Int. J. Dev. Biol. 66: 35 - 42 (2022)
Special Issue: Developmental Biology in Greece
Cortical interneuron development: a role for small Rho GTPases
Review | Published: 26 October 2021
Abstract
GABAergic interneurons control cortical excitation/inhibition balance and are implicated in severe neurodevelopmental disorders. In contrast to the multiplicity of signals underlying the generation and migration of cortical interneurons, the intracellular proteins mediating the response to these cues are largely unknown. We have demonstrated the unique and diverse roles of the Rho GTPases Rac1 and 3 in cell cycle and morphology in transgenic animals where Rac1 and Rac1/3 were ablated specifically in cortical interneurons. In the Rac1 mutant, progenitors delay their cell cycle exit, probably due to a prolonged G1 phase resulting in a cortex with 50% reductions in interneurons and an imbalance of excitation/inhibition in cortical circuits. This disruption in GABAergic inhibition alters glutamatergic function in the adult cortex, which could be reversed by enhancement of GABAergic functions during an early postnatal period. Furthermore, this disruption disturbs neuronal synchronization in the adult cortex. In the double mutant, additional severe cytoskeletal defects result in an 80% interneuron decrease. Both lines die postnatally from epileptic seizures. We have made progress towards characterizing the cell cycle defect in Rac1 mutant interneuron progenitors, determining the morphological and synaptic characteristics of single and double mutant interneurons and identifying some of the molecular players through which Racs exert their actions via proteomic analysis. In our present work, we review these studies and discuss open questions and future perspectives. We hope that our data will contribute to the understanding of the function of cortical interneurons, especially since preclinical models of interneuron-based cell therapies are being established.
Keywords
cortical interneurons, GABA, cell cycle, cytoskeleton, migration, Rac1, Rac3, Rho GTPases
Introduction
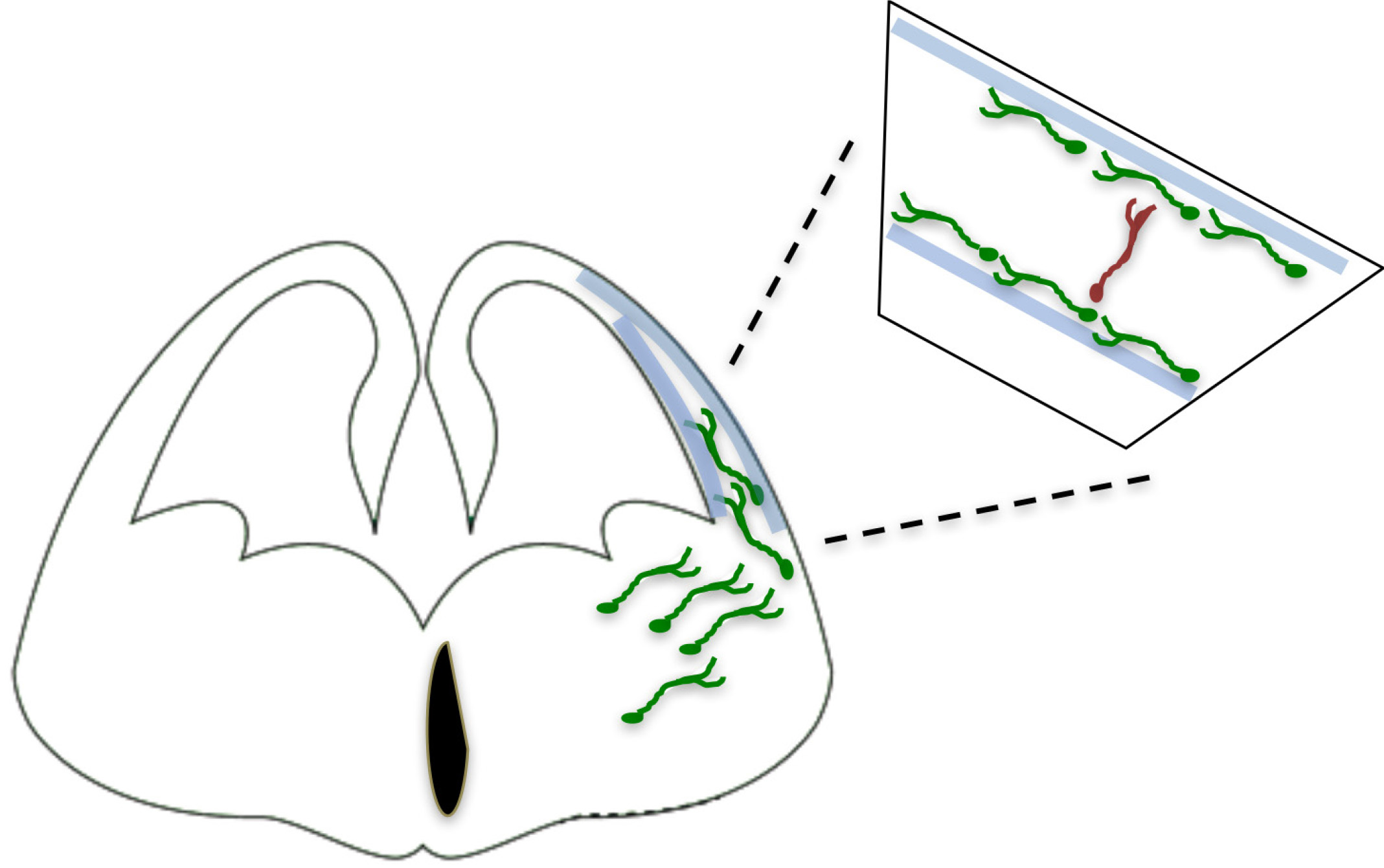
Gamma-aminobutyric acid-containing (GABAergic) interneurons, although comprising ~20% of all cortical neurons, play important roles in cortical function and have been implicated in severe disorders such as schizophrenia, epilepsy and autism spectrum disorders (Lewis et al., 2005; Marín, 2012). Interneurons are characterized by a remarkable morphological, molecular and functional diversity, and recent studies have uncovered some of the molecular components underlying the generation of this diversity (Wonders and Anderson 2006; Kepecs and Fishell 2014; Kessaris et al. 2014). All CINs (Fig. 1) originate mainly from the ganglionic eminences, which are well-defined domains of the basal telencephalon, and migrate tangentially to populate the developing cortex (Lim et al., 2018).
Fig. 1. GABAergic Interneurons originate from subpallium structures, medial ganglionic eminence (MGE) and caudal ganlionic eminence (CGE).
50-60% of all cortical interneurons originate in the MGE, and the main subtypes are the Parvalbumin (PV) and Somatostatin (SST) interneurons. They follow tangential routes towards and inside the pallium, in contrast to the pyramidal neurons, which move radially within the pallium (Marín and Rubenstein 2001; Corbin et al. 2001; Batista-Brito and Fishell 2009).
Around 60% of CINs are born in the MGE and include two major subtypes, defined by the expression of the calcium binding protein Parvalbumin (PV) and the neuropeptide Somatostatin (SST). At the top of this cascade is the transcription factor Nkx2.1, which is expressed transiently in MGE-derived IN progenitors, followed by the sequential and prolonged expression of LIM homeobox 6 (Lhx6), SRY-box transcription factor 6 (Sox6) and SC35-interacting protein 1 (Sip1) (Lim et al., 2018). Ablation of these transcription factors within CINs results in multiple defects at several developmental steps, such as migration, laminar acquisition, survival and marker expression, within both CIN subtypes.
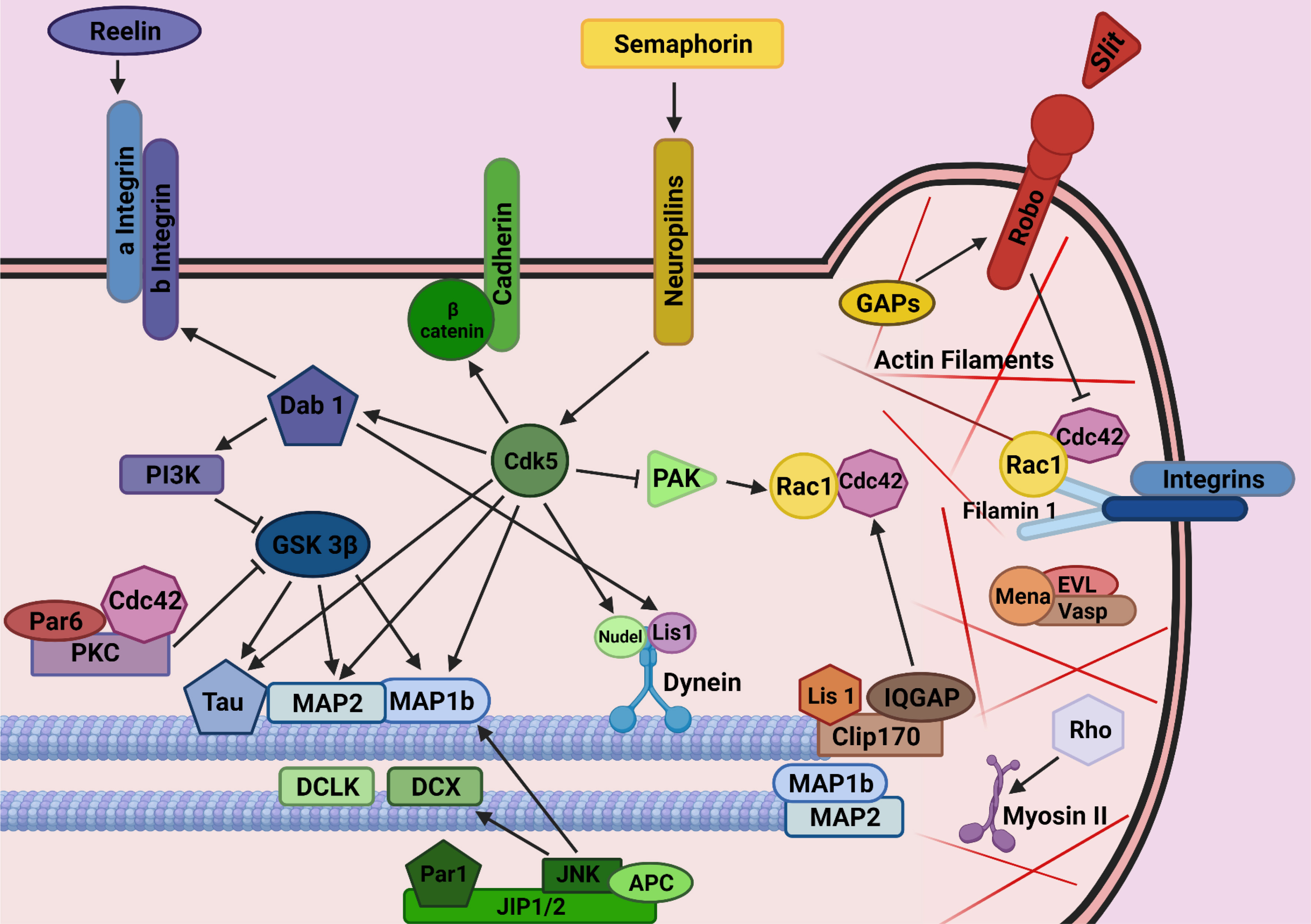
The cellular and molecular mechanisms guiding interneurons from their subpallial origins to the cortex have only recently started to be elucidated. The long list of cues involved includes long-range attractive and repulsive factors, surface-bound permissive and instructive molecules, and motogenic Factors (Marín, 2013). As part of our studies on the involvement of axon guidance cues in tangential migration, we found that the adhesion molecule transient axonal glycoprotein (TAG-1)/ contactin 2 (Cntn2), which is prominently expressed in corticofugal fibers, plays a role as a permissive cue for MGE-derived interneurons to move along these fibers as they enter the cortical area (Denaxa et al., 2005). However, in contrast to the multiplicity of extracellular signals, the intracellular proteins that mediate the response to these cues remain understudied (Peyre et al., 2015) (Fig. 2).
Fig. 2. Mechanisms by means of which intracellular molecules regulate interneuron development/migration/generation of their diversity have not yet been identified.
(Adapted from Ayala et al., 2007). (This figure was created with BioRender.com).
The Rac subfamily of Rho-GTPases is a family of proteins involved in a number of cellular functions such as regulation of actin dynamics, cell cycle entry and progression, polarity and axonogenesis (reviewed in (Jaffe and Hall 2005; Koh 2007; Govek et al. 2011). Recent data from our lab demonstrated that the small Rho-GTPases Rac1 and Rac3 have diverse roles as intracellular mediators of CIN migration (Vidaki et al. 2012; Tivodar et al. 2015). We review these data below.
RAC1 function in interneurons
The Rac subfamily consists of three members: Rac1 is ubiquitously expressed, while Rac2 is expressed mostly in the hematopoietic system, and Rac3 is highly enriched in the nervous system (Malosio et al., 1997). Disregulated Rho-GTPases and actin cytoskeleton have been correlated with the neuronal migration deficits in Lis1-deficient neurons (Kholmanskikh et al., 2003) and Rac1 is involved in the interkinetic nuclear migration of cortical progenitor cells (Minobe et al., 2009). Rac1 is required for the normal proliferation and differentiation of subventricular zone (SVZ) progenitors and for survival of both ventricular zone (VZ) and SVZ progenitors. Specific inactivation of Rac1 from the VZ of the telencephalon of Rac1fl/fl;Foxg1Tg(Cre) mice shows a wide spectrum of defects that range from severe defects in axonogenesis to perturbation in radial migration of cortical neurons, as well as increased cell death of nascent neurons (Chen et al., 2009, Leone et al., 2010). In this mouse line, however, it is hard to distinguish cell autonomous from non-cell autonomous defects (Chen et al., 2007). As Rac1 appears to play a crucial role in different cell biological processes in distinct neuronal populations and, possibly, distinct developmental time windows, and its putative role in cortical interneuron development was unclear, we embarked on a project to specifically ablate Rac1 in this population via Cre/loxP technology. We combined an Nkx2.1-Cre transgenic mouse (Fogarty et al., 2007) with a conditional allele of Rac1 (Walmsley et al., 2003) thus deleting the Rac1 gene in the MGE. The ROSA26fl-STOP-fl-YFP allele was also inserted as an independent marker (Srinivas et al., 2001) to allow visualization of the Rac1-mutant neurons, via yellow fluorescent protein (YFP) expression. Expression of this transgene recapitulates the pattern of expression of the endogenous Nkx2.1 gene and has been previously used to lineally mark MGE-derived cortical interneurons, which can be identified by the expression of calbindin (CB), PV, and SST (Sussel et al. 1999; Fogarty et al. 2007; Wonders et al. 2008; Butt et al. 2008).
Ablation of the Rac1 protein from the MGE of Rac1fl/fl;Nkx2.1Tg (Cre); R26R-YFP+/– animals occurs at embryonic day (E) 12 and by E13.5 no YFP+ MGE-derived cells co-express Rac1 and YFP, as assessed by immunohistochemistry. In E13.5 Rac1fl/fl;Nkx2.1Tg(Cre);R26R-YFP+/– embryos, the migration of MGE-derived interneurons is disrupted, since YFP+ cells are found only ventrally with respect to the pallial—subpallial boundary. Consistently, analysis of the expression of MGE-specific interneuronal markers, such as Lhx6 (Lavdas et al. 1999; Cobos et al. 2005; Liodis et al. 2007) and SST as well as glutamic acid decarboxylase 67 (GAD67) mRNA that should mark all GABA+ cells, showed absence of positive cells within the developing neocortex of E13.5 mutant embryos. Although by E16.5 and post natal day (P) 0 some YFP+ cells are found in the developing cortex, their numbers are significantly decreased, while the majority of them are aggregated ventrally in their place of birth, resulting in a 50% reduction in the number of GABAergic interneurons found in the mature cortex (Vidaki et al., 2012). Rac1 ablation from the MGE affects all cell populations (such as oligodendrocytes) originating there.
MGE explants were grown ex vivo to analyze the migratory behavior of control and mutant interneurons outside their normal environment. Although the speed of migratory cells of both genotypes was not affected, mutant cells were delayed in their exiting the explant and migrating out. These data indicate that the interneuron defect observed in Rac1 mutant animals is intrinsic to the MGE-derived cells and not due to a secondary defect in the cellular environment. In addition, they suggest that upon deletion of Rac1, the initiation of migration of MGE cells occurs later than in control cases. Importantly, these ex vivo observations correlate with the observed in vivo phenotype, where mutant cells start their migration later than the control ones.
Since the majority of Rac1 mutant animals die at around weaning time, we decided to analyze the brains of postnatal mice at P15. No differences in the overall structure of the brain between control and mutant animals were observed, as assessed by histological staining. The number and distribution of interneurons in the neocortex were examined either by expression of the lineage marker YFP and the general marker GABA or markers that are specifically expressed in MGE-derived interneuronal subpopulations such as Lhx6, PV, and SST. We have also examined calretinin (CR) and neuropeptide Y (NPY), which are primarily expressed by cortical interneurons derived from the CGE (Fogarty et al. 2007; Butt et al. 2008) focusing primarily on the barrel cortex. The quantifications revealed a 50% reduction in MGE-derived interneurons, but the ability of Rac1-deficient precursors to differentiate into different mature interneuron subtypes is not compromised, despite their reduced numbers.
We then assessed the pool size of proliferating progenitors and their mitotic potential in the MGE of control and Rac1 conditional mutant embryos in order to study whether the reduction in the number of cortical interneurons is due to perturbed proliferation in the VZ. No significant differences were observed. Thus, the reduced number of GABAergic cells in the cortex of mutant mice is not due to defective proliferation or aberrant mitotic activity of the Rac1-deficient cells. In addition, we examined apoptotic cell death at several embryonic stages of these animals with no apparent differences. However, by comparing the cell cycle dynamics of MGE progenitors in the two genotypes, we found that inactivation of Rac1 perturbs G1 phase progression and reduces cell cycle exit of MGE-derived progenitors. For this, we pulse-labeled neural progenitors with Bromodeoxyuridine (BrdU) at E12.5 and collected the embryos 24 h later for anti-BrdU and anti-Ki67 double-labeling. The fraction of BrdU+ cells that do not express Ki67 (Ki67–) was estimated in the MGE of control and mutant embryos, as well as in the lateral ganglionic eminence (LGE), which served as an internal control. This analysis revealed a specific decrease of BrdU+; Ki67– progenitors from the MGE in mutant embryos, indicating that fewer progenitors had exited the cell cycle in the absence of Rac1. We also found that Rac1 mutant embryos have greatly diminished amounts of phosphorylated Rb, a critical checkpoint of the cell cycle, in their MGE cells, compared with control littermates. We also analyzed the levels of cyclin D proteins, since cyclins are crucial for phosphorylation of Rb and progression of the cell cycle through the G1 phase. We found a two-fold reduction in the levels of cyclin D proteins in the MGE of mutant embryos compared with control littermates Taken together, these data indicate a specific role for Rac1 in the progression of the MGE-derived cells through the cell cycle, possibly during the G1 phase. In addition, a large percentage of Rac1-deficient neurons, most likely the population that remains aggregated in the ventral telencephalon, appear to have affected growth cones and abnormal axon extension, which is consistent with previously reported roles of Rac1 in cytoskeletal dynamics (Jaffe and Hall 2005; Koh 2007; Tahirovic et al. 2010). In short, we established that Rac1 is required cell autonomously for cortical interneuron development and cell cycle progression (Vidaki et al., 2012).
In order to verify the necessity of Rac1 in the Nkx2.1-positive progenitors of the MGE and to exclude an additional essential role for Rac1 in postmitotic cells, we specifically deleted Rac1 in postmitotic MGE-derived interneurons expressing Lhx6 using Lhx6-Cre transgenic mice (Fogarty et al., 2007). No differences in the migratory capacity or eventual numbers in the mature cortex between the two genotypes were observed, indicating that the requirement of Rac1 activity for the development of cortical interneurons is restricted to the progenitors that reside in the VZ of the MGE (Vidaki et al., 2012).
As mentioned above, most mutants die in early postnatal life due to epileptic seizures, but some survive until adulthood. We thus turned our attention to the putative adaptations that may occur in the adult glutamatergic system in the presence of a developmental defect in interneurons that prevents them from reaching the cerebral cortex in normal numbers. We used the aforementioned Rac1 conditional mutant to examine how the developmental loss of interneurons may affect basal synaptic transmission, synaptic plasticity and neuronal morphology in the mouse prefrontal cortex (PFC). Despite the 50% decrease in the number of interneurons, basal synaptic transmission, as examined by recording field excitatory postsynaptic potentials (fEPSPs) from layer II networks, is not altered in the PFC of Rac1 mutant mice. However, there is decreased paired-pulse ratio (PPR) and decreased long-term potentiation (LTP), in response to tetanic stimulation, in the layer II PFC synapses of Rac1 mutant mice. Furthermore, expression of the N-methyl-D-aspartate receptor (NMDA) subunits is decreased and dendritic morphology is altered, changes that could underlie the decrease in LTP in the Rac1 mutant mice. Finally, we found that treating Rac1 mutant mice with diazepam in early postnatal life can reverse changes in dendritic morphology observed in non-treated Rac1 mutants. Therefore, our data indicates that disruption in GABAergic inhibition alters glutamatergic function in the adult PFC, an effect that could be reversed by enhancement of GABAergic function during an early postnatal period (Konstantoudaki et al., 2016).
Our work also indicated significant alterations in both inhibitory and excitatory systems in the adult barrel cortex (BC) of Rac1 mutant mice (Kalemaki et al., 2018). We investigated the functional changes in adult cortical network activity in response to developmentally reduced inhibition. The decrease in the number of interneurons increased basal synaptic transmission, as examined by recording fEPSPs from layer II networks in the Rac1 mutant mouse cortex, decreased LTP in response to tetanic stimulation, but did not alter the PPR. Furthermore, under spontaneous recording conditions, Rac1 mutant brain slices exhibit enhanced sensitivity and susceptibility to emergent spontaneous activity. We also find that this developmental decrease in the number of cortical interneurons results in local neuronal networks with alterations in neuronal oscillations, exhibiting decreased power in low frequencies (delta, theta, alpha) and gamma frequency range (30–80 Hz) with an extra aberrant peak in the high gamma frequency range (80–150 Hz). Therefore, our data show that disruption in GABAergic inhibition alters synaptic properties and plasticity, while it also disrupts cortical neuronal synchronization in the adult BC (Kalemaki et al., 2018).
RAC1 and RAC3 combined action in interneurons
As detailed above, Rac1 ablation in interneurons results in 50% reduction of these cells in the mature cortex (Vidaki et al., 2012). We reasoned that other Rho-GTPase proteins may compensate for each other’s function. A microarray-based comparative profiling of gene expression of dorsal forebrain from E15.5 wild-type and Lhx6 mutant mice was carried out to identify novel genes implicated in cortical interneuron development (Denaxa et al., 2012). Among the genes affected by the deletion of Lhx6 in the dorsal forebrain was the Rho-GTPase protein Rac3. Since Lhx6 null mice show defects in the tangential migration of cortical interneurons (Liodis et al. 2007; Zhao et al. 2008), we hypothesized that this might be partially due to the lack of Rac3 protein in Lhx6 mutant interneurons. Nevertheless, Rac3 mutant mice have been reported not to have obvious defects in cortical interneuron migration (Corbetta et al., 2005), in contrast to our studies on the Rac1 mutant. We thus set out to study the role of both Rho-GTPase proteins in cortical interneuron development.
The Rac1 conditional knockout (Rac1fl/fl; Nkx2.1+/Cre, (Vidaki et al., 2012) was crossed to Rac3-deficient mice (Rac3−/−, Rac3KO; (Corbetta et al., 2005). Thus, we obtained a line where cortical interneurons deriving from the MGE are missing both Rac1 and Rac3 proteins (Rac1fl/fl; Rac3−/−;Nkx2.1+/Cre, double mutant) and performed similar studies as described above for the single mutant (Tivodar et al., 2015).
We observed that the double mutant cells entered the dorsal telencephalon ~1 day later than the single Rac1 mutant YFP+ cells. Analysis with a postmitotic marker of MGE-derived GABAergic interneurons, Lhx6, and a subtype specific marker, SST, verified the severely reduced number of migrating interneurons at E14.5 in the dorsal telencephalon of the double mutants. Later in embryogenesis (E16.5), in the double mutants, only a few YFP+, Lhx6+, or SST+ cells were found inside the cortex, but not extending as dorsally as in control mice. In addition, a significant accumulation of these cells was observed in the ventral telencephalon of double-mutant embryos and not in the control ones.
In the postnatal cortex, the number of MGE-derived GABAergic interneuron subpopulations is severely reduced in the Rac1/Rac3, while their differentiation is unaffected (Tivodar et al., 2015). A striking 80% of YFP+ cells were absent in the double mutant barrel cortex compared with the control one. Given the great loss of interneurons, we decided to look for further abnormalities in the cortices of double mutants. We observed that all layers were present at the correct place, while the cortex width in the double-mutant animals was not significantly smaller compared with control animals. In addition, there was no difference in the cell cycle exit index between the Rac1 conditional mutants and the double mutants, suggesting that this effect is likely due to the absence of Rac1 alone.
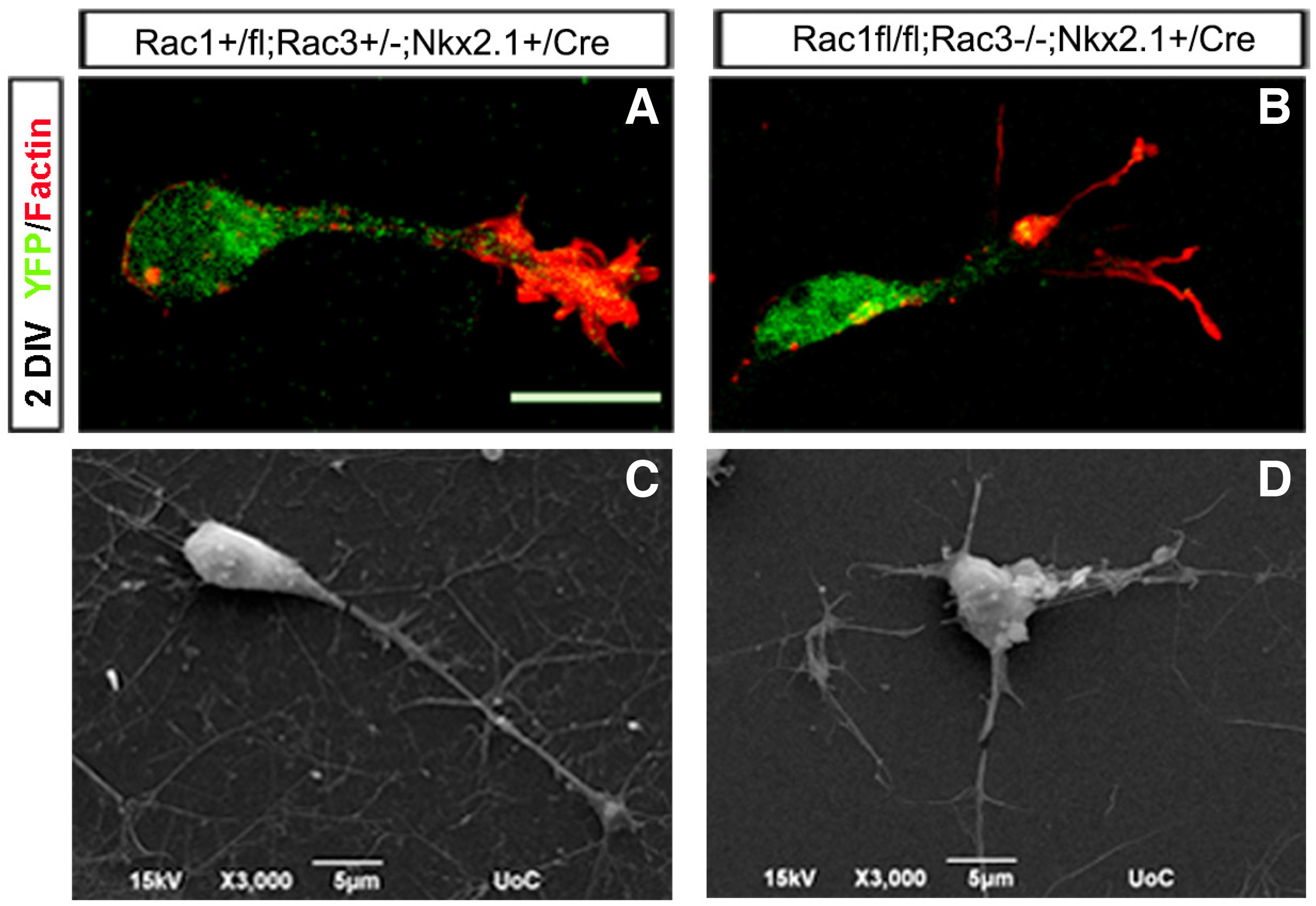
Furthermore, the MGE-derived interneurons missing Rac1 and Rac3 have specific morphological defects. Studies with MGE explants comparing control, Rac1 mutant, Rac3 mutant and double mutants showed that the double mutants had significantly shorter neurites and were the least motile, lacking the characteristic growth cone extension/retraction movements. To further study the morphological defects of Rac1/Rac3-deficient interneurons and their intrinsic or extrinsic nature, we grew ventrally aggregated YFP+ cells from the MGE on collagen-coated coverslips. We processed these cultures either for scanning electron microscopy (SEM) analysis or for immunostaining for various cytoskeletal markers. High magnification analysis of these cultures using SEM strongly indicated the presence of morphological defects in the absence of both Rac proteins These morphological defects included an increased number of neurites per cell, splitting of the leading processes and absence of an obvious axonal growth cone (Tivodar et al., 2015) (Fig. 3).
Fig. 3. Morphological defects of Rac1/Rac3-deficient MGE-derived interneurons.
The morphology of control (A, C) and double-mutant (B, D) interneurons was visualized by YFP/Phalloidin immunohistochemistry (A, B) and scanning electron microscopy (SEM (C, D). Scale bars: A and B:50 μm; C and D:5 μm. (Adapted from Tivodar et al., 2015).
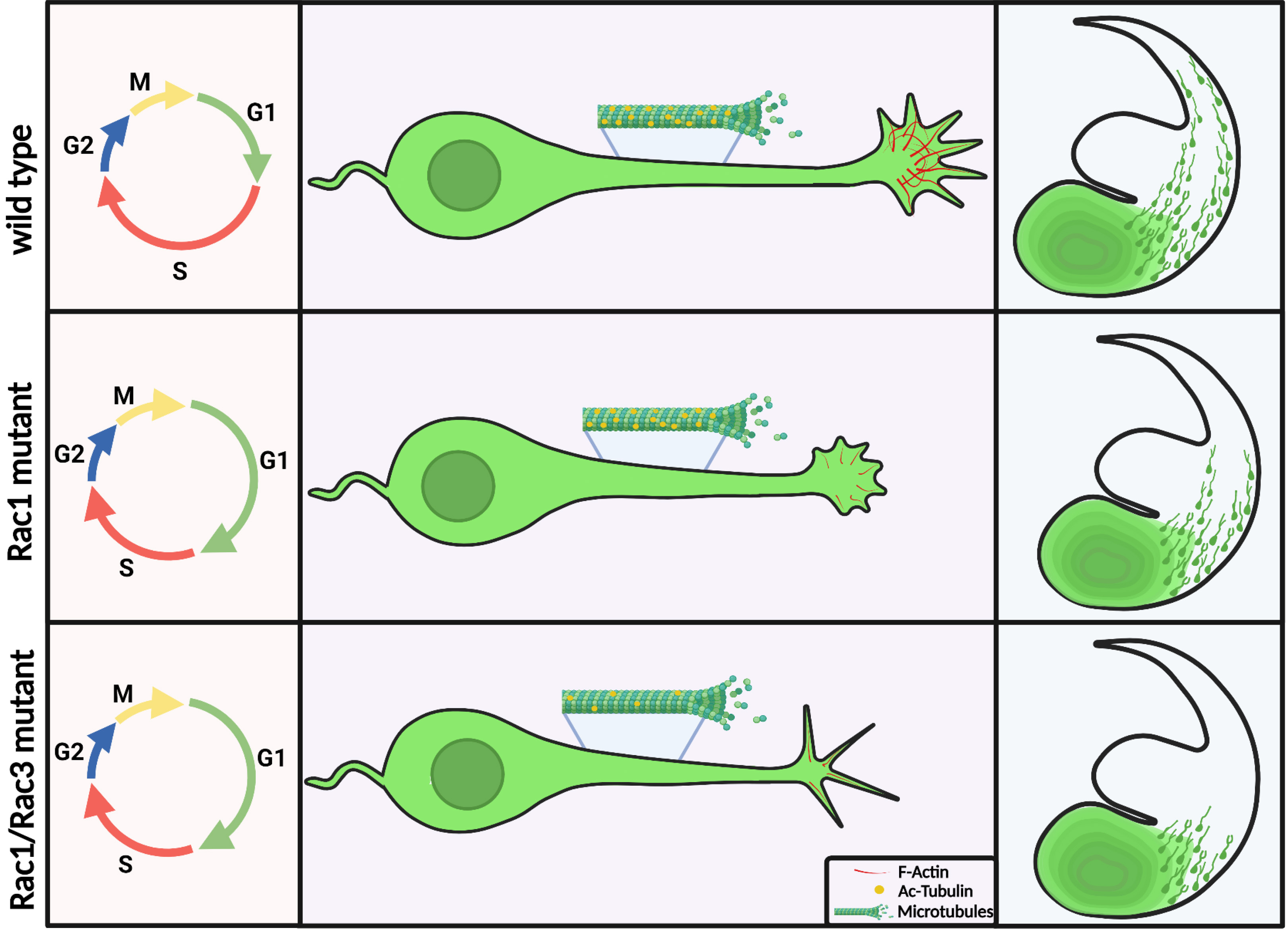
In particular, our data indicate that the stability of microtubules is affected in the double mutant. While the signal of acetylated tubulin (Ac-tubulin), a post-translational modification correlated with stable microtubules, was reduced in western blots, there was a change in its distribution, as in IHC the signal was enriched at the axon initial segment. We treated MGE-derived cells from control and double mutants in culture with taxol, which is known to stabilize microtubules by reducing their dynamics (Etienne-Manneville, 2010). We observed a partial rescue after taxol treatment, assessed by the length and the number of leading processes in the double mutant cultures. Leading process length was increased and the number of processes was reduced in the presence of taxol in cells where both Rac1 and Rac3 proteins were deleted. These findings indicate that Rac proteins make an essential contribution to interneuron migration by regulating actin–microtubule dynamics in developing MGE-derived interneurons (Tivodar et al., 2015). In addition, proteins of the PAK-interacting exchange factor (PIX)/ G-protein coupled receptor kinase-interacting protein (GIT) network expressed in MGE-derived cells and functionally linked to Rac1/3 have been shown to be involved in proper neuritic development (Franchi et al., 2016).
Time-lapse studies revealing the migratory behavior of double mutants
Cortical interneurons undergo a three-step migratory cycle in order to migrate from their birthplace to the neocortex. In the first step, the leading process extends and a cytoplasmic swelling appears in the proximal part thereof, toward which the centrosome and the Golgi apparatus move. Secondly, the nucleus translocates forward in a saltatory manner and invades the swelling, and lastly, the trailing process is retracted (Bellion 2005; Valiente and Marín 2010). When grown in 2D substrates in vitro, Rac1/Rac3-deficient interneurons show splitting of the leading process, abnormal growth cone, reduction of axon length, and reduction of microtubule stability. Furthermore, in a 3D environment, a significant delay in axon outgrowth is observed. Additionally, in Rac1/Rac3-deficient interneurons, the centrosome and Golgi complex positioning are also defective. Subsequently, their migratory behavior in ex vivo time-lapse imaging assays is severely perturbed, as several motility parameters (velocity, frequency and amplitude of nuclear translocation) are significantly decreased (ZK and DK, unpublished). The defects in microtubules also affect lysosomal transport in still extending axons, as well as lysosomal localization in migrating cortical interneurons. RNA-seq analysis indicated putative downstream effectors, among them, the two-pore channel protein TPC2, a lysosomal channel responsible for the endo-lysosomal calcium release, which is implicated in metastatic cell migration (Nguyen et al., 2017). In Rac1/Rac3-deficient interneurons, TPC2 levels are reduced and protein distribution is altered. Pharmacological inhibition of the channel negatively affects axon outgrowth of wild type CINs and also results in defective migration in ex vivo time-lapse imaging experiments (ZK and DK, unpublished). These findings indicate, once again, the essential role played by Rac protein in regulating cytoskeletal dynamics during cortical interneuron migration, and also provides additional evidence that migrating cells utilize similar mechanisms in order to move, independent of their cell identities per se (i.e. cancer cells and neurons) (Fig. 4).
Unanswered questions and future perspectives
Despite our increasing understanding of the function of Rho GTPases in interneuron development, several questions remain unanswered. A major challenge is to uncover the exact molecular mechanism via which the specific defects occur in the Rac1 single mutant line as well as the Rac1/3 double mutant line we have generated. As it pertains to the prolonged G1 phase that we uncovered in the Rac1 mutant, we need to be able to observe individual cells. However, due to the high density of the ventrally aggregated cells, it was not possible to study the morphological features and proliferative dynamics of cycling progenitors. These can be labelled in utero with low titer replication-incompetent, CRE-dependent, fluorophore-expressing retroviruses to uncover the cell division profiles of mutant progenitors and intermediate-progenitors in vivo. In addition, using the same viruses we can infect cells in organotypic brain slices ex vivo and examine the division patterns of these progenitors by monitoring their cell divisions by means of time-lapse microscopy.
An additional question worth investigating is the status of individual CINs (Rac1 and Rac1/3 mutants) and whether they are properly integrated in the mature cortical circuits. Although the majority of both mutant mice die before weaning at early postnatal stages, a small percentage survive until at least three weeks after birth. Therefore, it is possible to investigate the morphological and functional properties of the remaining CINs in the brain for both Rac1 and Rac1/3 deficient mice, in order to identify additional cellular processes in which Rac1 and Rac3 proteins are implicated. These experiments can be performed by injecting CRE-dependent fluorophore-expressing adeno-associated viruses and examining the brains a few weeks later for whole neuron morphology (dendritic growth and patterning), as well as via dendritic spine and synaptic puncta analysis. In addition, with the same approach, we can investigate whether any defects we may discern are cell autonomous or not, by transplanting mutant labelled cells intracranially into wild type brains and vice versa (Denaxa et al., 2019). In this way, we can force the mutant cells to find themselves in a wild type environment and assess their morphological properties. For the functional analysis, mutant interneurons can be subjected to patch-clamp recordings. Intrinsic electrophysiological properties via whole-cell patch-clamp in current-clamp mode, including passive and active properties, can be studied. Via whole-cell patch-clamp in voltage-clamp mode, the spontaneous excitatory and inhibitory post-synaptic currents (EPSCs and IPSCs) can be obtained, as a result of input by presynaptic neurons.
Furthermore, it is important to determine the role of Rac1 and Rac3 proteins in the development of synapse formation in CINs, as studies point to the involvement of Racs in synaptogenesis and dendritogenesis in other neurons (Tolias et al. 2005; Corbetta et al. 2009; Um et al. 2014; Zamboni et al. 2016; Nakamura et al. 2017; Tu et al. 2020). CRE-dependent fluorophore-expressing adeno-associated viruses can be used to infect mixed cortical cultures and measure synapse density, by quantifying co-localized puncta of pre- and postsynaptic excitatory or inhibitory synaptic markers that overlap with labeled interneurons.
Finally, a quantitative proteomic analysis in GFP+ CINs from the single Rac1 mutant could be undertaken to reveal up or downregulated effectors, in addition to a phosphomics approach to detect post-translational modifications. Determining a set of phosphorylated proteins that might be altered in the absence of the Rac1 protein could provide important information about the signaling pathways that are active under this condition. Such a candidate list will enable us to test the top choices in brain slices and ascertain whether we can rescue the migratory defects by overexpressing these candidate molecules.
In conclusion, the two genetic models we have generated have been useful in understanding novel aspects of interneuron development and the involvement of Rho GTPases in this process. These new data will not only contribute with novel information about the development of cortical assemblies but, in combination, will provide insight into unique neuron-type defects to specific neurodevelopmental diseases.
Acknowledgements
The authors would like to acknowledge funding from the Manassaki Foundation of the UoC (fellowship to ZK) and the Hellenic Foundation for Research and Innovation (grant 1621 to DK).
Abbreviations
Ac-tubulin, acetylated tubulin; BC, barrel cortex; BrdU, Bromodeoxyuridine; CB, calbindin; CR, calretinin; CGE, caudal ganglionic eminence; Cntn2, contactin2; CIN, cortical interneuron; E, embryonic day; fEPSPs, field excitatory postsynaptic potentials; Gamma-aminobutyric acid, GABA; GIT, G-protein coupled receptor kinase-interacting protein,; IN, interneuron; LGE, lateral ganglionic eminence; Lhx6, LIM homeobox 6; LTP, long-term potentiation; MGE, medial ganglionic eminence; NPY, neuropeptide Y; NMDA, N-methyl-D-aspartate receptor; PPR, paired-pulse ratio; PIX, PAK-interacting exchange factor,; PV, Parvalbumin; P, postnatal; PFC, prefrontal cortex; Sip1, SC35-interacting protein 1; SEM, scanning electron microscopy; SST, Somatostatin; Sox6, SRY-box transcription factor 6; SVZ, subventricular zone; TAG1, transient axonal glycoprotein; VZ, ventricular zone; YFP, yellow fluorescent protein.References
Ayala R., Shu T., Tsai L. H. (2007). Trekking across the Brain: The Journey of Neuronal Migration. Cell 128: 29-43.
Batista-Brito R., Fishell G. (2009). Chapter 3 The Developmental Integration of Cortical Interneurons into a Functional Network. In Development of Neural Circuitry. Elsevier.
Bellion A. (2005). Nucleokinesis in Tangentially Migrating Neurons Comprises Two Alternating Phases: Forward Migration of the Golgi/Centrosome Associated with Centrosome Splitting and Myosin Contraction at the Rear. Journal of Neuroscience 25: 5691-5699.
Butt S. J.B., Sousa V. H., Fuccillo M. V., Hjerling-Leffler J., Miyoshi G., Kimura S., Fishell G. (2008). The Requirement of Nkx2-1 in the Temporal Specification of Cortical Interneuron Subtypes. Neuron 59: 722-732.
Chen L., Liao G., Waclaw R. R., Burns K. A., Linquist D., Campbell K., Zheng Y., Kuan C.Y. (2007). Rac1 Controls the Formation of Midline Commissures and the Competency of Tangential Migration in Ventral Telencephalic Neurons. Journal of Neuroscience 27: 3884-3893.
Chen L., Melendez J., Campbell K., Kuan C.Y., Zheng Y. (2009). Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Developmental Biology 325: 162-170.
Cobos I., Calcagnotto M. E., Vilaythong A. J., Thwin M. T., Noebels J. L., Baraban S. C., Rubenstein J. L. R. (2005). Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature Neuroscience 8: 1059-1068.
Corbetta S., Gualdoni S., Albertinazzi C., Paris S., Croci L., Consalez G. G., de Curtis I. (2005). Generation and Characterization of Rac3 Knockout Mice. Molecular and Cellular Biology 25: 5763-5776.
Corbetta S., Gualdoni S., Ciceri G., Monari M., Zuccaro E., Tybulewicz V. L. J., De Curtis I. (2009). Essential role of Rac1 and Rac3 GTPases in neuronal development. The FASEB Journal 23: 1347-1357.
Corbin J. G., Nery S., Fishell G. (2001). Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nature Neuroscience 4: 1177-1182.
Denaxa M., Kalaitzidou M., Garefalaki A., Achimastou A., Lasrado R., Maes T., Pachnis V. (2012). Maturation-Promoting Activity of SATB1 in MGE-Derived Cortical Interneurons. Cell Reports 2: 1351-1362.
Denaxa M., Kyriakopoulou K., Theodorakis K., Trichas G., Vidaki M., Takeda Y., Watanabe K., Karagogeos D. (2005). The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Developmental Biology 288: 87-99.
Denaxa M., Neves G., Burrone J., Pachnis V. (2019). Transplantation of Chemogenetically Engineered Cortical Interneuron Progenitors into Early Postnatal Mouse Brains. Journal of Visualized Experiments e59568.
Etienne-Manneville S. (2010). From signaling pathways to microtubule dynamics: the key players. Current Opinion in Cell Biology 22: 104-111.
Fogarty M., Grist M., Gelman D., Marin O., Pachnis V., Kessaris N. (2007). Spatial Genetic Patterning of the Embryonic Neuroepithelium Generates GABAergic Interneuron Diversity in the Adult Cortex. Journal of Neuroscience 27: 10935-10946.
Franchi S. A., Astro V., Macco R., Tonoli D., Barnier J.V., Botta M., de Curtis I. (2016). Identification of a Protein Network Driving Neuritogenesis of MGE-Derived GABAergic Interneurons. Frontiers in Cellular Neuroscience 10: 289.
Govek E.E., Hatten M. E., Van Aelst L. (2011). The role of Rho GTPase proteins in CNS neuronal migration. Developmental Neurobiology 71: 528-553.
Jaffe A. B., Hall A. (2005). RHO GTPASES: Biochemistry and Biology. Annual Review of Cell and Developmental Biology 21: 247-269.
Kalemaki K., Konstantoudaki X., Tivodar S., Sidiropoulou K., Karagogeos D. (2018). Mice With Decreased Number of Interneurons Exhibit Aberrant Spontaneous and Oscillatory Activity in the Cortex. Frontiers in Neural Circuits 12: 96.
Kepecs A., Fishell G. (2014). Interneuron cell types are fit to function. Nature 505: 318-326.
Kessaris N., Magno L., Rubin A. N., Oliveira M. G. (2014). Genetic programs controlling cortical interneuron fate. Current Opinion in Neurobiology 26: 79-87.
Kholmanskikh S. S., Dobrin J. S., Wynshaw-Boris A., Letourneau P. C., Ross M. E. (2003). Disregulated RhoGTPases and Actin Cytoskeleton Contribute to the Migration Defect in Lis1-Deficient Neurons. The Journal of Neuroscience 23: 8673-8681.
Koh C.G. (2007). Rho GTPases and Their Regulators in Neuronal Functions and Development. Neurosignals 15: 228-237.
Konstantoudaki X., Chalkiadaki K., Tivodar S., Karagogeos D., Sidiropoulou K. (2016). Impaired synaptic plasticity in the prefrontal cortex of mice with developmentally decreased number of interneurons. Neuroscience 322: 333-345.
Lavdas A. A., Grigoriou M., Pachnis V., Parnavelas J. G. (1999). The Medial Ganglionic Eminence Gives Rise to a Population of Early Neurons in the Developing Cerebral Cortex. The Journal of Neuroscience 19: 7881-7888.
Leone D. P., Srinivasan K., Brakebusch C., McConnell S. K. (2010). The Rho GTPase Rac1 is required for proliferation and survival of progenitors in the developing forebrain. Developmental Neurobiology 70: 659-678.
Lewis D. A., Hashimoto T., Volk D. W. (2005). Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience 6: 312-324.
Lim L., Mi D., Llorca A., Marín O. (2018). Development and Functional Diversification of Cortical Interneurons. Neuron 100: 294-313.
Liodis P., Denaxa M., Grigoriou M., Akufo-Addo C., Yanagawa Y., Pachnis V. (2007). Lhx6 Activity Is Required for the Normal Migration and Specification of Cortical Interneuron Subtypes. Journal of Neuroscience 27: 3078-3089.
Malosio M. L., Gilardelli D., Paris S., Albertinazzi C., de Curtis I. (1997). Differential Expression of Distinct Members of Rho Family GTP-Binding Proteins during Neuronal Development: Identification of Rac1B , a New Neural-Specific Member of the Family . The Journal of Neuroscience 17: 6717-6728.
Marín O. (2012). Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience 13: 107-120.
Marín O. (2013). Cellular and molecular mechanisms controlling the migration of neocortical interneurons. European Journal of Neuroscience 38: 2019-2029.
Marín O., Rubenstein J. L. R. (2001). A long, remarkable journey: Tangential migration in the telencephalon. Nature Reviews Neuroscience 2: 780-790.
Minobe S., Sakakibara A., Ohdachi T., Kanda R., Kimura M., Nakatani S., Tadokoro R., Ochiai W., Nishizawa Y., Mizoguchi A., Kawauchi T., Miyata T. (2009). Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neuroscience Research 63: 294-301.
Nakamura T., Ueyama T., Ninoyu Y., Sakaguchi H., Choijookhuu N., Hishikawa Y., Kiyonari H., Kohta M., Sakahara M., de Curtis I., Kohmura E., Hisa Y., Aiba A., Saito N. (2017). Novel role of Rac-Mid1 signaling in medial cerebellar development. Development 144: 1863-1875.
Nguyen O. N. P., Grimm C., Schneider L. S., Chao Y.K., Atzberger C., Bartel K., Watermann A., Ulrich M., Mayr D., Wahl-Schott C., Biel M., Vollmar A. M. (2017). Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Research 77: 1427-1438.
Peyre E., Silva C. G., Nguyen L. (2015). Crosstalk between intracellular and extracellular signals regulating interneuron production, migration and integration into the cortex. Frontiers in Cellular Neuroscience 9: 129.
Srinivas S., Watanabe T., Lin C.S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology 1: 4.
Sussel L., Marin O., Kimura S., Rubenstein J.L. (1999). Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126: 3359-3370.
Tahirovic S., Hellal F., Neukirchen D., Hindges R., Garvalov B. K., Flynn K. C., Stradal T. E., Chrostek-Grashoff A., Brakebusch C., Bradke F. (2010). Rac1 Regulates Neuronal Polarization through the WAVE Complex. Journal of Neuroscience 30: 6930-6943.
Tivodar S., Kalemaki K., Kounoupa Z., Vidaki M., Theodorakis K., Denaxa M., Kessaris N., de Curtis I., Pachnis V., Karagogeos D. (2015). Rac-GTPases Regulate Microtubule Stability and Axon Growth of Cortical GABAergic Interneurons. Cerebral Cortex 25: 2370-2382.
Tolias K. F., Bikoff J. B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R. J., Greenberg M. E. (2005). The Rac1-GEF Tiam1 Couples the NMDA Receptor to the Activity-Dependent Development of Dendritic Arbors and Spines. Neuron 45: 525-538.
Tu X., Yasuda R., Colgan L. A. (2020). Rac1 is a downstream effector of PKCα in structural synaptic plasticity. Scientific Reports 10: 1777.
Um K., Niu S., Duman J. G., Cheng J. X., Tu Y.K., Schwechter B., Liu F., Hiles L., Narayanan A. S., Ash R. T., Mulherkar S., Alpadi K., Smirnakis S. M., Tolias K. F. (2014). Dynamic Control of Excitatory Synapse Development by a Rac1 GEF/GAP Regulatory Complex. Developmental Cell 29: 701-715.
Valiente M., Marín O. (2010). Neuronal migration mechanisms in development and disease. Current Opinion in Neurobiology 20: 68-78.
Vidaki M., Tivodar S., Doulgeraki K., Tybulewicz V., Kessaris N., Pachnis V., Karagogeos D. (2012). Rac1-Dependent Cell Cycle Exit of MGE Precursors and GABAergic Interneuron Migration to the Cortex. Cerebral Cortex 22: 680-692.
Walmsley M. J., Ooi S. K. T., Reynolds L. F., Smith S. H., Ruf S., Mathiot A., Vanes L., Williams D. A., Cancro M. P., Tybulewicz V. L. J. (2003). Critical Roles for Rac1 and Rac2 GTPases in B Cell Development and Signaling. Science 302: 459-462.
Wonders C. P., Anderson S. A. (2006). The origin and specification of cortical interneurons. Nature Reviews Neuroscience 7: 687-696.
Wonders C. P., Taylor L., Welagen J., Mbata I. C., Xiang J. Z., Anderson S. A. (2008). A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Developmental Biology 314: 127-136.
Zamboni V., Armentano M., Sarò G., Ciraolo E., Ghigo A., Germena G., Umbach A., Valnegri P., Passafaro M., Carabelli V., Gavello D., Bianchi V., D’Adamo P., de Curtis I., El-Assawi N., Mauro A., Priano L., Ferri N., Hirsch E., Merlo G. R. (2016). Disruption of ArhGAP15 results in hyperactive Rac1, affects the architecture and function of hippocampal inhibitory neurons and causes cognitive deficits. Scientific Reports 6: 34877.
Zhao Y., Flandin P., Long J. E., Cuesta M. D., Westphal H., Rubenstein J. L.R. (2008). Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. The Journal of Comparative Neurology 510: 79-99.