Int. J. Dev. Biol. 67: 137 - 146 (2023)
Strontium-doped hydroxyapatite and its role in osteogenesis and angiogenesis
Open Access | Review | Published: 14 November 2023
Abstract
For the past 50 years, hydroxyapatite (HA) has been widely used in bone defect repair because it is the main inorganic component of the mineral phase of a human bone. Extensive preclinical and clinical studies have shown that strontium (Sr) can safely and effectively help prevent and treat bone diseases, including osteoporosis. These findings have resulted in the concept of integrating Sr and HA for bone disease management. The doped Sr can improve the physicochemical properties of HA and enhance its angiogenic and bone regeneration ability. Nevertheless, no study has reviewed the design strategy of Sr-doped HA (Sr-HA) to understand its biological roles. Therefore, in this article, we review recent developments in Sr-HA preparation and its effect on osteogenesis and angiogenesis in vitro and in vivo along with key suggestions for future research and development.
Keywords
hydroxyapatite, strontium, biological mechanism, osteogenesis, angiogenesis
Introduction
The clinical treatment of serious segmental bone defects caused by trauma or bone tumors is challenging. Moreover, seeking an alternative to autologous bone grafting is one of the major goals of bone tissue engineering. Bone engineering has been extensively studied by researchers over the past few decades. Consequently, many artificial bone materials have been developed. HA has garnered extensive attention from researchers because this main inorganic component of bone tissues (Jarcho et al., 1977; Zhan et al., 2005; Kutikov et al., 2015; Deville et al., 2006; Li et al., 2013; Poddar et al., 2023) is the most important bone repair/regeneration material owing to its excellent biocompatibility and osteoinductive properties. However, its poor mechanical properties and slow degradation limit its application to clinical practice (Rezwan et al., 2006). Researchers think that modifying HA may solve the mentioned problems.
Currently, the biological properties of HA-based materials can be improved by several methods, including doping other elements, heat treatment, and material coating (Shavandi et al., 2015; Shi et al., 2020; He et al., 2021; Boanini et al., 2010). Adding certain metal ions can improve the physicochemical properties of HA and enhance its antibacterial, angiogenic and bone regeneration capacity (Turhan et al., 2023). For example, strontium (Sr), magnesium (Mg), and zinc (Zn) ions can promote bone regeneration by regulating osteoblast and osteoclast activity.
Mg is the fourth most abundant element in the human body, with 60% of magnesium deposited in the bones (Laskus and Kolmas, 2017). Mg alloys began to be used in orthopedics and blood vessels in the mid-nineteenth century (Walker et al., 2014). Mg forms bone by promoting osteoblast differentiation. He et al., demonstrated that Mg metal enhanced the viability and osteogenic differentiation of human bone marrow-derived stromal cells (hBMSCs) (He et al., 2016), as did Yang et al., (Yang et al., 2010). It is reported that Mg2+ replacement of Ca2+ in HA occurs only to a limited extent, up to 10 at.% (Bigi et al., 1996; Yasukawa et al., 1996). Despite the limited substitution, the doping of Mg leads to an increase in the solubility of HA (Landi et al., 2008), which may be related to the decrease in crystallinity. Meanwhile, the morphology of HA is changed to a spherical shape (Berg et al., 2020).
Zn is an essential trace element in the human body, involved in DNA and RNA replication, protein synthesis, and bone metabolism (Prasad, 1995). Zn promotes bone metabolism by increasing osteoblast activity and collagen synthesis, as well as inhibiting osteoclast formation (Yamaguchi and Gao, 1998; Kishi and Yamaguchi, 1994). Zn2+ probably replace up to 20% of the Ca2+ in the HA lattice (Boanini et al., 2010). Since it causes defects in the structure, it makes the lattice more susceptible to disruption (Gutsalova et al., 2021), which makes Zn-doped HA less soluble than conventional HA (Osorio et al., 2014; Hu et al., 2012). Zn-doped HA crystals are irregular and form agglomerates, usually in the form of rods (Hu et al., 2012).
The trace element Sr is present in the human body at 0.008% - 0.01%, which is much lower than that of Ca. However, Sr has a strong affinity for bone, especially in metabolically active tissues (Dahl et al., 2001). Sr has a dual effect on bone: stimulation of bone formation and inhibition of bone resorption (Saidak and Marie, 2012; Hassan et al., 2023). On the one hand, Sr activates signaling pathways such as OPG/RANKL/RANK, NFκB to inhibit osteoclast activity; on the other hand, Sr enhances alkaline phosphatase (ALP) activity, collagen synthesis, and the formation of osteogenic markers to promote bone production (Huang et al., 2020; Zarins et al., 2019; Zarins et al., 2018; Rybchyn et al., 2011). Because Sr2+ (0.12 nm) has an ionic radius similar to Ca2+(0.099 nm), it can replace Ca in the HA structure throughout the compositional range (Boanini et al., 2010). A study showed that Sr2+ enters the HA lattice and enhances the mechanical strength (Geng et al., 2016). Landi et al., confirmed the increase in the solubility of Sr-HA (Landi et al., 2007). Dai et al., analyzed HX-BGC, a bioactive glass with 1.6 % Sr, was in the form of bands and plates (Dai et al., 2021b). Although Sr can replace up to 100% of Ca (Frangopol et al., 2016), in one study it was found that the HA structure was maintained at Sr molar ratios of 2% and 4% in the compound, while it disappeared at other different Sr molar ratios (Nagyné-Kovács et al., 2018). In addition, the Sr content has an effect on osteogenesis. When the Sr concentration exceeds a certain threshold, there will be toxic inhibition (Liu et al., 2016). Almeida et al., demonstrated that the optimal concentration range of Sr is 1-10 mM, and within this range, Sr can effectively enhance the proliferation and activity of preosteoblasts, and promote the maturation of osteoblasts into osteocytes (Almeida et al., 2016).
Therefore, in this review, we systematically introduce the preparation method of Sr-HA and provide insights into the effects of implanted Sr-HA on osteogenesis and angiogenesis. Furthermore, the role of Sr in osteogenesis and vascularization in bone repair has also been discussed in detail.
Preparation of Sr-Doped hydroxyapatite
HA is an important component of the human skeleton. Because of its low solubility in physiological environments, as it is a bioactive material with a high osseointegration capacity that does not induce inflammatory reactions on direct contact with hard tissues. However, the properties of HA can be easily improved. The Ca2+ in the structure can be replaced by Sr2+, Zn2+, Cu2+, Fe2+, Ag+, Mg2+ and other ions (Dapporto et al., 2022; Ungureanu et al., 2023), so Sr can change the HA lattice to modify its biological properties in order to overcome any potential disadvantages; therefore, researchers have integrated Sr and HA. Sr-HA can be prepared by several methods, and four methods, namely chemical precipitation, wet processing, hydrothermal preparation, and wet microwave synthesis, have been discussed below. Furthermore, their advantages and disadvantages are presented in Table 1.
Table 1
Advantages and disadvantages of the preparation methods of strontium-doped hydroxyapatite (Sr-HA)
| Method | Advantage | Disadvantage |
|---|---|---|
|
Chemical precipitation method |
1. Certain interactions exist among the groups 2. Good permeability and adsorption 3. Certain inducement |
1. Unknown results 2. Low product purity |
Wet process preparation |
1. High crystallinity and stability 2. Resistant to corrosion 3. Biocompatible |
1. The product is not stable 2. Slow reaction rate and large product particle size |
Hydrothermal method |
1. No cytotoxicity 2. Good biocompatibility |
1. Thermal stability performance becomes poor 2. Easy to decompose |
Wet microwave synthesis |
1. Able to fuse with natural bone 2. Increased cell growth rate 3. Strontium content is controllable 4. Shorten reaction time |
Currently not widely used |
Chemical precipitation method
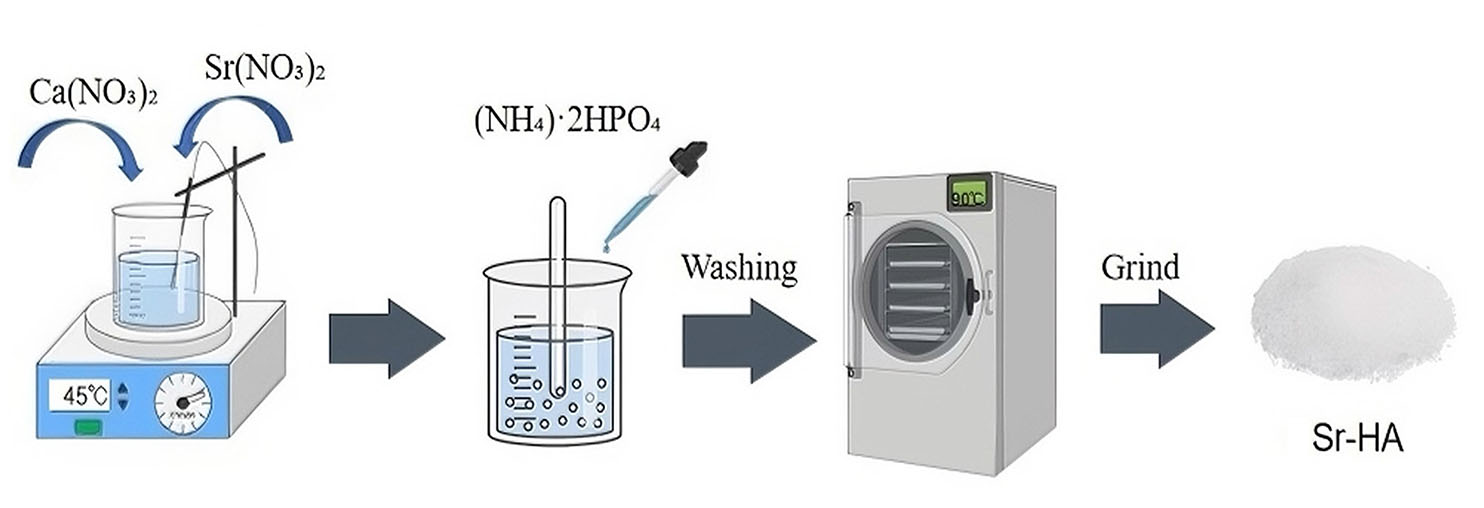
Chemical precipitation method is to use the reaction between the reactant solution, by controlling the pH value of the solution, the solution precipitates. The crystallization process of precipitation is carried out by controlling the reaction rate, temperature, and aging time. It is the most widely used and simplest method to prepare Sr-HA. Scaffolds prepared by this method exhibit good dispersion and ably meet the requirements of tissue engineering.
In this method, (CaNO3)2·4H2O and (NH4)2HPO4 are used as the main reactants. First, 0.5 mol/L Ca(NO3)2 and Sr(NO3)2 solutions are mixed at 45°C together. After the reaction, the pH of the solution is adjusted to 10-11 with (NH4)2HPO4. After, precipitates are filtered, washed with anhydrous ethanol to remove impurities, vacuum-filtered and -dried, and ground to obtain Sr-HA powder (Fig. 1). The use of Sr/[Ca+Sr] and [Ca+Sr]/P at atomic ratios of 0.5 and 1.67, respectively, can yield 0.5-nm Sr-HA (Zhu et al., 2022; Catros et al., 2010). Ehret and Maqbool et al., (Ehret et al., 2017; Maqbool et al., 2021) prepared Sr-HA with good biocompatibility by this method without changing the phase composition and crystallinity of HA. The reaction mechanism of Sr-HA preparation by chemical precipitation is as follows:
(NH4) 2HPO4+NH4OH+ (10-α) Ca (NO3) 2+αSr (NO3) 2→H2O+SrαCa10-α (PO4) 6 (OH) 2+NH4NO3, α≤10
Wet process method
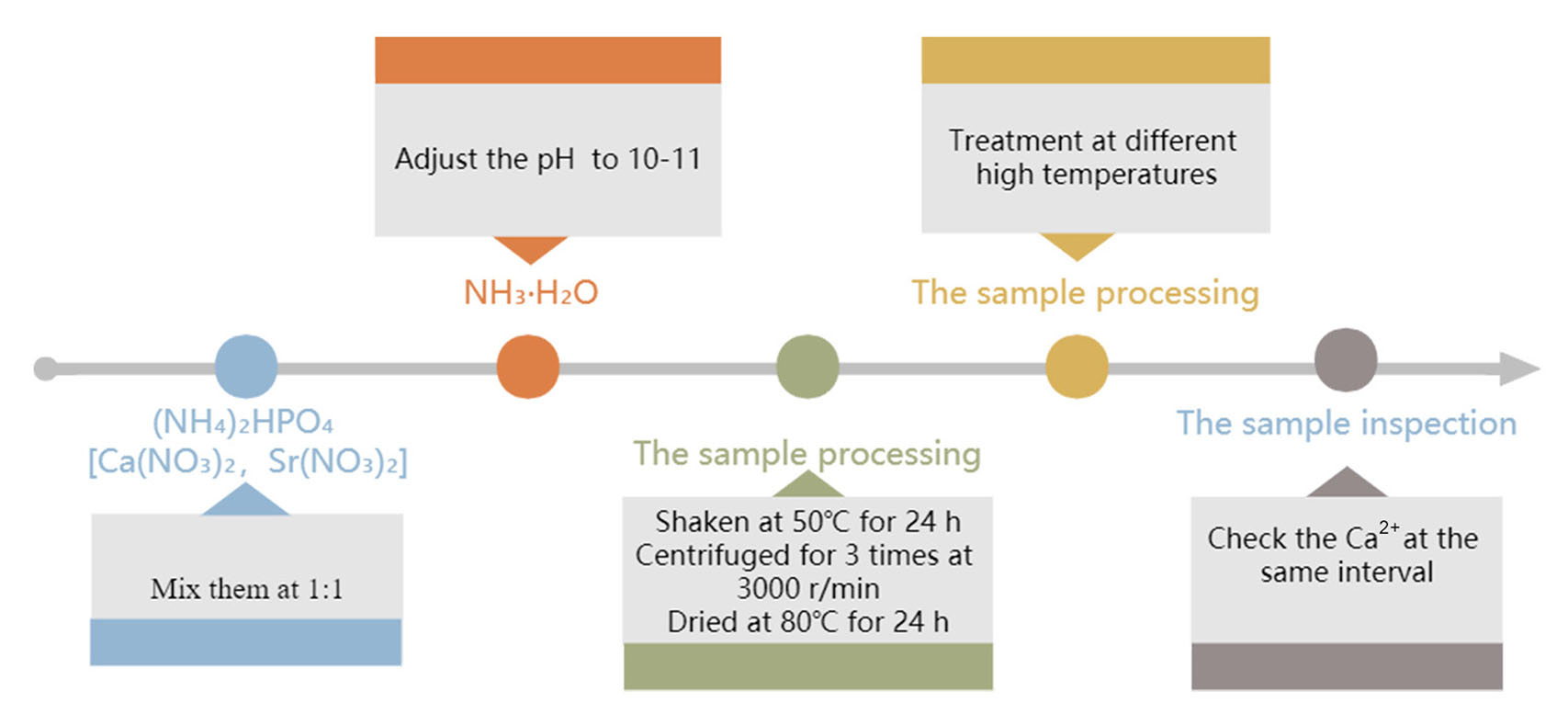
In this method, (NH4)2HPO4, [Ca(NO3)2, and Sr(NO3)2] solutions are mixed at a molar ratio of 1:1, and the molar ratio of Sr/(Ca+Sr) is set to 1:100.The solution is then kept in a shaker incubator at 50°C for 24 h, centrifuged thrice at 3000 r/min, and dried at 80°C for 24 h. The obtained blocks are then ground and sieved. Part of the powdered samples are cold-pressed into blocks at 200 kg pressure and heat treatment at 300°C, 600°C, and 900°C for 1 h (Fig. 2) (Pan et al., 2009). Li et al., (Li et al., 2019) used this method to prepare Sr-HA, which exhibited good biocompatibility and stability, but its preparation time is long and the reaction speed is slow. It is not widely used in industrial production.
Hydrothermal method
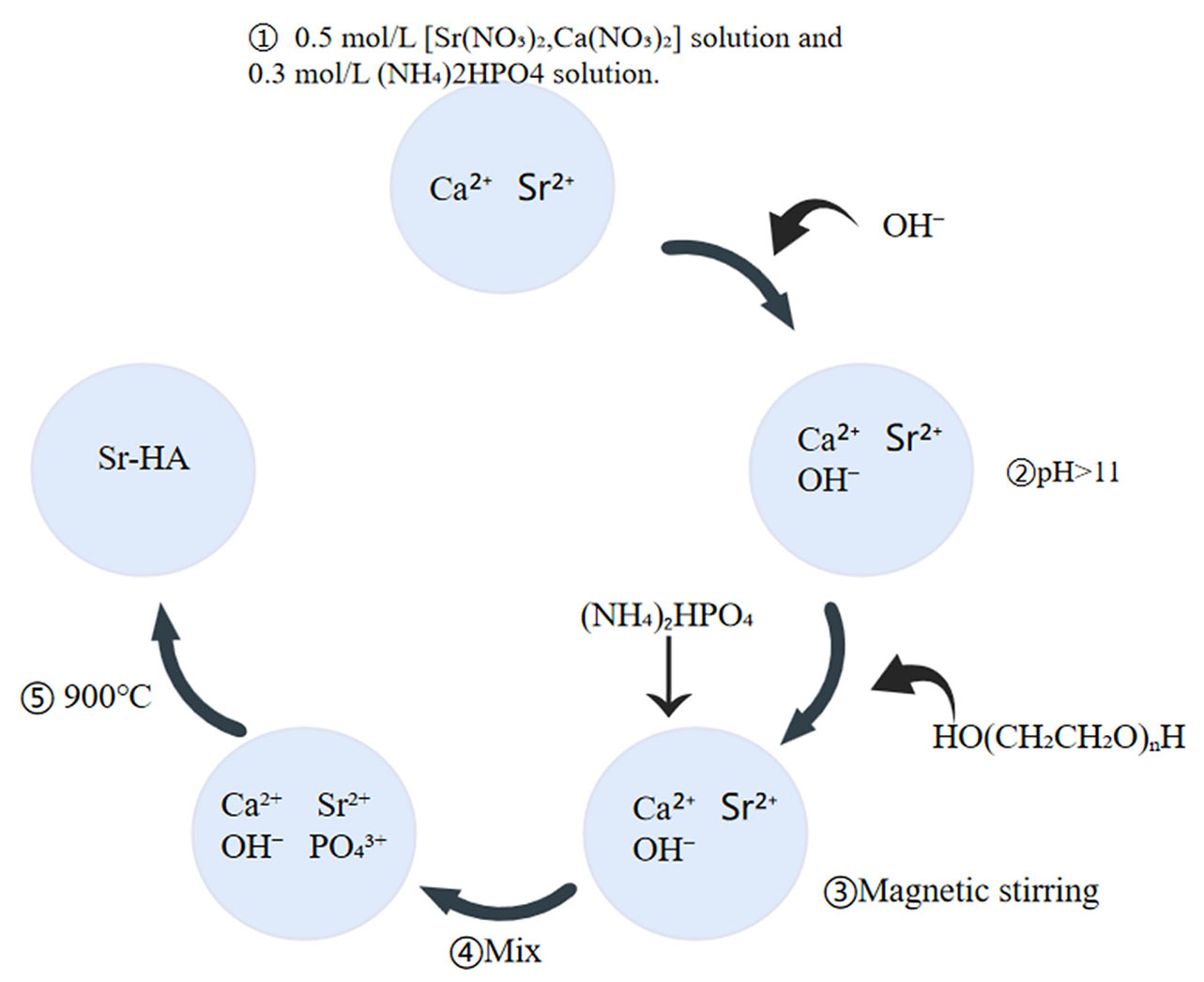
In this method, a 0.5 mol/L [Sr(NO3)2, Ca(NO3)2] solution and a 0.3 mol/L (NH4)2HPO4 solution are prepared. The pH values of these Ca and Sr solutions are adjusted to >11 using concentrated ammonia and to >10 using a phosphorus solution. Next, a certain amount from both solutions is mixed thoroughly, followed by the addition of 0.4 g of polyethylene glycol and magnetic stirring to dissolve polyethylene glycol. Under the stirring condition, the (NH4)2HPO4 solution is added slowly in a dropwise manner to maintain the n (Ca+Sr)/n (P) atom ratio at about 1.67, and again the mixture is stirred well. Then, it is transferred to a teflon-lined stainless steel hydrothermal kettle and heat-treated at 900°C. The product is then washed with water, followed by an ethanol wash, and filtered. Then the powdered product is dried at 80°C overnight (Fig. 3) (Li et al., 2022; Donazzon et al., 1998). The reaction mechanism of Sr-HA preparation by hydrothermal method is as follows:
(10-x)Ca2++xSr2++6PO43++2OH-→Ca10-xSrx(PO4)6(OH)2
Wet microwave synthesis method
This method is characterized by the simultaneous generation of heat at different depths of the material being heated. Compare the other three methods, this method allows for faster and more uniform heating (Fig. 4).
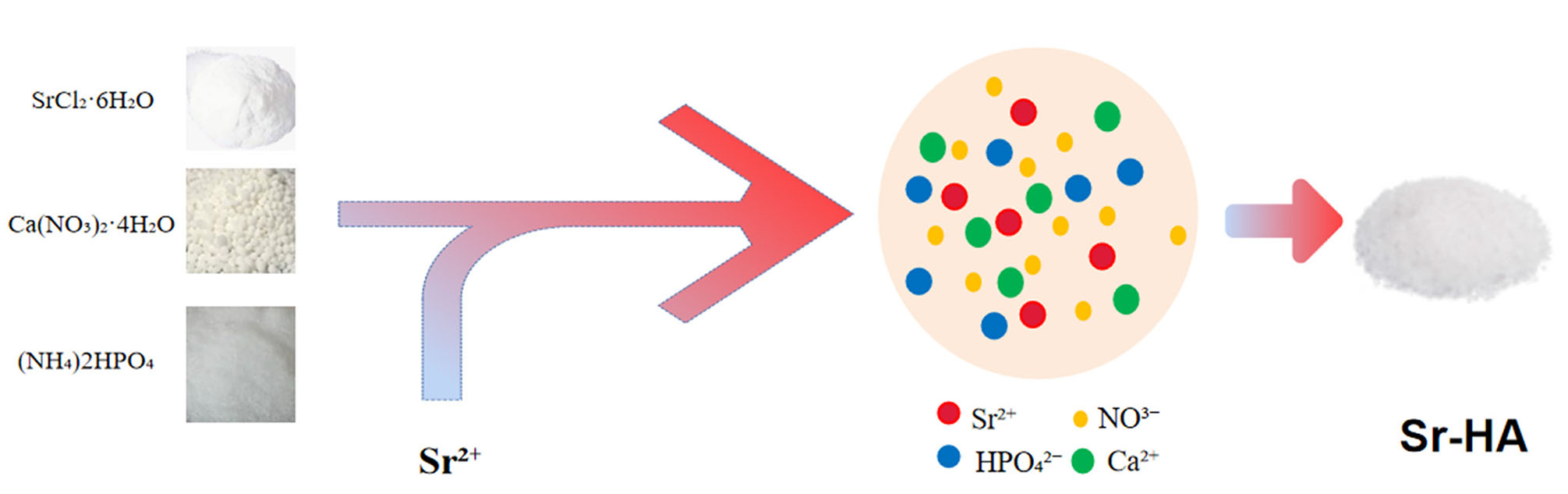
The crystals of SrCl2, Ca(NO3)2·4H2O, (NH4)2HPO4 are used in this method. First, [Ca(NO3)2·SrCl2] and (NH4)2HPO4 solutions of the 0.5 mol/L are prepared and pH is adjusted to 11 using concentrated ammonia. Next, the (NH4)2HPO4 solution is rapidly poured int°o the [Ca(NO3)2·SrCl2] solution and kept in a heating water bath at 80°C. The reaction is performed in a microwave oven with the power set to 800 W. The slurry is allowed to stand for a while, followed by filtration. The sediment is washed with distilled water and then with anhydrous ethanol, dried at 800oC, and finally ground (Yu et al., 2017; Agrawal et al., 2018). However, the resulting product is unstable; hence, this method is not used widely.
Osteogenic effects of Sr and Sr-HA
Owing to the similarity between Sr and Ca, Sr exhibits osteogenic properties similar to that exhibited by Ca, which can increase the levels of runt-related transcriptional factor 2 (RUNX2) and further stimulate Ca sensing receptor (CaSR), thereby inducing mitogen-activated protein kinase phosphorylation and activating cell signaling pathways, and ultimately increasing primary osteoblast formation (Gu et al., 2013). Sr promotes collagen secretion in osteoblasts via CaSR, thus increasing the levels of osteopontin (OPN), ALP, bone sialoprotein, salivary protein, and osteocalcin during osteoblast differentiation (Li et al., 2015). Thus, when Sr-HA is implanted, HA forms a strong bond with bone tissues. After coming in contact with body fluids, HA is degraded over time, thereby increasing the local Sr2+ concentration at the action site. The released Sr2+ exerts its osteogenic properties, inducing osteoblast differentiation (Fig. 5) (Wan et al., 2020). Brennan observed a dose-dependent increase in RUNX2/CBFA1 levels in human primary osteoblasts after Sr-Ran administration for 10 days. Moreover, OPN mRNA levels were increased by 50% after Sr-Ran (1 mM) administration (Brennan et al., 2009). Additionally, Sr promotes bone matrix synthesis (Barbara et al., 2004). Frasnelli observed that the cultures of rat skull osteoblasts exposed to Sr-Ran showed an increased synthesis of collagen Ⅰ, a marker of osteoblast differentiation (Frasnelli et al., 2017).
Fig. 5. The degradation and precipitation reaction of bioactive strontium-doped hydroxyapatite (Sr-HA) regulates local ion concentrations and influences surrounding physiological processes, including osteoblast differentiation.
Abbreviations: bMSC, bone marrow-derived stromal cell; Sr, strontium. Source: Wan et al., 2020. Licensed under CC BY.
Effects of Sr on osteoclasts
Osteoclasts are specialized cells within the bone tissue responsible for bone resorption or the breakdown of bone matrix. They play a crucial role in the dynamic process of bone remodeling, which involves the continuous removal of old bone tissue and the formation of new bone. Osteoclasts achieve bone resorption by secreting enzymes and acids that dissolve the mineralized matrix, making room for the subsequent action of bone-forming cells like osteoblasts.
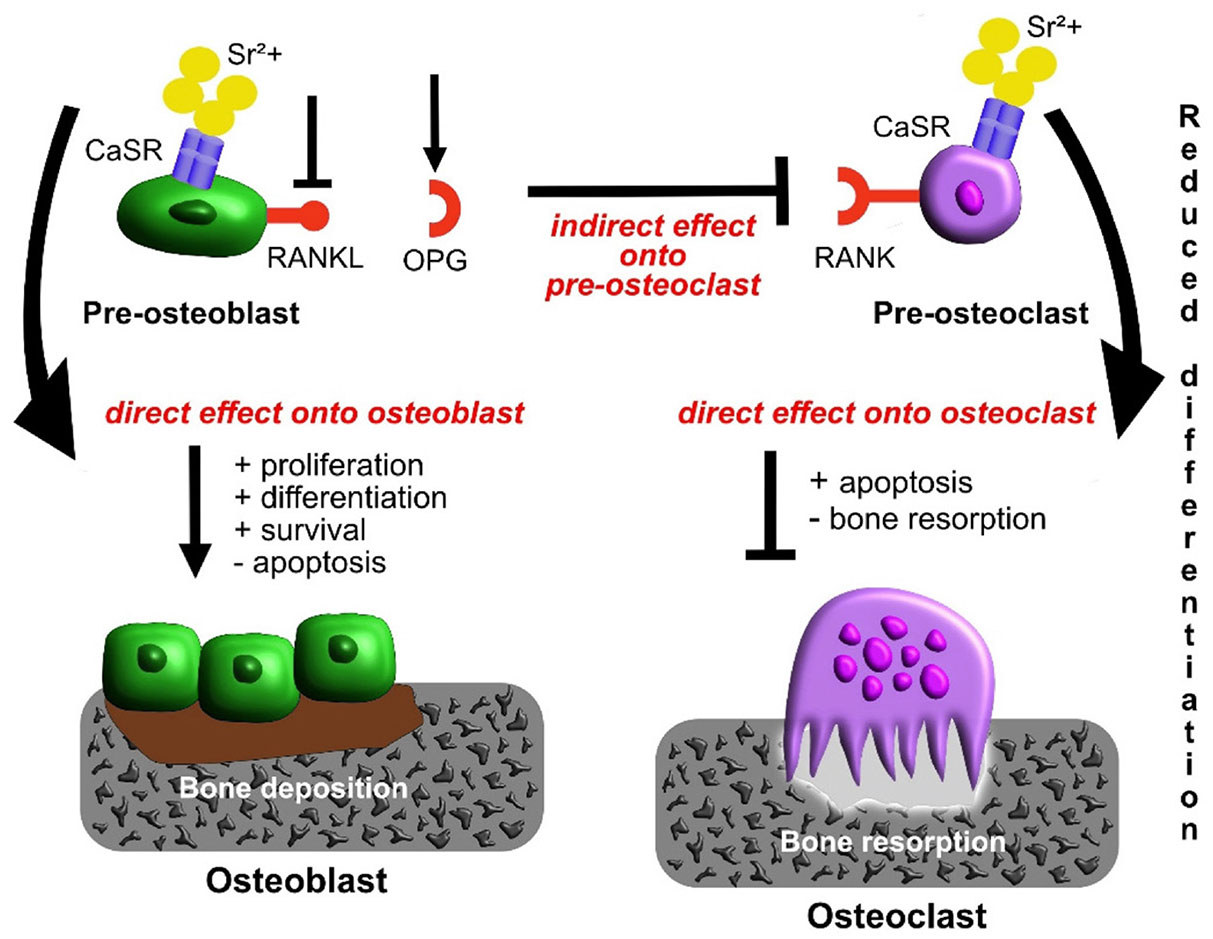
Studies have shown that Sr promotes osteoclast apoptosis, inhibiting their proliferation and differentiation, thereby reducing bone resorption (Bonnelye et al., 2008). Owing to their similar atomic and ionic properties, both Ca and Sr are agonists of CaSR (Luo et al., 2018). Sr probably acts on CaSR to affect the apoptosis of mature osteoclasts (Fig. 6) (Neves et al., 2017; Borciani et al., 2022). Furthermore, Sr regulates inflammatory states to promote the osteogenesis of BMSCs and accelerate the inhibition of macrophage RAW264.7 differentiation (Li et al., 2016). In another study, consistent with in vitro results, Capuccini proved that the activity and differentiation of MG-63 were increased and the differentiation of osteoclasts was inhibited by Sr (Capuccini et al., 2008), confirming that Sr incorporation reduced the immune response to the material, thereby promoting bone regeneration in vitro (Lee et al., 2021). Additionally, Sr reduces the levels of carbonic anhydrase II and glass agglutinin receptors in osteoclasts, thereby inhibiting cell differentiation and reducing osteoclast resorption by up to 66% (Baron and Tsouderos, 2002). The above studies suggest that Sr promotes osteogenesis.
Fig. 6. Schematic diagram of influence of strontium (Sr) administration on osteoblasts and osteoclasts and their crosstalk.
Source: Borciani et al., 2022. Licensed under CC BY 4.0.
Effects of Sr-HA on osteogenesis
The chemical and physical properties of Sr and Ca are similar; however, Sr has a larger ionic radius than Ca (112 vs. 99 pm). The partial substitution of Ca by Sr results in higher solubility compared with solubility in the presence of Sr-free HA owning to unit cell enlargement (Wan et al., 2020). Thus, Sr binds to hyaluronic acid by adsorption on mineral surfaces. Moreover, the surface of Sr-HA is active and biodegradable, which forms bone-like apatite on its surface because of continuous dissolution and precipitation (Kołodziejska et al., 2021). Osteogenesis, the highly regulated process of bone formation, is a complex and crucial aspect of the skeletal system's development and maintenance. It involves the intricate interplay of various cellular components, including osteoblasts and osteoclasts, as well as the deposition and resorption of bone matrix. Many studies have shown that, compared with HA, Sr-HA promotes the osteogenesis of BMSCs, reduces the bone-healing period, and enhances implant bone fusion (Table 2). Furthermore, Sr concentration in the range of 3%–7% stimulates osteoblast activity and differentiation, and the 1% Sr concentration affects osteoblast proliferation (Boanini et al., 2011). These results imply that the doping of the trace element Sr promotes osteoblast activity and differentiation, whereas it inhibits osteoclast differentiation, exerting a potential positive effect in vivo (Gu et al., 2013).
Table 2
Studies on the osteogenic effects of strontium-doped hydroxyapatite (Sr-HA)
| Advances on Sr-HA | Effects on Osteogenesis | Reference |
| Biomimetic mineralized Sr-HA bone defect repair on porous polylactic acid scaffolds | Micro-computed tomography (micro-CT) results revealed that Sr-HA /PLLA porous scaffolds could form more new bone tissues. | (Ge et al., 2018) |
| Application of Sr-HA bioactive bone cement in hip arthroplasty | Sr-HA bioactive bone cement displayed good bioactivity in the goat model of improved hip arthroplasty. | (Ni et al., 2006) |
| Strontium instead of calcium sulfate hydroxyapatite scaffold promotes bone regeneration | Pre-BMSCs, Sr-CSH /HA complex extraction significantly increased cell migration, upregulated the expression of osteogenic marker genes and increased the area of mineralized nodules. | (Chang et al., 2020) |
| Sr-HA promotes osteogenesis on polypropylene fumarate nanocomposite scaffold | Sr-HA scaffold was superior to the normal groups in supporting the adhesion, proliferation, and differentiation of MC3T3-E1 cells. | (Li et al., 2019) |
| Osteogenesis of Sr-HA coating on bone ceramic surface in vitro and in vivo | The area ratio of new bone in the Sr10-TBC group (10 mol% Sr2+ in apatite coating) was significantly higher than that in the normal group. | (Li et al., 2017) |
| 3D printing Sr-HA for repairing rabbit skull defects | Sr-HA had better osteogenic ability and stimulated much more new bone formation within 12 weeks. | (Luo et al., 2018) |
| Sr-HA scaffolds prepared by the SPS technique | The material effectively repaired bone defects and displayed good biodegradable properties. | (Hu et al., 2020) |
| Sr-HA-graft-Poly(γ-benzyl-l-glutamate) nanocomposite microcarriers | Controlled Sr2+ release accelerated bone formation and promoted the repair of bone nonunion. | (Gao et al., 2017) |
| Synthesis of hydroxyapatite co-doped with trace elements Si and Sr | The measurement of osteoblast adhesion and proliferation indicated that, when compared with undoped HA, the osteoblast proliferation ability of Si HA and Si + Sr HA was increased by approximately 1.3 times and 1.8 times, respectively. | (Gao et al., 2016) |
| Effect of Sr-HA coating on bone bonding of implants in low bone mass rats | This finding revealed that 20% strontium coating had the best implant bone integration performance among the tested coatings of osteoporosis rats. | (Tao et al., 2016) |
| Effect of Sr incorporation into HA on osteoblasts in vitro | This study revealed that the incorporation of Sr in HA ceramics enhanced osteoblast differentiation and mineralization. | (Ni et al., 2011) |
| Osteogenesis of rat mesenchymal stem cells and osteoblastic cells on Sr-doped nanohydroxyapatite-coated titanium surfaces | The Sr-HA coating prepared by electrochemical deposition significantly enhanced the adhesion, spreading, and alkaline phosphatase activity of Sr-HA. | (Jiang et al., 2015) |
| Response of osteoprotective cells to Sr-containing HA ceramics | The results revealed that the presence of Sr stimulated the differentiation of OPC1 cells and increased the expression of ALP and OPN. | (Xue et al., 2010) |
| In vivo cancellous bone reconstruction with Sr-HA bioactive bone cement | Sr-HA forms a thick osteoid layer on the surface of bone cement, osteoblasts form along the bone and guide along the surface of bone cement, reflecting the stimulation of Sr-HA. | (Wong et al., 2004) |
| A novel injectable Sr-HA bone cement | Sr-HA bone cement slightly promoted the osteoblastic differentiation of MC3T3 cells, suggesting that Sr-HA bone cement could promote its combination with the surrounding bone. | (Dai et al., 2021a) |
| Incorporation of Sr into biomimetic carbonated calcium-deficient HA-coated carbon cloth: biocompatibility with human primary osteoblasts | The materials displayed a strong affinity with human primary osteoblasts, the incorporation of Sr in the carbonated calcium-deficient HA phase structure had a beneficial role in cell proliferation. | (Olivier et al., 2020) |
| Sr substitution for HA promotes the maturation of human osteoblasts | Qualitative evaluation using primary human osteoblasts exposed to Sr-HA for 28 days showed that, compared with HA, the presence of Sr directly promoted osteoblasts to mature into osteoblasts in vitro. | (Stipniece et al., 2021) |
| Synthesis and characterization of Sr-HA nanoparticles for bone regeneration | Sr-HA nanoparticles may be used to transport Sr to bone tissues and promote its regeneration. | (Frasnelli et al., 2017) |
| Sr sintered calcium sulfate bone graft promotes osteogenesis in a rat femoral defect model | The concentration of Sr2+ below 10-4M had a positive effect on the osteoblastic differentiation of MC3T3E1 cells. | (Ming-Kai et al., 2022) |
Effects of Sr and Sr-HA on blood vessels
Pro-vascularization function of bone repairing biomaterials
Fracture healing is a gradual process of restoring normal bone form and function. It is important to realize that in addition to the development of new bones, the regeneration of bones involves blood vessel remodeling, implying that cells in new bone tissues require nutrients and excrete waste materials via blood vessels; in other words, the ideal bone tissue material should exhibit the function of pro-vascularization (Mao et al., 2009). The endothelial cells are pivotal in angiogenesis; therefore, the prerequisite for biomaterial graft vascularization is good affinity and compatibility with endothelial cells (Chen et al., 2006). Angiogenesis is mainly affected by vascularizing growth factors, and among all known members of vascular endothelial and angiogenic growth factors, vascular endothelial growth factor (VEGF) is the most specific pro-angiogenic factor, which plays a central regulatory role in physiological and pathological angiogenesis (Hirschi and D’Amore, 1997).
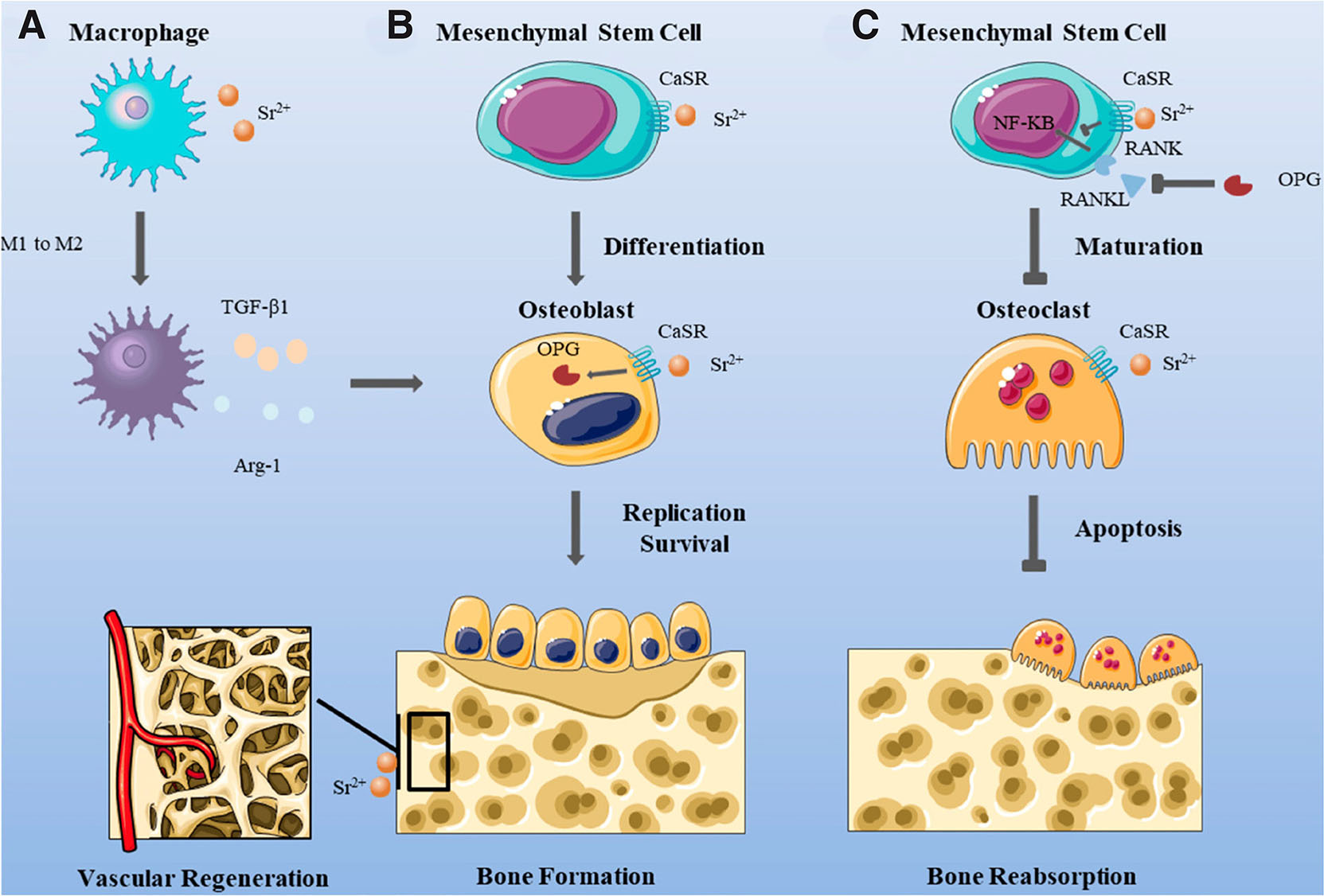
Notably, biomaterials are widely used in the treatment of bone defects and bone regeneration, where immune modulation, especially the abatement of inflammation, and the pro-vascularizing effect of biomaterials are crucial, where macrophages and neutrophils have a key role in the process of tissue-engineered angiogenesis. It is widely accepted that M2 macrophages are associated with promoting angiogenesis, while M1 macrophages have a comparatively weaker effect on angiogenesis (Wang et al., 2017). Li et al., (Li et al., 2021) prepared Sr-HA nanofibrous gelatin scaffolds to study Sr-mediated regulation of neutrophil polarization and subsequent effects on angiogenesis and macrophage polarization. Sr-HA-doped gelatin scaffolds released by Sr were found to polarize neutrophils to an N2 phenotype and act on subsequent macrophages and effector cells, thereby promoting angiogenesis and tissue regeneration. Liu et al., (Liu et al., 2023) summarized the mechanisms associated with Sr in bone regeneration (Fig. 7): Sr induces macrophage differentiation and promotes vascular regeneration.
Fig. 7. Strontium (Sr) induces macrophages to differentiate toward M2 instead of M1, which benefits the promotion of osteoblast proliferation; Sr also raises early vascular regeneration.
(A) Sr induces macrophage differentiation towards M2 not M1, which is conducive to promoting osteoblast proliferation, and Sr also promotes early vascular regeneration. (B) Sr promotes the differentiation of mesenchymal stem cell and the proliferation of osteoblasts, which is conducive to bone formation. (C) Sr can inhibit the differentiation of mesenchymal stem cell and the proliferation of osteoclasts, thus reducing bone resorption. Source: Liu et al., 2023. Licensed under CC BY 4.0.
Mature osteoblasts and endothelial cells can express VEGF and basic fibroblast growth factor (bFGF). Sr-HA signaling increases VEGF and bFGF levels, which play a crucial role in the vascularization of the material. VEGF promotes blood vessel development by inducing endothelial cell proliferation and participates in bone development via pro-angiogenesis. Furthermore, it is involved in bone formation and metabolism as a paracrine factor. BFGF promotes the proliferation and differentiation of as well as protein synthesis in its own and surrounding cells in autocrine and paracrine ways (Hu and Olsen, 2016). Therefore, Sr-HA with pro-vascularization function can be used as a potential material, providing a new idea to solve the problem associated with vascularization in bone tissue engineering.
The role of Sr and Sr-HA in vascularization
It has been reported that bioactive ions released by some bone repair materials have an important role in angiogenesis during bone regeneration. We also found that both Sr and Sr-doped materials have an effect on angiogenesis in bone defects or osteoporotic bone regeneration. Mao et al., (Mao et al., 2017) investigated the role of Sr ions, as well as other ions, in bioceramic materials, and showed that Sr ions enhance angiogenesis and inhibit osteoblast formation. Liu et al., (Liu et al., 2021) selected strontium-substituted calcium silicate (Sr-CS) in their study and systematically investigated the biological functions of BMSCs-derived exosomes after Sr-CS stimulation. The results showed that Sr-CS significantly promoted in vitro angiogenesis of human umbilical vein endothelial cells. HA has been found to be biologically active and biocompatible under both in vitro and in vivo conditions, which is an advantageous material for bone repair (Ramesh et al., 2018). Thus, investigating the role of HA in vascularization and related mechanisms is essential. Sr addition into HA can improve the osteogenic capability of HA. With the excellent biocompatibility and osteoconductivity of HA, whereas Sr exhibits osteoinductive properties (Yedekçi et al., 2021); therefore, doping Sr greatly improves the osteoinductive characteristics of Sr-HA. The early development of several blood vessels at the implantation site of Sr-doped biological tissue engineering materials increases local nutrient supply and ion exchange, facilitates material degradation, and draws osteoblasts from the blood circulation to initiate osteogenesis. Cui et al., (Cui et al., 2022) prepared a novel biomimetic bone scaffold containing decellularized small intestinal submucosal matrix (SIS-ECM) and Sr2+/Fe3+ co-doped hydroxyapatite (SrFeHA), and a series of in vitro and in vivo experiments were performed to reveal that the composites had sufficiently strong vasculogenic properties, and these positive results confirmed that the incorporation of Sr-HA enhanced the angiogenic effect. The incorporation of 10 mol% of Sr-HA significantly promoted angiogenesis by promoting cell proliferation, migration and angiogenic differentiation.
It was found that granular Sr-HA was effective in promoting the formation of bone tissue and blood vessels. Bai et al., (Bai et al., 2018) deposited Sr-HA nanostructures onto the titanium foil surface, changed its morphology (from granular to short rods) by adjusting heating time, and verified its osteogenic and angiogenic effects in vitro and in vivo. The results showed that Sr in the 1% Sr-doped Ca polyphosphate material degradation solution remarkably promoted the secretion of the vascularization factor matrix metalloproteinase 2, a Zn-containing protease that degrades extracellular matrix components and the basement membrane and is a crucial pro-angiogenic factor that strongly promotes the outgrowth of new capillaries to form vascular networks. In terms of osteogenesis and angiogenesis can be found, Sr-HA is full of great potential in bone repair materials and may become a promising alternative for new bone tissue engineering.
Conclusions
Herein, we systematically review Sr-HA preparation and provide insights into the effects of implanted Sr-HA and Sr on osteogenesis and angiogenesis. Among the four preparation methods mentioned, the chemical precipitation method yields more efficient and stable materials; thus, it is the most widely used method. Additionally, Sr promotes osteogenesis by inhibiting osteoclast differentiation and promoting osteoblast differentiation. Hence, a better osteogenic effect can be achieved by adding Sr to HA. The formation and reconstruction of blood vessels play a vital role in bone healing. Sr-HA increases VEGF and BFGF levels noticeably; thus, Sr-HA can be used as a potent pro-angiogenic material. However, the involvement of inflammation and angiogenesis in bone healing leads to the complex mechanism of Sr-HA-mediated osteogenesis. Therefore, the specific osteogenic mechanism of Sr-HA needs further elucidation to maximize the effect of HA and Sr on osteogenesis.
Acknowledgements
This work was supported by Natural Science Foundation of Sichuan Province, China (2022NSFSC1510), Medical Scientific Research Project of Chengdu City, China (2021043), the Industry-university Cooperative education program of Ministry of Education, China (220506007260254), the Innovation team project of Clinical Medical college and Affiliated hospital of Chengdu University (CDFYCX202208), Sichuan Provincial Science and Technology Foundation (22NZZH0031), Higher education talent training quality and teaching reform project of education department of Sichuan province, China (JG2021-1102). The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Abbreviations
BMSC, bone marrow-derived stromal cell ; Sr-HA, strontium-doped hydroxyapatite ;References
Agrawal S., Kelkar M., De A., Kulkarni A. R., Gandhi M. N. (2018). Newly emerging mesoporous strontium hydroxyapatite nanorods: microwave synthesis and relevance as doxorubicin nanocarrier. Journal of Nanoparticle Research 20: 230.
Almeida M. M., Nani E. P., Teixeira L. N., Peruzzo D. C., Joly J. C., Napimoga M. H., Martinez E. F. (2016). Strontium ranelate increases osteoblast activity. Tissue and Cell 48: 183-188.
Bai L., Liu Y., Du Z., Weng Z., Yao W., Zhang X., Huang X., Yao X., Crawford R., Hang R., Huang D., Tang B., Xiao Y. (2018). Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomaterialia 76: 344-358.
Barbara A., Delannoy P., Denis B.G., Marie P.J. (2004). Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 53: 532-537.
Baron R., Tsouderos Y. (2002). In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. European Journal of Pharmacology 450: 11-17.
Berg C., Unosson E., Engqvist H., Xia W. (2020). Amorphous Calcium Magnesium Phosphate Particles for Treatment of Dentin Hypersensitivity: A Mode of Action Study. ACS Biomaterials Science & Engineering 6: 3599-3607.
Bigi A., Falini G., Foresti E., Gazzano M., Ripmonti A., Roveri N. (1996). Rietveld structure refinements of calcium hydroxylapatite containing magnesium. Acta Crystallographica Section B Structural Science 52: 87-92.
Boanini E., Gazzano M., Bigi A. (2010). Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomaterialia 6: 1882-1894.
Boanini E., Torricelli P., Fini M., Bigi A. (2011). Osteopenic bone cell response to strontium-substituted hydroxyapatite. Journal of Materials Science: Materials in Medicine 22: 2079-2088.
Bonnelye E., Chabadel A., Saltel F., Jurdic P. (2008). Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42: 129-138.
Borciani G., Ciapetti G., Vitale-Brovarone C., Baldini N. (2022). Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View. Materials (Basel) 15: 1724.
Brennan T.C., Rybchyn M.S., Green W., Atwa S., Conigrave A.D., Mason R.S. (2009). Osteoblasts play key roles in the mechanisms of action of strontium ranelate. British Journal of Pharmacology 157: 1291-1300.
Capuccini C., Torricelli P., Sima F., Boanini E., Ristoscu C., Bracci B., Socol G., Fini M., Mihailescu I.N., Bigi A. (2008). Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomaterialia 4: 1885-1893.
Catros S., Guillemot F., Lebraud E., Chanseau C., Perez S., Bareille R., Amédée J., Fricain J.C. (2010). Physico-chemical and biological properties of a nano-hydroxyapatite powder synthesized at room temperature. IRBM 31: 226-233.
Chang H., Xiang H., Yao Z., Yang S., Tu M., Zhang X., Yu B. (2020). Strontium-substituted calcium sulfate hemihydrate/hydroxyapatite scaffold enhances bone regeneration by recruiting bone mesenchymal stromal cells. Journal of Biomaterials Applications 35: 97-107.
Chen Y., Shi G., Qin Y., Ding Y., Yu X., Zhang X., Wan C., (2006). Microstructure, in vitro degradation properties, and vascular endothelial cell compatibility of strontium-doped calcium polyphosphate. Advanced Engineering Sciences 98-103.
Cui W., Yang L., Ullah I., Yu K., Zhao Z., Gao X., Liu T., Liu M., Li P., Wang J., Guo X. (2022). Biomimetic porous scaffolds containing decellularized small intestinal submucosa and Sr 2+ /Fe 3+ co-doped hydroxyapatite accelerate angiogenesis/osteogenesis for bone regeneration . Biomedical Materials 17: 025008.
Dahl S.G., Allain P., Marie P.J., Mauras Y., Boivin G., Ammann P., Tsouderos Y., Delmas P.D., Christiansen C. (2001). Incorporation and distribution of strontium in bone. Bone 28: 446-453.
Dai J., Fu Y., Chen D., Sun Z. (2021a). A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Materials Science and Engineering: C 124: 112052.
Dai L. L., Mei M. L., Chu C. H., Lo E. C. M. (2021b). Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. Journal of Dentistry 108: 103633.
Dapporto M., Tavoni M., Restivo E., Carella F., Bruni G., Mercatali L., Visai L., Tampieri A., Iafisco M., Sprio S. (2022). Strontium-doped apatitic bone cements with tunable antibacterial and antibiofilm ability. Frontiers in Bioengineering and Biotechnology 10: 969641.
Deville S., Saiz E., Nalla R. K., Tomsia A. P. (2006). Freezing as a Path to Build Complex Composites. Science 311: 515-518.
Donazzon B., Dechambre G., Lacout J. (1998). Calcium-strontium hydroxyapatite: Hydrothermal preparation. Annales de Chimie Science des Mat�riaux 23: 53-56.
Ehret C., Aid-Launais R., Sagardoy T., Siadous R., Bareille R., Rey S., Pechev S., Etienne L., Kalisky J., de Mones E., Letourneur D., Amedee Vilamitjana J. (2017). Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLOS ONE 12: e0184663.
Frangopol P. T., Mocanu A., Almasan V., Garbo C., Balint R., Borodi G., Bratu I., Hoovitz O., Tomoaia-Cotisel M., (2016). Synthesis and structural characterization of strontium substituted hydroxyapatites. Revista de Chimie 61: 337-344.
Frasnelli M., Cristofaro F., Sglavo V. M., Dirè S., Callone E., Ceccato R., Bruni G., Cornaglia A. I., Visai L. (2017). Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Materials Science and Engineering: C 71: 653-662.
Gao J., Wang M., Shi C., Wang L., Wang D., Zhu Y. (2016). Synthesis of trace element Si and Sr codoping hydroxyapatite with non-cytotoxicity and enhanced cell proliferation and differentiation. Biological Trace Element Research 174: 208-217.
Gao L., Huang Z., Yan S., Zhang K., Xu S., Li G., Cui L., Yin J. (2017). Sr-HA- graft -Poly(γ-benzyl- l -glutamate) Nanocomposite Microcarriers: Controllable Sr 2+ Release for Accelerating Osteogenenisis and Bony Nonunion Repair . Biomacromolecules 18: 3742-3752.
Ge M., Ge K., Gao F., Yan W., Liu H., Xue L., Jin Y., Ma H., Zhang J. (2018). Biomimetic mineralized strontium-doped hydroxyapatite on porous poly(L-lactic acid) scaffolds for bone defect repair. International Journal of Nanomedicine Volume 13: 1707-1721.
Geng Z., Wang R., Li Z., Cui Z., Zhu S., Liang Y., Liu Y., Huijing B., Li X., Huo Q., Liu Z., Yang X. (2016). Synthesis, characterization and biological evaluation of strontium/magnesium-co-substituted hydroxyapatite. Journal of Biomaterials Applications 31: 140-151.
Gu Z., Zhang X., Li L., Wang Q., Yu X., Feng T. (2013). Acceleration of segmental bone regeneration in a rabbit model by strontium-doped calcium polyphosphate scaffold through stimulating VEGF and bFGF secretion from osteoblasts. Materials Science and Engineering: C 33: 274-281.
Gutsalova A. A., Fedorishin D. A., Lytkina D. N., Kurzina I. A. (2021). Bioactive materials for bone regeneration based on zinc-modified hydroxyapatite. Mendeleev Communications 31: 382-384.
Hassan M., Khaleel A., Karam S. M., Al-Marzouqi A. H., ur Rehman I., Mohsin S. (2023). Bacterial Inhibition and Osteogenic Potentials of Sr/Zn Co-Doped Nano-Hydroxyapatite-PLGA Composite Scaffold for Bone Tissue Engineering Applications. Polymers 15: 1370.
He J., Hu X., Cao J., Zhang Y., Xiao J., Peng J., Chen D., Xiong C., Zhang L. (2021). Chitosan-coated hydroxyapatite and drug-loaded polytrimethylene carbonate/polylactic acid scaffold for enhancing bone regeneration. Carbohydrate Polymers 253: 117198.
He L.Y., Zhang X.M., Liu B., Tian Y., Ma W.H. (2016). Effect of magnesium ion on human osteoblast activity. Brazilian Journal of Medical and Biological Research 49: e5257.
Hirschi K. K., D’Amore P. A. (1997). Control of angiogenesis by the pericyte: Molecular mechanisms and significance. In Regulation of Angiogenesis. (Ed. Goldberg Itzhak D., Rosen Eliot M.) Birkhäuser Basel, Basel.
Hu B., Meng Z.D., Zhang Y.Q., Ye L.Y., Wang C.J., Guo W.C. (2020). Sr-HA scaffolds fabricated by SPS technology promote the repair of segmental bone defects. Tissue and Cell 66: 101386.
Hu K., Olsen B. R. (2016). Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. Journal of Clinical Investigation 126: 509-526.
Hu W., Ma J., Wang J., Zhang S. (2012). Fine structure study on low concentration zinc substituted hydroxyapatite nanoparticles. Materials Science and Engineering: C 32: 2404-2410.
Huang D., Zhao F., Gao W., Chen X., Guo Z., Zhang W. (2020). Strontium-substituted sub-micron bioactive glasses inhibit ostoclastogenesis through suppression of RANKL-induced signaling pathway. Regenerative Biomaterials 7: 303-311.
Jarcho M., Kay J. F., Gumaer K. I., Doremus R. H., Drobeck H. P., (1977). Tissue, cellular and subcellular events at a bone-ceramic hydroxylapatite interface. Journal of bioengineering 1: 79-92.
Jiang Q.H., Gong X., Wang X.X., He F.M. (2015). Osteogenesis of Rat Mesenchymal Stem Cells and Osteoblastic Cells on Strontium-Doped Nanohydroxyapatite-Coated Titanium Surfaces. The International Journal of Oral & Maxillofacial Implants 30: 461-471.
Kishi S., Yamaguchi M. (1994). Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochemical Pharmacology 48: 1225-1230.
Kołodziejska B., Stępień N., Kolmas J. (2021). The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. International Journal of Molecular Sciences 22: 6564.
Kutikov A. B., Skelly J. D., Ayers D. C., Song J. (2015). Templated Repair of Long Bone Defects in Rats with Bioactive Spiral-Wrapped Electrospun Amphiphilic Polymer/Hydroxyapatite Scaffolds. ACS Applied Materials & Interfaces 7: 4890-4901.
Landi E., Logroscino G., Proietti L., Tampieri A., Sandri M., Sprio S. (2008). Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. Journal of Materials Science: Materials in Medicine 19: 239-247.
Landi E., Tampieri A., Celotti G., Sprio S., Sandri M., Logroscino G. (2007). Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomaterialia 3: 961-969.
Laskus A., Kolmas J. (2017). Ionic Substitutions in Non-Apatitic Calcium Phosphates. International Journal of Molecular Sciences 18: 2542.
Lee N.H., Kang M. S., Kim T.H., Yoon D. S., Mandakhbayar N., Jo S. B., Kim H. S., Knowles J. C., Lee J.H., Kim H.W. (2021). Dual actions of osteoclastic-inhibition and osteogenic-stimulation through strontium-releasing bioactive nanoscale cement imply biomaterial-enabled osteoporosis therapy. Biomaterials 276: 121025.
Li J., Liu X., Park S., Miller A. L., Terzic A., Lu L. (2019). Strontium‐substituted hydroxyapatite stimulates osteogenesis on poly(propylene fumarate) nanocomposite scaffolds. Journal of Biomedical Materials Research Part A 107: 631-642.
Li J., Yang L., Guo X., Cui W., Yang S., Wang J., Qu Y., Shao Z., Xu S. (2017). Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo . Biomedical Materials 13: 015018.
Li M., Wang Y., Liu Q., Li Q., Cheng Y., Zheng Y., Xi T., Wei S. (2013). In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalized graphene oxide. J. Mater. Chem. B 1: 475-484.
Li T., He H., Yang Z., Wang J., Zhang Y., He G., Huang J., Song D., Ni J., Zhou X., Zhu J., Ding M. (2021). Strontium-doped gelatin scaffolds promote M2 macrophage switch and angiogenesis through modulating the polarization of neutrophils. Biomaterials Science 9: 2931-2946.
Li Y., Luo E., Zhu S., Li J., Zhang L., Hu J. (2015). Cancellous Bone Response to Strontium-Doped Hydroxyapatite in Osteoporotic Rats. Journal of Applied Biomaterials & Functional Materials 13: 28-34.
Li Y., Shui X., Zhang L., Hu J. (2016). Cancellous bone healing around strontium‐doped hydroxyapatite in osteoporotic rats previously treated with zoledronic acid. Journal of Biomedical Materials Research Part B: Applied Biomaterials 104: 476-481.
Li Y., Wang W., Han J., Li Z., Wang Q., Lin X., Ge K., Zhou G. (2022). Synthesis of Silver- and Strontium-Substituted Hydroxyapatite with Combined Osteogenic and Antibacterial Activities. Biological Trace Element Research 200: 931-942.
Liu J., Rawlinson S. C.F., Hill R. G., Fortune F. (2016). Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dental Materials 32: 412-422.
Liu L., Yu F., Li L., Zhou L., Zhou T., Xu Y., Lin K., Fang B., Xia L. (2021). Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomaterialia 119: 444-457.
Liu X., Huang H., Zhang J., Sun T., Zhang W., Li Z. (2023). Recent Advance of Strontium Functionalized in Biomaterials for Bone Regeneration. Bioengineering 10: 414.
Luo Y., Chen S., Shi Y., Ma J. (2018). 3D printing of strontium-doped hydroxyapatite based composite scaffolds for repairing critical-sized rabbit calvarial defects. Biomedical Materials 13: 065004.
Mao L., Xia L., Chang J., Liu J., Jiang L., Wu C., Fang B. (2017). The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomaterialia 61: 217-232.
Mao Z., Shi H., Guo R., Ma L., Gao C., Han C., Shen J. (2009). Enhanced angiogenesis of porous collagen scaffolds by incorporation of TMC/DNA complexes encoding vascular endothelial growth factor. Acta Biomaterialia 5: 2983-2994.
Maqbool M., Nawaz Q., Atiq Ur Rehman M., Cresswell M., Jackson P., Hurle K., Detsch R., Goldmann W. H., Shah A. T., Boccaccini A. R. (2021). Synthesis, Characterization, Antibacterial Properties, and In Vitro Studies of Selenium and Strontium Co-Substituted Hydroxyapatite. International Journal of Molecular Sciences 22: 4246.
Ming-Kai H., W. Chi-Yun, W. Chia-Jung, C. Ying-Cen, W. Shinn-Chih, T. Wei-Hsing, L. Po-Liang, (2022). Materials Today Communications 30: 2352-4928.
Nagyné-Kovács T., Studnicka L., Kincses A., Spengler G., Molnár M., Tolner M., Lukács I. E., Szilágyi I. M., Pokol G. (2018). Synthesis and characterization of Sr and Mg-doped hydroxyapatite by a simple precipitation method. Ceramics International 44: 22976-22982.
Neves N., Linhares D., Costa G., Ribeiro C. C., Barbosa M. A. (2017). In vivo and clinical application of strontium-enriched biomaterials for bone regeneration . Bone & Joint Research 6: 366-375.
Ni G.X., Chiu K.Y., Lu W.W., Wang Y., Zhang Y.G., Hao L.B., Li Z.Y., Lam W.M., Lu S.B., Luk K.D.K. (2006). Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 27: 4348-4355.
Ni G.X., Yao Z.P., Huang G.T., Liu W.G., Lu W. W. (2011). The effect of strontium incorporation in hydroxyapatite on osteoblasts in vitro. Journal of Materials Science: Materials in Medicine 22: 961-967.
Olivier F., Rochet N., Delpeux-Ouldriane S., Chancolon J., Sarou-Kanian V., Fayon F., Bonnamy S. (2020). Strontium incorporation into biomimetic carbonated calcium-deficient hydroxyapatite coated carbon cloth: Biocompatibility with human primary osteoblasts. Materials Science and Engineering: C 116: 111192.
Osorio R., Osorio E., Cabello I., Toledano M. (2014). Zinc Induces Apatite and Scholzite Formation during Dentin Remineralization. Caries Research 48: 276-290.
Pan H.B., Li Z.Y., Lam W.M., Wong J.C., Darvell B.W., Luk K.D.K., Lu W.W. (2009). Solubility of strontium-substituted apatite by solid titration. Acta Biomaterialia 5: 1678-1685.
Poddar D., Singh A., Rao P., Mohanty S., Jain P. (2023). Modified-Hydroxyapatite-Chitosan Hybrid Composite Interfacial Coating on 3D Polymeric Scaffolds for Bone Tissue Engineering. Macromolecular Bioscience In Press: e2300243.
Prasad A. S., (1995). Zinc: an overview. Nutrition (Burbank, Los Angeles County, Calif.) 11: 93-99.
Ramesh N., Moratti S. C., Dias G. J. (2018). Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. Journal of Biomedical Materials Research Part B: Applied Biomaterials 106: 2046-2057.
Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A. R. (2006). Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27: 3413-3431.
Rybchyn M. S., Slater M., Conigrave A. D., Mason R. S. (2011). An Akt-dependent Increase in Canonical Wnt Signaling and a Decrease in Sclerostin Protein Levels Are Involved in Strontium Ranelate-induced Osteogenic Effects in Human Osteoblasts. Journal of Biological Chemistry 286: 23771-23779.
Saidak Z., Marie P. J. (2012). Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacology & Therapeutics 136: 216-226.
Shavandi A., Bekhit A. E.D. A., Sun Z. F., Ali A. (2015). A Review of Synthesis Methods, Properties and Use of Hydroxyapatite as a Substitute of Bone. Journal of Biomimetics, Biomaterials and Biomedical Engineering 25: 98-117.
Shi H., Ye X., Zhang J., Wu T., Yu T., Zhou C., Ye J. (2020). A thermostability perspective on enhancing physicochemical and cytological characteristics of octacalcium phosphate by doping iron and strontium. Bioactive Materials 6: 1267-1282.
Stipniece L., Wilson S., Curran J.M., Chen R., Salma-Ancane K., Sharma P.K., Meenan B.J., Boyd A.R. (2021). Strontium substituted hydroxyapatite promotes direct primary human osteoblast maturation. Ceramics International 47: 3368-3379.
Tao Z.S., Bai B.L., He X.W., Liu W., Li H., Zhou Q., Sun T., Huang Z.L., Tu K., Lv Y.X., Cui W., Yang L. (2016). A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Medical & Biological Engineering & Computing 54: 1959-1968.
Turhan E. A., Akbaba S., Tezcaner A., Evis Z. (2023). Boron nitride nanofiber/Zn-doped hydroxyapatite/polycaprolactone scaffolds for bone tissue engineering applications. Biomaterials Advances 148: 213382.
Ungureanu E., Vladescu (Dragomir) A., Parau A. C., Mitran V., Cimpean A., Tarcolea M., Vranceanu D. M., Cotrut C. M. (2023). In Vitro Evaluation of Ag- and Sr-Doped Hydroxyapatite Coatings for Medical Applications. Materials (Basel) 16: 5428.
Walker J., Shadanbaz S., Woodfield T. B. F., Staiger M. P., Dias G. J. (2014). Magnesium biomaterials for orthopedic application: A review from a biological perspective. Journal of Biomedical Materials Research Part B: Applied Biomaterials 102: 1316-1331.
Wan B., Wang R., Sun Y., Cao J., Wang H., Guo J., Chen D. (2020). Building Osteogenic Microenvironments With Strontium-Substituted Calcium Phosphate Ceramics. Frontiers in Bioengineering and Biotechnology 8: 591467.
Wang J., Qian S., Liu X., Xu L., Miao X., Xu Z., Cao L., Wang H., Jiang X. (2017). M2 macrophages contribute to osteogenesis and angiogenesis on nanotubular TiO 2 surfaces . Journal of Materials Chemistry B 5: 3364-3376.
Wong C. T., Lu W. W., Chan W. K., Cheung K. M. C., Luk K. D. K., Lu D. S., Rabie A. B. M., Deng L. F., Leong J. C. Y. (2004). In vivo cancellous bone remodeling on a strontium‐containing hydroxyapatite (sr‐HA) bioactive cement . Journal of Biomedical Materials Research Part A 68A: 513-521.
Xue W., Moore J. L., Hosick H. L., Bose S., Bandyopadhyay A., Lu W.W., Cheung K. M.C., Luk K. D.K. (2010). Osteoprecursor cell response to strontium‐containing hydroxyapatite ceramics. Journal of Biomedical Materials Research Part A 79A: 804-814.
Yamaguchi M., Gao Y. H. (1998). Potent Effect of Zinc Acexamate on Bone Components in the Femoral–Metaphyseal Tissues of Elderly Female Rats. General Pharmacology: The Vascular System 30: 423-427.
Yang C., Yuan G., Zhang J., Tang Z., Zhang X., Dai K. (2010). Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomedical Materials 5: 045005.
Yasukawa A., Ouchi S., Kandori K., Ishikawa T. (1996). Preparation and characterization of magnesium–calcium hydroxyapatites. J. Mater. Chem. 6: 1401-1405.
Yedekçi B., Tezcaner A., Alshemary A. Z., Yılmaz B., Demir T., Evis Z. (2021). Synthesis and sintering of B, Sr, Mg multi-doped hydroxyapatites: Structural, mechanical and biological characterization. Journal of the Mechanical Behavior of Biomedical Materials 115: 104230.
Yu N., Cai S., Wang F., Zhang F., Ling R., Li Y., Jiang Y., Xu G. (2017). Microwave assisted deposition of strontium doped hydroxyapatite coating on AZ31 magnesium alloy with enhanced mineralization ability and corrosion resistance. Ceramics International 43: 2495-2503.
Zarins J., Pilmane M., Sidhoma E., Salma I., Locs J. (2018). Immunohistochemical evaluation after Sr-enriched biphasic ceramic implantation in rabbits femoral neck: comparison of seven different bone conditions. Journal of Materials Science: Materials in Medicine 29: 119.
Zarins J., Pilmane M., Sidhoma E., Salma I., Locs J. (2019). The Role of Strontium Enriched Hydroxyapatite and Tricalcium Phosphate Biomaterials in Osteoporotic Bone Regeneration. Symmetry 11: 229.
Zhan J., Tseng Y.H., Chan J.C.C., Mou C.Y. (2005). Biomimetic Formation of Hydroxyapatite Nanorods by a Single‐Crystal‐to‐Single‐Crystal Transformation. Advanced Functional Materials 15: 2005-2010.
Zhu Z., Jiang H., Zhu Y., Zhang L., Tang S., Zhou X., Fan Y. (2022). Strontium-doped hydroxyapatite as adsorbent effectively to remove lead ions from water. Environmental Science and Pollution Research 29: 81063-81075.