevelopment of the offspring.
A total of 404 DEGs were screened through placental transcriptome analysis. Among these, 125 genes were found to be upregulated, while 279 were downregulated. GO enrichment analysis was performed on these DEGs, revealing the significant involvement of molecular functions such as receptor binding to advanced glycation end products receptor binding(RAGE) and G protein coupled peptide receptor activity. Additionally, important cellular components, including the clathrin-coated endocytic vesicle membrane and the transport vesicle membrane, were identified. In terms of biological processes, the Photosynthesis C-activating G protein coupled receptors signaling pathway, positive regulation of cyclosolid calcium ion concentration, and regulation of cyclosolid calcium were found to be major contributors. Previous research has demonstrated that the interaction between advanced glycation endproducts (AGE) and RAGE can activate the inflammatory signaling pathways, leading to neurological disorders such as traumatic brain injury(Reddy, Aryal, and Soni 2023). Following glutamate activation, G-protein coupled peptide receptors transform into metabolic glutamate receptors (mGluRs), which are crucial regulators of neuronal and synaptic plasticity. Dysregulation of mGluRs signaling has been associated with various neurological diseases, and numerous studies have indicated a close relationship between the expression/activity of mGluRs and epilepsy development(Huang et al., 2024). Moreover, mGluRs play a vital role in regulating neurodevelopment, synaptic transmission, and overall brain function(Abd-Elrahman and Ferguson 2022), influencing sensory and motor processing, cognition, memory, pain, behavior, etc. (Guo et al., 2018; Seven et al., 2021; Liang et al., 2022). The vesicle membrane plays a pivotal role in intercellular communication and the release of neurotransmitters (Zorec and Wu 2023). Neurotransmitters are encapsulated within synaptic vesicles, and the influx of Ca2+ triggers adhesion and localized fusion between synaptic vesicles and axonal membranes(Katz 1971), thereby facilitating the release of neurotransmitters(Zhou 2023). These neurotransmitters include glutamate, dopamine, and 5-HT (Xue et al., 2023; Vidyadhara et al., 2023; Pidathala et al., 2023). The results of KEGG enrichment analysis revealed alterations in the neuroactive ligand-receptor interaction pathway within GDM placental tissue. Previous research has demonstrated the pivotal role of the neuroactive ligand-receptor interaction pathway in the initial formation of auditory memory(Graham et al., 2023). Transcriptome analysis of the hippocampus in an animal model of type I diabetes revealed that DEGs are primarily enriched in neural ligand receptor interaction and other pathways, leading to cognitive dysfunction (Han et al., 2023). However, the exact mechanism by which the neuroactive ligand receptor interaction pathway affects neurodevelopment in GDM offspring remains unclear.
A PPI network was constructed to screen 8 genes involved in neurodevelopment, namely DLGAP1, SCG2, SMN2, SLC18A2, NXNL2, LYNX1, GRM1 and BIRC7, from the top 50 DEGs. DLGAP1 and NXNL2 primarily participate in neuronal maturation (Nakamori et al., 2022; Jaillard et al., 2021; Kim et al., 1997), and SCG2, SLC18A2, LYNX1, GRM1 contribute to neural cell signaling (Fleming et al., 2023; Manchishi, Prater, and Colledge 2024; Bychkov et al., 2023; Chen et al., 2023; Wang et al., 2023). SMN2 and BIRC7 are involved in neuronal apoptosis (Kölbel et al., 2024) (Li et al., 2020). DLGAP1, GRM1, and SLC18A2 have been classified as placental brain axis genes (Bonnin and Levitt 2011). DLGAP1 is a crucial component of the postsynaptic scaffold in neuronal cells (Kim et al., 1997), and the DLGAP1-DLG4-NMDA complex plays a key role in cognitive function (Fan et al., 2018). GRM1 is activated by the neurotransmitter glutamate and coupled with heterotrimeric G protein, regulating excitatory synaptic transmission, plasticity, neuronal development, and learning. The DLGAP1-SHANK-PSD95 glutamate receptor network and GRM1are interconnected, influencing neuronal activity and plasticity (Harbers et al., 2022; Bockaert, Perroy, and Ango 2021). The GRM1 receptor triggers the opening of the GluD2 channel through the classical Gq/PLC/PKC pathway, and the loss of GRM1 receptor function impairs motor coordination and cerebellar learning processes (Dadak et al., 2017). SLC18A2 regulates the extracellular secretion of monoamine and acetylcholine, responsible for the uptake and storage of neurotransmitters such as 5-HT in synaptic/secretory vesicles. The expression level of the SLC18A2 gene affects cognitive function in fruit flies and mice (Baronio et al., 2022; Oliveras-Cañellas et al., 2023; Eiden et al., 2004; Georgantzi et al., 2019). Research has shown that Phe stimulates presynaptic Glu release through the Gq/PLC/PKC pathway (Luo et al., 2015), and the release of 5-HT activates the 5-HT2A/Gq/PLC/PKC pathway, inhibiting the occurrence of chronic pain (Yuan et al., 2022).
The DLGAP1, GRM1, and SLC18A2 genes exhibit abnormal regulation in the placenta, thereby impacting the development of the fetal brain (Bonnin and Levitt 2011; Zhu et al., 2023; Baier et al., 2012; Bonnin et al., 2011; Muller et al., 2016). Analysis of the DLGAP1, GRM1, and SLC18A placental brain axis genes through the KEGG database confirm their role in regulating the release of Glu and 5-HT neurotransmitters via the Gq/PLC/PKC signaling pathway, consequently influencing the neurodevelopment of offspring. The findings of this study will contribute valuable evidence towards understanding the mechanism underlying abnormal neurobehavioral development in GDM offspring.
The placental brain axis genes DLGAP1, GRM1, SLC18A2 regulate the release of Glu and 5-HT neurotransmitters, having been found to affect GDM offspring neurodevelopment by regulating the Gq/PLC/PKC pathway.
Materials and Methods
Sample collection and processing
This study included 6 women with GDM and 6 normal pregnant women. 2 GDM patients with vaginitis were excluded, and the placental tissues were rinsed off the blood stains with physiological saline after they were removed, and put them into cryopreservation tubes containing RNAlater solution, and cryopreservation tubes were stored in a refrigerator set at -80℃.
Total RNA extraction and transcriptome high-throughput sequencing
We selected 6 GDM and 6 normal pregnant placentas, the separated placental tissues were thoroughly ground, and total RNA was extracted using the Trizol method according the manufacturer’s instructions. RNA samples were detected through the A260/A280 absorbance ratio with Nanodrop ND-2000. Library was construction through qualified. Paired-end libraries were prepared, etc. Finally, sequencing was performed using the mRNA-seq technique.
Data processing and screening of DEGs
The expression of known genes and transcripts were quantified with Stringtie's analysis process. Gene expression levels of were compared through different grouped samples through FPKM distribution of all genes. Differentially expressed genes (DEGs) between the GDM group and Normal group were analyzed using DESeq2 software. DEGs were identified based on P value<0.05 and |log2 (fold change)|>1. Genes with a log2 (fold change)>1 were marked as upregulated, while those with a log2 (fold change) <-1 were marked as downregulated. Genes that did not meet these criteria were not considered as DEGs.
Functional enrichment analysis of the DEGs
The DEGs were subjected to GO and KEGG enrichment analysis using the cluster- profiler package in R. GO enrichment analysis classified the DEGs based on Cellular component (CC), Molecular function (MF), and Biological process (BP). The top 10 most significantly enriched items (based on P value) were screened from the BP, CC, MF categories and KEGG enrichment analysis.
Construction and analysis of PPI network of DEGs
Biological functions and pathways associated with neurodevelopment were screened. The top 50 DEGs were used to construct a PPI network and annotate the nodes using the STRING online software (https://cn.STRING-db.org). Hub genes associated with neurodevelopment were screened using the Cytoscape 3.7.1 software.
Verification of mRNA expression levels of DEGs involved in neurodevelopment
The qPCR analysis was performed on the mRNA verification using SYBR premix Ex Taq. The reaction system: 10µL 2X SYBR Green Mix, 0.4µL upstream and downstream primers (10 µmol/L), 2µL template, and RNase-free water added to a total volume of 20µL. The reaction conditions: pre-denaturation at 94oC for 4 min, followed by amplification at 95oC for 5 s, 60oC for 34 s, and 72oC for 30 s, 40 cycles. Gene expression was analyzed using the 2-∆∆CT method.
Analysis of the association between Hub genes involved in neurodevelopment and placental brain axis genes
Genes and pathways related to the placental brain axis were screened through literatures search. The association between Hub genes involved in neurodevelopment and placental brain axis genes were analyzed by aligning literatures. Abnormalities in these genes and pathways may contribute to abnormal neurodevelopment in children, including the impact of neurotransmitters, such as serotonin, dopamine, and norepinephrine/adrenaline, which are secreted by the placenta during pregnancy and play a role in fetal brain development.
Prediction of pathways related to placental brain axis genes affecting neurodevelopment in offspring of mothers with GDM
The pathways through which the Hub genes of the placental brain axis affect neurodevelopment in offspring of mothers with GDM were analyzed using the KEGG database (https://www.kegg.jp/kegg). Keywords were entered into the PATHWAY navigation bar of the KEGG database to search for Hub genes, and the pathways affecting neurodevelopment in offspring of mothers with GDM were predicted based on these Hub genes.
Statistical analysis
Statistical analysis was performed using SPSS 23.0 software, and the results were presented as means ± standard deviation (SD). GraphPad Prism 8 was utilized to create graphical representations of the statistical data. The independent Student’s t-test was employed to compare equivalent variables between the two groups, with statistical significance defined as P<0.05. DEGs were screened based on the criteria of |log2 (Fold change)| >1 and P<0.05, indicating statistically significant.
Supplementary Material
Acknowledgements
The authors thank the funding from the Science and Technology Department of Gansu Province.
Declarations
Author contributions
The original draft and experiment were carried out by Jianhua Li. Qian Liu conducted the bioinformatic analysis. Xuhui Liu and Yunyun Wang collected placenta samples. The qPCR experiment was conducted by Weikai Wang and Yuxia Jin. Bin Yi provided statistical assistance. Yanxia Wang revised the manuscript and gave the final approval for publication.
Ethical statement
This research obtained approval from the hospital ethics committee ((2021) GSFY Ethical Review No. 66), and all participants had signed informed consent forms.
Patient consent
All participants had signed informed consent forms prior to the collection of maternal placental samples.
Funding
This work was supported by the Gansu Province Key Research and Development Projects under Grant (22YF7FA093), Gansu Province Science and Technology Plan Project (24JRRA938), General Project of Clinical Medical Research of National Center for Clinical Medical Research on Child Health and Diseases under Grant (NCRCCHD-2020-GP-15, NCRCCHD-2022-GP-17), Natural Science Foundation of Gansu Province under Grant (20JR10RA427, 21JR11RA167, 22JR11RA181), Gansu Provincial Science and Technology Major Project under Grant (22ZD6FA034).
References
Abd-Elrahman K. S., Ferguson S. S. G. (2022). Noncanonical Metabotropic Glutamate Receptor 5 Signaling in Alzheimer's Disease. Annual Review of Pharmacology and Toxicology 62: 235-254.
Baier C. J., Katunar M. R., Adrover E., Pallarés M. E., Antonelli M. C. (2012). Gestational Restraint Stress and the Developing Dopaminergic System: An Overview. Neurotoxicity Research 22: 16-32.
Baronio D., Chen Y.C., Decker A. R., Enckell L., Fernández‐López B., Semenova S., Puttonen H. A. J., Cornell R. A., Panula P. (2022). Vesicular monoamine transporter 2 (SLC18A2) regulates monoamine turnover and brain development in zebrafish. Acta Physiologica 234: e13725.
Behura S. K., Dhakal P., Kelleher A. M., Balboula A., Patterson A., Spencer T. E. (2019). The brain-placental axis: Therapeutic and pharmacological relevancy to pregnancy. Pharmacological Research 149: 104468.
Bockaert J., Perroy J., Ango F. (2021). The Complex Formed by Group I Metabotropic Glutamate Receptor (mGluR) and Homer1a Plays a Central Role in Metaplasticity and Homeostatic Synaptic Scaling. The Journal of Neuroscience 41: 5567-5578.
Bonnin A., Levitt P. (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197: 1-7.
Bonnin A., Goeden N., Chen K., Wilson M. L., King J., Shih J. C., Blakely R. D., Deneris E. S., Levitt P. (2011). A transient placental source of serotonin for the fetal forebrain. Nature 472: 347-350.
Bychkov M. L., Kirichenko A. V., Paramonov A. S., Kirpichnikov M. P., Lukmanova E. N. (2023). Accumulation of β-Amyloid Leads to a Decrease in Lynx1 and Lypd6B Expression in the Hippocampus and Increased Expression of Proinflammatory Cytokines in the Hippocampus and Blood Serum. Doklady Biochemistry and Biophysics 511: 145-150.
Chen C., Zhu B., Tang X., Chen B., Liu M., Gao N., Li S., Gu J. (2023). Genome-Wide Assessment of Runs of Homozygosity by Whole-Genome Sequencing in Diverse Horse Breeds Worldwide. Genes 14: 1211.
Dadak S., Bouquier N., Goyet E., Fagni L., Levenes C., Perroy J. (2017). mGlu1 receptor canonical signaling pathway contributes to the opening of the orphan GluD2 receptor. Neuropharmacology 115: 92-99.
Eiden L. E., Schäfer M. K. H., Weihe E., Schütz B. (2004). The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Archiv European Journal of Physiology 447: 636-640.
Fan Z., Qian Y., Lu Q., Wang Y., Chang S., Yang L. (2018). DLGAP 1 and NMDA receptor‐associated postsynaptic density protein genes influence executive function in attention deficit hyperactivity disorder. Brain and Behavior 8: e00914.
Fleming T., Tachizawa M., Nishiike Y., Koiwa A., Homan Y., Okubo K. (2023). Estrogen-dependent expression and function of secretogranin 2a in female-specific peptidergic neurons. PNAS Nexus 2: pgad413.
Georgantzi K., Tsolakis A. V., Jakobson Åke, Christofferson R., Janson E. T., Grimelius L. (2019). Synaptic Vesicle Protein 2 and Vesicular Monoamine Transporter 1 and 2 Are Expressed in Neuroblastoma. Endocrine Pathology 30: 173-179.
Graham G., Chimenti M.S., Knudtson K.L., Grenard D.N., Co L., Sumner M., Tchou T., Bieszczad K.M. (2023). Learning induces unique transcriptional landscapes in the auditory cortex. Hearing Research 438: 108878.
Guo B., Wang J., Yao H., Ren K., Chen J., Yang J., Cai G., Liu H., Fan Y., Wang W., Wu S. (2018). Chronic Inflammatory Pain Impairs mGluR5-Mediated Depolarization-Induced Suppression of Excitation in the Anterior Cingulate Cortex. Cerebral Cortex 28: 2118-2130.
Han Q., Ding Q., Yu L., Li T., Sun B., Tang Z. (2023). Hippocampal transcriptome analysis reveals mechanisms of cognitive impairment in beagle dogs with type 1 diabetes. Journal of Neuropathology & Experimental Neurology 82: 774-786.
Harbers M., Nakao H., Watanabe T., Matsuyama K., Tohyama S., Nakao K., Kishimoto Y., Kano M., Aiba A. (2022). mGluR5 Is Substitutable for mGluR1 in Cerebellar Purkinje Cells for Motor Coordination, Developmental Synapse Elimination, and Motor Learning. Cells 11: 2004.
Huang L., Xiao W., Wang Y., Li J., Gong J., Tu E., Long L., Xiao B., Yan X., Wan L. (2024). Metabotropic glutamate receptors (mGluRs) in epileptogenesis: an update on abnormal mGluRs signaling and its therapeutic implications. Neural Regeneration Research 19: 360-368.
Jaillard C., Ouechtati F., Clérin E., Millet-Puel G., Corsi M., Aït-Ali N., Blond F., Chevy Q., Gales L., Farinelli M., Dalkara D., Sahel J.A., Portais J.C., Poncer J.C., Léveillard T. (2021). The metabolic signaling of the nucleoredoxin-like 2 gene supports brain function. Redox Biology 48: 102198.
Katz B. (1971). Quantal Mechanism of Neural Transmitter Release. Science 173: 123-126.
Kim E., Naisbitt S., Hsueh Y.P., Rao A., Rothschild A., Craig A. M., Sheng M. (1997). GKAP, a Novel Synaptic Protein That Interacts with the Guanylate Kinase-like Domain of the PSD-95/SAP90 Family of Channel Clustering Molecules. The Journal of Cell Biology 136: 669-678.
Kinkade J. A., Seetharam A. S., Sachdev S., Bivens N. J., Phinney B. S., Grigorean G., Roberts R. M., Tuteja G., Rosenfeld C. S. (2024). Extracellular vesicles from mouse trophoblast cells: Effects on neural progenitor cells and potential participants in the placenta–brain axis. Biology of Reproduction 110: 310-328.
Kölbel H., Kopka M., Modler L., Blaschek A., Schara-Schmidt U., Vill K., Schwartz O., Müller-Felber W. (2024). Impaired Neurodevelopment in Children with 5q-SMA - 2 Years After Newborn Screening. Journal of Neuromuscular Diseases 11: 143-151.
Li Z., Yang C., Li X., Du X., Tao Y., Ren J., Fang F., Xie Y., Li M., Qian G., Xu L., Cao X., Wu Y., Lv H., Hu S., Lu J., Pan J. (2020). The dual role of BI 2536, a small-molecule inhibitor that targets PLK1, in induction of apoptosis and attenuation of autophagy in neuroblastoma cells. Journal of Cancer 11: 3274-3287.
Liang X., Miao A., Zhang W., Li M., Xing Y. (2022). Effect of family integrated care on physical growth and language development of premature infants: a retrospective study. Translational Pediatrics 11: 965-977.
Luo F., Li S., Tang H., Deng W., Zhang Y., Liu Y. (2015). Phenylephrine enhances glutamate release in the medial prefrontal cortex through interaction with N‐type Ca 2+ channels and release machinery. Journal of Neurochemistry 132: 38-50.
Manchishi S., Prater M., Colledge W. H. (2024). Transcriptional profiling of Kiss1 neurons from arcuate and rostral periventricular hypothalamic regions in female mice. Reproduction 167: e230342.
Muller C. L., Anacker A. M.J., Rogers T. D., Goeden N., Keller E. H., Forsberg C. G., Kerr T. M., Wender C. L.A., Anderson G. M., Stanwood G. D., Blakely R. D., Bonnin A., Veenstra-VanderWeele J. (2016). Impact of Maternal Serotonin Transporter Genotype on Placental Serotonin, Fetal Forebrain Serotonin, and Neurodevelopment. Neuropsychopharmacology 42: 427-436.
Nakamori M., Shimizu H., Ogawa K., Hasuike Y., Nakajima T., Sakurai H., Araki T., Okada Y., Kakita A., Mochizuki H. (2022). Cell type-specific abnormalities of central nervous system in myotonic dystrophy type 1. Brain Communications 4: fcac154.
Oliveras-Cañellas N., Castells-Nobau A., de la Vega-Correa L., Latorre-Luque J., Motger-Albertí A., Arnoriaga-Rodriguez M., Garre-Olmo J., Zapata-Tona C., Coll-Martínez C., Ramió-Torrentà L., Moreno-Navarrete J. M., Puig J., Villarroya F., Ramos R., Casadó-Anguera V., Martín-García E., Maldonado R., Mayneris-Perxachs J., Fernández-Real J. M. (2023). Adipose tissue coregulates cognitive function. Science Advances 9: eadg4017.
Pidathala S., Liao S., Dai Y., Li X., Long C., Chang C.L., Zhang Z., Lee C.H. (2023). Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2. Nature 623: 1086-1092.
Reddy V. P., Aryal P., Soni P. (2023). RAGE Inhibitors in Neurodegenerative Diseases. Biomedicines 11: 1131.
Ren J., Wang Y., Zhang Y., Jin H., Cheng J., Tao F., Zhu Y. (2024). Placental Transcriptomic Signatures of Prenatal Phthalate Exposure and Identification of Placenta-Brain Genes Associated with the Effects of Phthalate Exposure on Neurodevelopment in Children. Environmental Science & Technology 58: 19141-19151.
Rosenfeld C. S. (2020). The placenta‐brain‐axis. Journal of Neuroscience Research 99: 271-283.
Seven A. B., Barros-Álvarez X., de Lapeyrière M., Papasergi-Scott M. M., Robertson M. J., Zhang C., Nwokonko R. M., Gao Y., Meyerowitz J. G., Rocher J.P., Schelshorn D., Kobilka B. K., Mathiesen J. M., Skiniotis G. (2021). G-protein activation by a metabotropic glutamate receptor. Nature 595: 450-454.
Shallie P. D., Naicker T. (2019). The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. International Journal of Developmental Neuroscience 73: 41-49.
Vidyadhara D.J., Somayaji M., Wade N., Yücel B., Zhao H., Shashaank N., Ribaudo J., Gupta J., Lam T.K. T., Sames D., Greene L. E., Sulzer D. L., Chandra S. S. (2023). Dopamine transporter and synaptic vesicle sorting defects underlie auxilin-associated Parkinson’s disease. Cell Reports 42: 112231.
Wang C., Liu Y., Liu X., Zhang Y., Yan X., Deng X., Shi J. (2023). Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons. Molecules 28: 3181.
Xue M., Cao Y., Shen C., Guo W. (2023). Computational Advances of Protein/Neurotransmitter-membrane Interactions Involved in Vesicle Fusion and Neurotransmitter Release. Journal of Molecular Biology 435: 167818.
Yu H.T., Gong J.Y., Xu W.H., Chen Y.R., Li Y.T., Chen Y.F., Liu G.L., Zhang H.Y., Xie L. (2024). Gestational Diabetes Mellitus Remodels the Fetal Brain Fatty Acid Profile Through Placenta-Brain Lipid Axis in C57BL/6J Mice. The Journal of Nutrition 154: 590-599.
Yuan X.C., Wang Y.Y., Tian L.X., Yan X.J., Guo Y.X., Zhao Y.L., Baba S. S., Jia H., Wang H.S., Li M., Huo F.Q. (2022). Spinal 5-HT 2A receptor is involved in electroacupuncture inhibition of chronic pain. Molecular Pain 18: 17448069221087583.
Zhou Q. (2023). Calcium Sensors of Neurotransmitter Release. In Molecular Mechanisms of Neurotransmitter Release. (Ed. Wang Zhao-Wen) Springer International Publishing, Cham.
Zhu Y., Zhang Y., Jin Y., Jin H., Huang K., Tong J., Gan H., Rui C., Lv J., Wang X., Wang Q., Tao F. (2023). Identification and prediction model of placenta-brain axis genes associated with neurodevelopmental delay in moderate and late preterm children. BMC Medicine 21: 326.
Zorec R., Wu L.G. (2023). Barriers to exocytotic vesicle discharge. Cell Calcium 112: 102737.