Int. J. Dev. Biol. 66: 115 - 124 (2022)
Special Issue: Developmental Biology in Greece
On the role of pleiotrophin and its receptors in development and angiogenesis
Review | Published: 20 September 2021
Abstract
The secreted growth factor pleiotrophin (PTN) is expressed in all species and is evolutionarily highly conserved, suggesting that it plays a significant role in the regulation of important processes. The observation that it is highly expressed at early stages during development and in embryonic progenitor cells highlights a potentially important contribution to development. There is ample evidence of the role of PTN in the development of the nervous system and hematopoiesis, some, albeit inconclusive, evidence of its role in the skeletomuscular system, and limited evidence of its role in the development of other organs. Studies on its role in the cardiovascular system and angiogenesis suggest that PTN has a significant regulatory effect by acting on endothelial cells, while its role in the functions of smooth or cardiac muscle cells has not been studied. This review highlights what is known to date regarding the role of PTN in the development of various organs and in angiogenesis. Wherever possible, evidence on the crosstalk between the receptors that mediate PTN’s functions is also quoted, highlighting the complex regulatory pathways that affect development and angiogenesis.
Keywords
angiogenesis, development, growth factors, pleiotrophin, receptors
Introduction
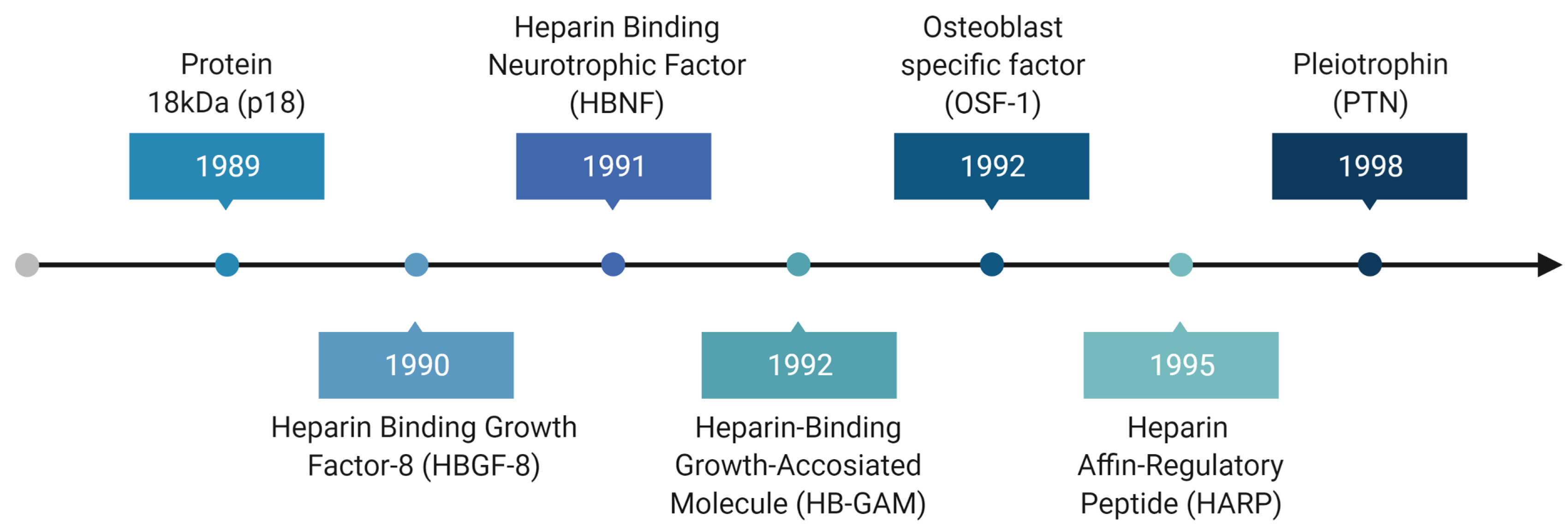
The secreted growth factor pleiotrophin (PTN) was initially isolated from bovine uterus and fetal mouse brain by different groups in 1989. It was given several different names, but the one that dominated and is currently being universally used is PTN (Fig. 1).
PTN is highly conserved among species and shows high amino acid homology with midkine (MK), the other member of the same family of heparin-binding proteins. The mature peptide consists of 136 amino acids, following cleavage of the signal peptide, and contains 24% cationic amino acids mainly at the amino- and carboxy-terminals, as well as 10 cysteines involved in the formation of 5 disulfide bonds. PTN comprises of two β-structures linked by a flexible region. Each β-structure contains three antiparallel β-folds, homologous to the thrombospondin type I repeat (TSR-1) motif. The ends of the molecule have numerous basal amino acids and lack configuration. The carboxy terminus of PTN has been shown to be responsible for its interaction with chondroitin sulfate chains. The human ptn gene has been identified on chromosome 7, in arm q33, is at least 42 kb and contains 7 exons. The open reading frame includes 4 exons, mostly exons 3 and 4, and the borders between introns/exons are well maintained. Different PTN isoforms have been suggested but have not been studied. Despite high homology in the coding region, the 3΄ and 5΄ untranslated regions differ between species. The promoter of the human ptn gene contains response elements for myogenic differentiation factor 1, trihelix transcription factor GT-1, activator protein 1, serum, homeobox A5 and Sox10. Possible binding sites for nuclear receptor κB, cAMP binding protein, serum response factor and retinoic acid have been suggested but not proved (revised in Papadimitriou et al., 2009; 2016). In relation to the latter, experimental evidence suggests that PTN expression is not enhanced by retinoic acid (Li et al., 1992) and this notion is supported by our data showing that all-trans retinoic acid inhibits PTN expression (Theodorakopoulou et al., 2006).
PTN receptors
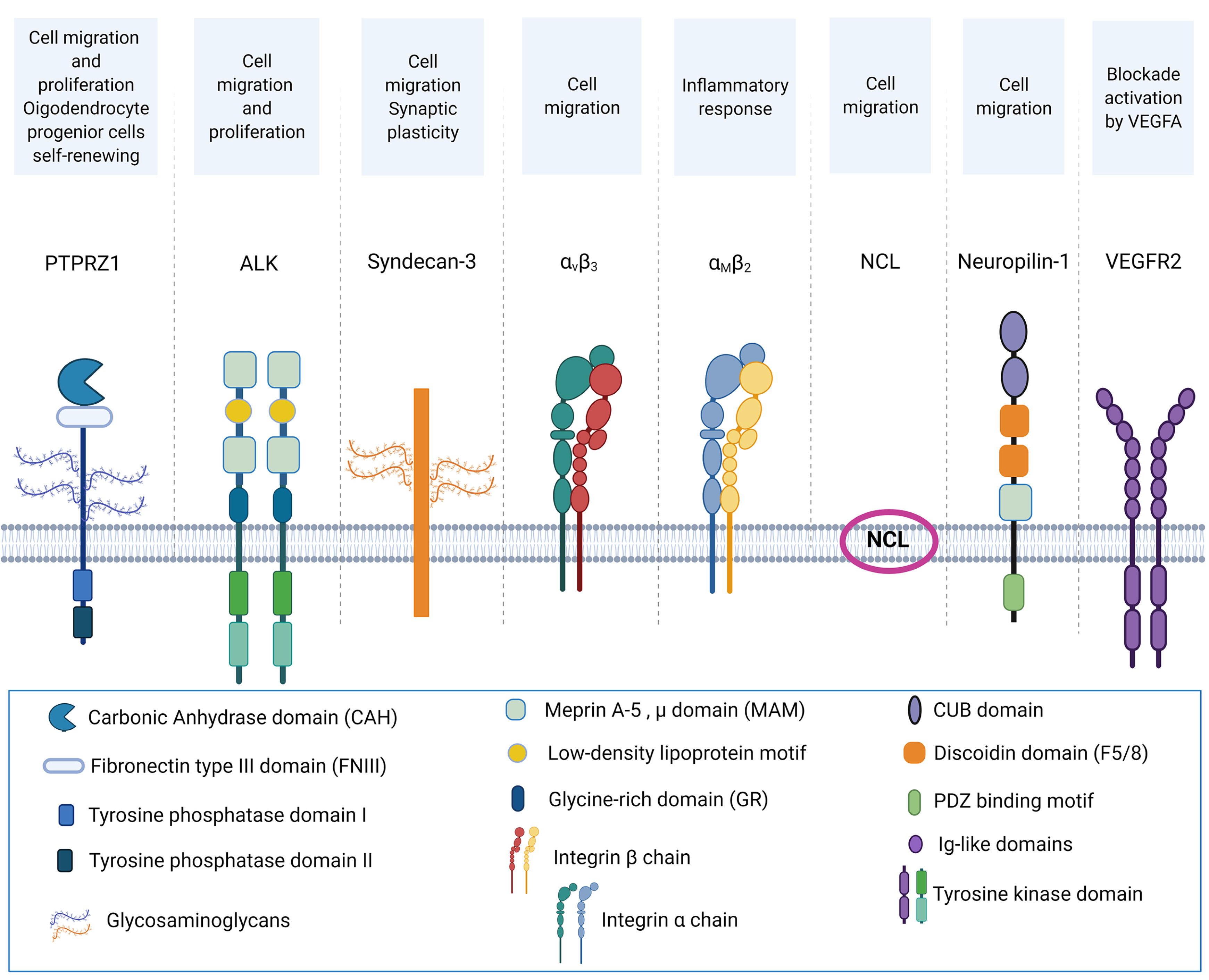
PTN has been shown to act through numerous receptors (Fig. 2). The first molecule identified as a functional PTN receptor in brain neurons was N-syndecan or syndecan-3, SDC3), which was found to interact with PTN in a solid phase binding assay. Using a similar assay, PTN was also found to interact with SDC1 in a heparan sulfate-dependent manner. It was later shown that the chondroitin sulphate chains on SDC1 and SDC4 are involved in their interaction with PTN, while the core protein of SDC1 may also play a role. A cooperative action of both TSR-1 domains of PTN is required for its interaction with the heparan sulphate chains of SDC3 and the regulation of synaptic plasticity. Despite these studies showing interaction of PTN with different syndecans, only interaction with SDC3 has been linked to PTN functions, as discussed later (revised in Papadimitriou et al., 2009; 2016; Pantazaka and Papadimitriou, 2014).
Fig. 2. Schematic representation of the known PTN receptors and their proven biological effects upon PTN binding.
All shown receptors are functional PTN receptors that mainly affect the PTN-mediated regulation of migration of different types of cells. SDC1 and SDC4 have been shown to interact with PTN but there are no data on their involvement in any PTN actions; therefore, they are not presented in this figure. Data on ALK as a PTN receptor are conflicting (see text), but PTN may activate ALK through PTPRZ1 and it seems that ALK mediates some of PTN’s actions. NCL conformation and exact mode of localization on the cell surface is not known. The figure was created with BioRender.com.
Another promptly identified PTN receptor in the nervous system is receptor protein tyrosine phosphatase zeta 1 (PTPRZ1). PTN binds to high and low-affinity sites that involve both the chondroitin sulphate chains and the protein core of PTPRZ1. The carboxy-terminal PTN domain seems to be involved in the interaction with the PTPRZ1 chondroitin sulphate structures, which differ during development or in pathologies (revised in Pantazaka and Papadimitriou, 2014; Papadimitriou et al., 2016). Binding of PTN to PTPRZ1 increases tyrosine phosphorylation of numerous downstream molecules, such as c-Src and Fyn kinases, β-catenin, β-adducin, ανβ3 integrin, focal adhesion kinase, phosphoinositide 3-kinase and activation of protein kinase C alpha, beta or delta, ERK1/2 kinases (reviewed in Papadimitriou et al., 2009; 2016) and cyclin dependent kinase 5 (Lampropoulou et al., 2018). It was initially suggested that binding of PTN to PTPRZ1 inhibits the phosphatase activity of the receptor through dimerization/oligomerization (revised in Papadimitriou et al., 2016), and in response to the doubts with regard to how the highly negatively charged chondroitin sulphate moieties on PTPRZ1 could allow dimerization, it has been suggested more recently that the positively charged ligand PTN may neutralize electrostatic repulsion between chondroitin sulphate chains, thus inducing PTPRZ1 clustering, an effect also observed after removal of the PTPRZ1chondroitin sulphate chains (Kuboyama et al., 2016). However, this point requires further study and clarification, since it is also possible that the PTPRZ1 downstream signalling results from the regulation of tyrosine kinase receptors autophosphorylation by PTPRZ1. Such a mechanism has been described for the neuregulin receptor ErbB4 that interacts with PTPRZ1 through postsynaptic density-95 and results in decreased tyrosine phosphorylation of ErbB4 (Fujikawa et al., 2007). Similarly, PTPRZ1 has been shown to dephosphorylate anaplastic lymphoma kinase (ALK) autophosphorylation sites, an effect that may be inhibited upon PTN binding to PTPRZ1 or following PTPRZ1 deletion or tyrosine phosphatase inhibition, leading to ALK activation (Xia et al., 2019; Ntenekou et al., 2020).
ALK has been suggested as a PTN receptor in ligand-receptor binding studies performed in cell-free assays and in intact cells, in which PTN was shown to bind to the ALK extracellular domain. Although direct PTN binding to ALK has been questioned by numerous studies, ALK seems to be activated by PTN and to be involved in PTN’s signaling and actions. It has been suggested that PTN may activate ALK through PTPRZ1 (Papadimitriou et al., 2016; Xia et al., 2019) but the exact pathway requires further study.
Another cell surface protein that was identified at an early stage and has been discussed as a low affinity PTN receptor is nucleolin (NCL). NCL is a ubiquitous nucleolar protein that regulates several aspects of the DNA and RNA metabolism, ribosome assembly and the development of various tissues and organs. NCL is present on the surface of activated endothelial and cancer cells following ανβ3 integrin activation by PTN. Cell surface NCL interacts with ανβ3 and PTPRZ1 and seems to affect both PTN signaling and its translocation to the nucleus (Koutsioumpa and Papadimitriou, 2014; Papadimitriou et al., 2016) through mechanisms that remain unclear.
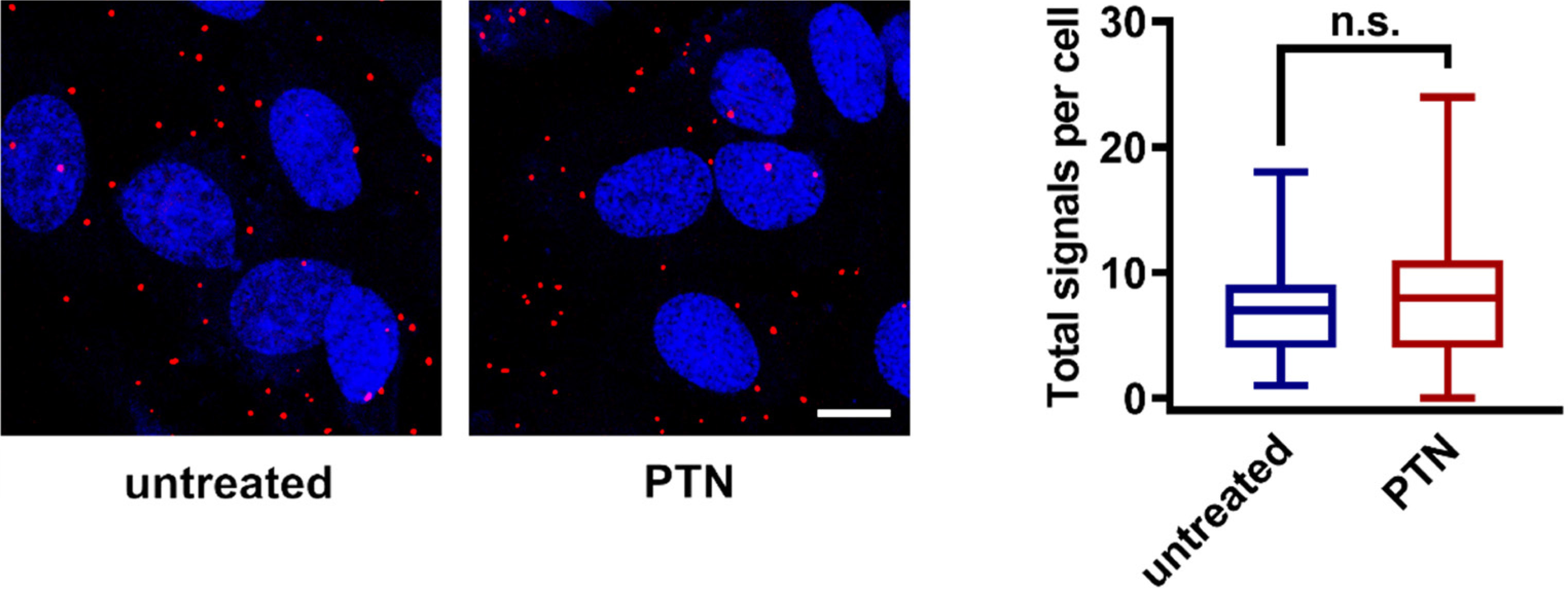
Integrins are heterodimeric cell-membrane receptors that mediate cell-cell interactions and adhesion between the extracellular matrix and the cytoskeleton and play important roles during development (Maartens and Brown, 2015). Integrins that have been characterized as PTN receptors are αvβ3 on endothelial cells (Mikelis et al., 2009) and αMβ2 on leukocytes (Shen et al., 2017), mediating functions of PTN related to angiogenesis and inflammatory responses, respectively. PTN interacts with the extracellular domain of the β3 integrin subunit through its carboxy-terminal domain (Mikelis et al., 2011), and with the α5-β5 loop of the αMI-domain through its amino-terminal domain (Feng et al., 2021). In endothelial cells, ανβ3 interacts with PTPRZ1 even in the absence of PTN, and this interaction is not affected by PTN (Mikelis et al., 2009 and Fig. 3), although PTN induces PTPRZ1-dependent β3 Tyr774 phosphorylation (Mikelis et al., 2009).
Fig. 3. PTPRZ1 interacts with ανβ3 in endothelial cells independently of PTN.
Serum-starved human umbilical vein endothelial cells were incubated with PTN (100 ng/ml) for 10 min. Formation of β3-PTPRZ1 complexes as evidenced by in situ proximity ligation assay are shown on the left. The box plots shown on the right indicate the median, mean and range of the detected signals (n = 10-12 image fields with 4-5 cells per image per sample type, each sample run in duplicate). Details on the methodology used can be found at Koutsioumpa et al., 2015. Scale bar corresponds to 10 μm.
Neuropilin-1, a receptor for semaphorins that has significant role(s) in embryonic development of the nervous and the vascular system (Jubb et al., 2012) has been shown to bind PTN. This interaction requires the TSR-1 domains of PTN and leads to ERK1/2 activation and enhancement of endothelial cell migration (Elahouel et al., 2015), but has not been studied further.
More recently, we have shown that PTN selectively binds to vascular endothelial growth factor receptor 2 (VEGFR2) in endothelial and other types of cells, and inhibits vascular endothelial growth factor A (VEGFA)-induced VEGFR2 phosphorylation at Tyr1175 (Lamprou et al., 2020).
Expression and functions of PTN and its receptors during development
Without a doubt, the highest PTN expression is observed in the nervous system, the pituitary, the heart, the eye, the placenta, the seminal tissue and the testis, the bladder, and the stomach. PTPRZ1 is significantly expressed in the nervous system, the skin, and the eye. They are both highly expressed in induced pluripotent stem cells and embryonic stem cells (Papadimitriou et al., 2016), suggesting important role(s) in early development. However, only in few cases has a causal relationship between PTN and its receptors been linked to specific functions.
Nervous system
Since its discovery, PTN has been implicated in brain development and maturation. During development, it is highly expressed in the nervous system at sites of active mitogenesis, initially in the developing neuroepithelium and later in both glial cells and neurons in developing axonal tracts. In zebrafish embryos, overexpression of PTN induces extensive neurite outgrowth with complicated branching patterns. In the perinatal period, it is expressed by neurons, astrocytes and oligodendrocytes and has been characterized as a potent stimulator of neurite outgrowth in central nervous system neurons (revised in Winkler and Yao, 2004; González-Castillo et al., 2015) and in spiral ganglion neurons (Bertram et al., 2019). A recent study showed that neural stem cells secrete PTN into the niche and that this contributes to the newborn neurons’ maturation (Tang et al., 2019). In adults, PTN expression is limited to specific neuronal subpopulations in the brain cortex, the hippocampus, the cerebellum, and the olfactory bulb (González-Castillo et al., 2015).
A specific role of PTN has been described in relation to dopaminergic neurons. It is highly expressed in neural stem cells of mouse ventral mesencephalon and promotes the production of dopaminergic neurons (González-Castillo et al., 2015). It has also been shown to induce the differentiation of human umbilical cord mesenchymal stem cells into dopaminergic neuron-like cells (Yang et al., 2013), and in combination with stromal cell-derived factor 1, insulin-like growth factor 2, and ephrin B1, to promote the differentiation of human embryonic stem cells to functional tyrosine hydroxylase-positive neurons (Vazin et al., 2009). PTN is overexpressed in neurodegenerative diseases and confers a protective and/or nourishing effect on dopaminergic neurons in vivo and in vitro (Mourlevat et al., 2005). The effects of PTN on the development of the nigrostriatal dopaminergic pathway are shown to be mediated by SDC3 and PTPRZ1 (González-Castillo et al., 2015).
PTPRZ1 during brain development is expressed mainly to the ventricular and subventricular zone (Levy et al., 1993), on both neuronal and glial cells (Canoll et al., 1993). Ptprz1-knockout mice show neurological abnormalities and differences in behavior and learning, including increased exploratory activities to novelty, deficits in spatial and contextual learning, and reduced responses to methamphetamine (Tanga et al., 2019). PTN also maintains the self-renewing phenotype of oligodendrocyte progenitor cells through PTPRZ1 (McClain et al., 2012), which has made a significant contribution to the survival and recovery of oligodendrocytes from demyelinating disease (Harroch et al., 2002). More recently, PTPRZ1 has been shown to be important for the perineuronal net structures that are important for both development and plasticity of the brain in the adult (Eill et al., 2020).
PTN has been shown to mediate the neurite stimulating activity of the peptide Y-P30 by binding to SCD2 and SDC3 (Landgraf et al., 2008). Phosphacan, the alternatively spliced extracellular PTPRZ1 domain, inhibits neurite outgrowth (Margolis et al., 1996), and PTN has been shown to convert the neurite growth inhibitory effect of chondroitin sulfate proteoglycans into an activating effect (Paveliev et al., 2016). The expression pattern of PTN, PTPRZ1 and SDC3 in the postnatal mouse cerebellum appears to confirm their involvement in the morphogenesis of Purkinje cells, as well as in the control of the granule cell migration (Basille-Dugay et al., 2013), while disruption of PTN distribution extracellularly alters the development and function of the neuronal circuits of the cerebellum (Hamza et al., 2016). Besides the central nervous system, PTN is highly expressed in the peripheral nervous system during development and seems to promote the repair of neurons after injury of peripheral nerves. It may also have a significant role in muscle innervation, based on the observations that it is present at the neuromuscular junction and contributes to acetylcholine receptors clustering (revised in Jin et al., 2009).
The effects of PTN on neural development and neurite outgrowth have also been shown to be mediated by ALK (Yanagisawa et al., 2010). ALK has been implicated in the nervous system development, signaling during neuromuscular junction development in C. elegans, affecting the establishment of iridophores and normal pigmentation patterns in zebrafish, and neurogenesis in mice. It is highly expressed during embryonic mouse and chick development, in both the central and peripheral nervous system, while ALK expression levels are decreased after birth (revised in Kalamatianos et al., 2018). Whether any of these functions are related to PTN has not been studied.
Muscle
Outside the nervous system, soon after its discovery, PTN was found to be expressed in human intestinal smooth muscle cells (Li et al., 1992) and in satellite cells (Wanaka et al., 1993). However, although a potential role of PTN in myogenesis was suspected at an early stage, the first evidence came from studies showing that PTN mRNA and protein expression are increased during myogenesis and regeneration after crushing, in newly formed myotubes and in activated myoblasts before fusion in vivo. In vitro, PTN expression increases during the differentiation process, being maximal on fusion of myoblasts into myotubes (Caruelle et al., 2004).
PTN expression is found to increase during postnatal heart development and PTN increases postnatal cardiomyocytes’ DNA synthesis (Chen et al., 2004), suggesting that PTN may have a role in myocardial development and regeneration. PTN overexpression in slow-twitch soleus muscle leads to an increased number of pure type 1 fibers and decreased number of pure type 2A and 2X fibers, as well as increased sulfonylurea receptor 1, citrate synthase and cycloxygenase IV gene expression and increased vascularization, suggesting a shift toward a more oxidative metabolism and improved muscle performance (Camerino et al., 2013). The role of PTN receptors in heart development has not been studied. We have unpublished evidence using two different animal models, as well as human data showing that PTPRZ1 is expressed in the embryonic but not in the adult heart and plays a role in heart morphogenesis and subsequent function (Katraki-Pavlou et al., unpublished).
As is also mentioned above, PTN is a muscle protein present at the neuromuscular junction in close contact with the acetylcholine receptors and may have a significant role in muscle innervation (Jin et al., 2009).
Skeletal system
A role of PTN in bone development was suggested at an early stage on the basis of studies showing expression of its mRNA during bone growth in ancestral cartilage and pulp. PTN has initially been considered important for new bone formation on the basis of observations that it induces osteoblast attachment to the extracellular bone material through binding to a specific receptor, which may be SDC3. It was also shown to induce hypertrophy during the chondrogenetic differentiation of human bone marrow progenitor cells, an effect that has been associated with both ALK and PTPRZ1. PTN enhances the release of heparin-binding epidermal growth factor, which then activates its receptor in precursor osteoblasts and osteoblast-type cells, increases alkaline phosphatase activity and inhibits dexamethasone-induced cell death. PTN overexpressing transgenic mice develop a phenotype characterized by increased bone growth and higher salt and bone density. When PTN is overexpressed solely in the mouse skeletal system, increased bone length and maturation are observed at the early stages of bone development, a balanced phenomenon in adult life. PTN-deficient mice appear to have bone growth retardation at two months, and osteopenia and resistance to bone remodeling in adult life (revised in Lamprou et al., 2014). In transgenic mice that overexpress PTN, bone loss due to estrogen deficiency (Masuda et al., 1997) or almost zero gravity (Tavella et al., 2012) is compensated, in line with the observation that estrogen enhances PTN expression (Xi et al., 2020). It has been suggested that the increased expression of PTN and PTPRZ1 in osteoblasts depleted of insulin-like growth factor binding protein-2 helps maintain, partially at least, normal bone mass in female mice (Xi et al., 2020).
PTN is also involved in odontogenesis. It was initially shown that PTN is expressed during initiation, morphogenesis and cytodifferentiation stages of incisor development at structures that are important for tooth morphogenesis (Mitsiadis et al., 2008). PTN is also expressed in epithelial and mesenchymal dental cell lines and its expression is regulated by bone morphogenetic proteins. During maturation of the ameloblasts and odontoblasts, PTN is expressed in the inner enamel epithelium; PTN is also expressed in the terminally differentiated and enamel matrix-secreting ameloblasts and odontoblasts of the adult mouse incisors and molars (Erlandsen et al., 2012). More recently, it has been shown that PTN positively regulates dental pulp stem cell proliferation and potential to differentiate (Jin et al., 2020), protecting them from senescence (Zhang et al., 2021).
Hematopoiesis and immune system
PTN promotes hematopoietic stem cell (HSC) expansion in vitro and HSC regeneration in vivo (Himburg et al., 2010) via PTPRZ1-mediated activation of the RAS pathway (Himburg et al., 2014), supporting a role of the PTN/PTPRZ1 axis in hemopoiesis. The main source of PTN during steady-state hematopoiesis is the leptin receptor expressing bone marrow stromal cells, but regeneration of HSCs depends on endothelial cell-derived PTN (Himburg et al., 2018). In the same line, PTN secreted into the human tonsil mesenchymal stem cells conditioned medium increases HSC transplantation efficacy (Kim et al., 2020). It has been also shown that loss of stromal PTN results in changes in expression of genes that lead to myeloid engraftment dominance, suggesting that PTN plays a role in maintaining the balance of myeloid and lymphoid potential of regenerating HSCs (Istvanffy et al., 2011).
PTN is mitogenic for human peripheral blood mononuclear cells, and this initial observation suggested that it may be involved in the growth regulation of cell mediated immunity (Achour et al., 2001), further supported by a subsequent study showing enhanced expression of inflammatory cytokines, such as tumor necrosis factor α and interleukins 1β and 6 by PTN (Achour et al., 2008). A role of PTN in adaptive immunity was corroborated by the observation that interferon γ induces PTN expression by macrophages (Li et al., 2010) and a role in innate immunity has been suggested by its bactericidal properties (Svensson et al., 2010; Guyot et al., 2016). PTN has also been suggested as a potent regulator of neuroinflammation through PTPRZ1 (Herradon et al., 2019), and a regulator of the levels of functional cytokines in both M1 and M2 type microglia cells, strengthening M1/M2 transformation (Miao et al., 2019).
Mice that are knockout for PTPRZ1 have a higher number and proportion of mature B cells, and signaling of MK through PTPRZ1 leads to B cell survival (Cohen et al., 2012). Whether PTN has such an effect on B cells has not been studied. It has been only shown that PTN expression is significantly increased in B cells from both chronic and acute leukemia patients compared to healthy controls and suppression of PTN activity induced apoptosis of B cells from both leukemia patients and cell lines (Du et al., 2014).
Urogenital system
In the human mammary gland, PTN is expressed in epithelial and endothelial cells (Ledoux et al., 1997) and helps maintain the mammary epithelial cells in a progenitor phenotype, thus delaying mammary gland maturation (Rosenfield et al., 2012). Its expression in the uterus depends on the estrous cycle and is upregulated by progesterone (Milhiet et al., 1998). In the endometrial stromal cells, it is upregulated during decidualization (Bany and Schultz, 2001), apparently having a vital role in the progesterone-induced decidualization pathway (Yu et al., 2018). High mobility group box 3 regulates uterine stromal cells proliferation and differentiation by targeting PTN (Wang et al., 2019), further supporting a physiological role of PTN in uterine decidualization. CD49a+ Eomes+ natural killer (NK) cells in the human and mouse uterus secrete PTN and enhance fetal growth during early stages of fetal development (Fu et al., 2017). The CD49a+ PBX homeobox 1 enhances PTN transcription in decidual NK cells, thus promoting fetal growth (Zhou et al., 2020). Female mice that are deficient in both PTN and MK show reproductive abnormalities (Muramatsu et al., 2006).
PTN is expressed in testicular Leydig cells and plays a normal role in spermatogenesis. Male mice deficient in PTN are characterized by infertility, atrophic testes and apoptotic sperm cells (Zhang et al., 1999). PTN mRNA and protein are expressed in ventral mesenchymal pad and prostatic mesenchyme surrounding ductal epithelial tips that undergo branching morphogenesis in vivo. In vitro, PTN is upregulated by androgens and stimulates both stromal and epithelial cell proliferation, as well as branching morphogenesis (Orr et al, 2011).
In the embryonic kidney, PTN is expressed in the developing kidney mesenchyme (Mitsiadis et al., 1995) and localizes onto the basement membrane of the developing ureteric bud (Sakurai et al., 2001).
Respiratory system
PTN and PTPRZ1 have been shown to be highly expressed in mesenchymal and epithelial cells of fetal lungs, respectively. PTN promotes fetal type II cell proliferation and inhibits their trans-differentiation into alveolar epithelial type I cells, through multiple signaling pathways that include β-catenin and Notch (Weng et al., 2009).
Eye
PTN is mitogenic for bovine lens epithelial cells (Delbé et al., 1995), and blocks or promotes rod or bipolar cell differentiation respectively (Roger et al., 2006). In Drosophila, overexpression of the MK/PTN orthologue miple leads to defective eye patterning through Ptp99A, the Drosophila ortholog of PTPRZ1 (Muñoz-Soriano et al., 2013). During embryonic development, PTN is expressed in the iris mesenchyme, the optic nerve, and the neural retina, suggesting that it may have a role in epithelial-mesenchymal interactions and affect optic nerve and retinal development. Among PTN receptors, SDC1, and SDC3 are expressed in the cornea, whereas all receptors including RPTPZ1 and ALK are expressed in the retina (Cui and Lwigale, 2019) but a causal relation to the PTN effects on the eye has not been studied.
Liver
PTN was initially identified as a mitogen for adult hepatocytes (Sato et al., 1999) and parenchymal cells in both adult and embryonic liver (Asahina et al., 2002). It is expressed by mesenchymal cells in fetal liver; its expression is gradually reduced during development (Asahina et al., 2002; Ito et al., 2014) and seems to play a role in liver regeneration (Asahina et al., 2002; Michelotti et al., 2016) through its receptor PTPRZ1 (Michelotti et al., 2016). It was recently shown that in myofibroblasts, there is decreased expression of several growth factors, including PTN, compared to fibroblasts, and this leads to reduced hepatoblast proliferation and induced maturation of the differentiated cholangiocytes (Wang et al., 2020).
Other organs
PTN expression has been identified during all embryonic stages in the neurohypophysis primordium. It is expressed only in neurohypophyseal cells and might be involved in pituitary development (Fujiwara et al., 2014).
PTN mRNA is strongly expressed in the mouse cochlea one week after birth and gradually decreases, being undetectable by week 8 after birth. PTN knockout mice exhibit low to moderate auditory deficits and have higher hearing thresholds compared to the wild-type mice, with an almost normal appearance of the stria vascularis but weak expression of the Kir4.1 potassium channel (Zou et al., 2006). Mice knockout for both PTN and MK also appears with severe vacuolar degeneration in the intermediate cells (Sone et al., 2011).
PTN and angiogenesis
The vascular system is very important for fetal development and there is evidence that PTN has a significant regulatory role in vascular homeostasis, although the exact pathways involved are still being explored.
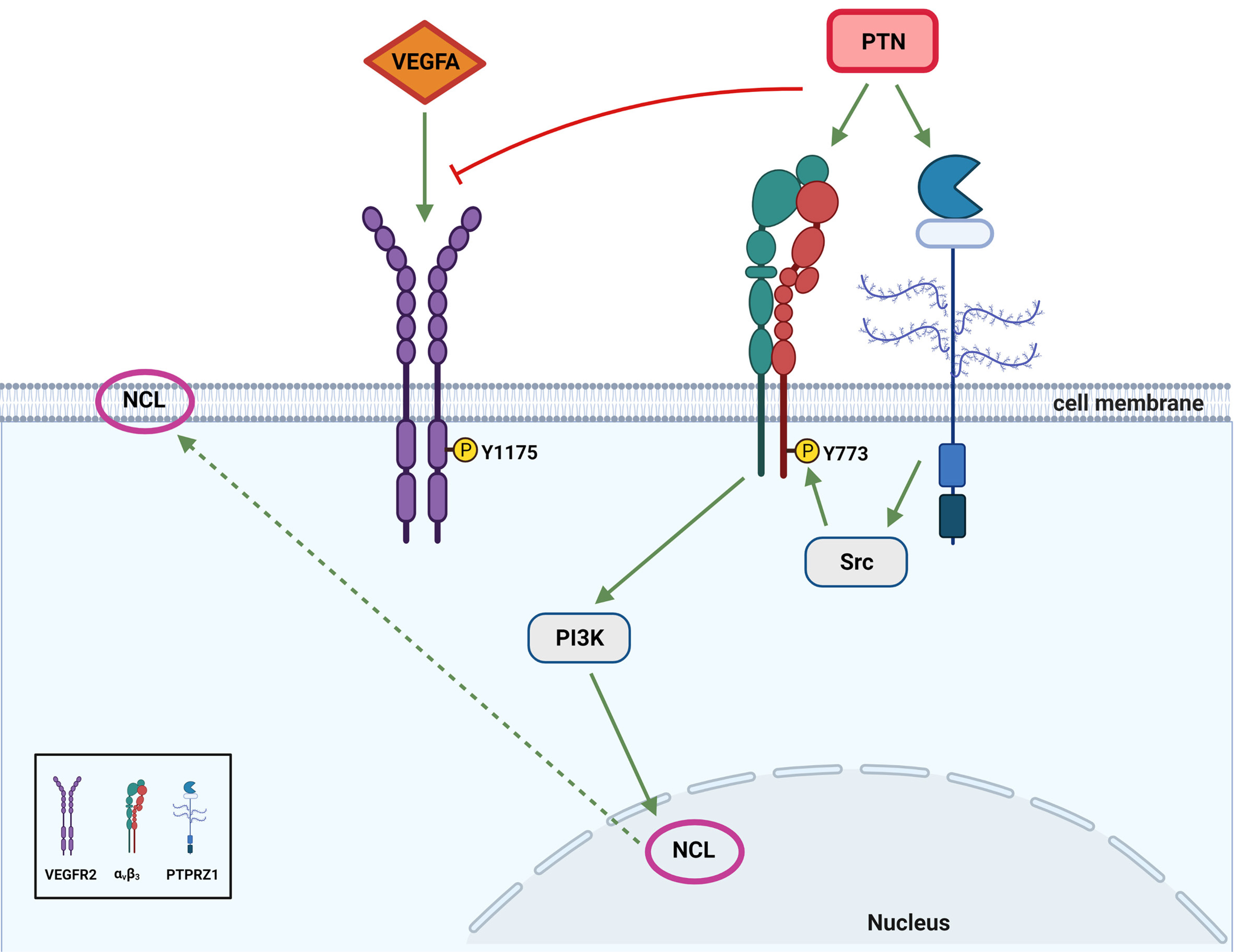
The initial evidence came from in vitro studies showing that PTN is mitogenic for endothelial cells. The mitogenic effect of PTN was questioned in subsequent studies and it seems that it may be cell-type and cell-context specific or depend on the origin of the recombinant protein used (revised in Papadimitriou et al., 2004; 2009). PTN induces endothelial cell migration and differentiation into tubes in various substrates in vitro, through its receptor PTPRZ1, which forms a functional complex with αvβ3 integrin on the surface of endothelial cells and leads to cell surface translocation of NCL (revised in Papadimitriou et al., 2009; 2016). PTN has also been shown to enhance VEGFA expression (Koutsioumpa et al., 2012; Palmieri et al., 2015) and this may contribute to its angiogenic activities, although the receptors/pathways involved in this action have not been studied. In contrast to the initial characterization of PTN as an angiogenesis-promoting growth factor, later studies suggested that PTN may also limit the angiogenic effect of VEGFA (Héroult et al., 2004; Koutsioumpa et al., 2015). This possibility was further supported by data showing that VEGFA competes with PTN for binding to PTPRZ1 (Koutsioumpa et al., 2015). Binding of VEGFA to PTPRZ1 inhibits endogenous PTN levels (Poimenidi et al., 2016) that limit the angiogenic effects of VEGFA, thus enhancing VEGA-induced endothelial cell migration (Koutsioumpa et al., 2015; Poimenidi et al., 2016). More recently, we have shown that PTN selectively binds VEGFR2 and inhibits its activation by VEGFA, highlighting another mechanism through which PTN limits the angiogenic effect of VEGFA (Lamprou et al., 2020). Although the cross talk between PTPRZ1, ανβ3 and VEGFR2 is still being studied, our up-to-date data support a model through which PTN has a moderate stimulatory effect on endothelial cells via PTPRZ1/ανβ3/cell surface NCL but limits the angiogenic effects of VEGFA through VEGFR2 (Fig. 4).
Fig. 4. Schematic representation of the up to date known PTN-induced pathway in endothelial cells.
PTN has a moderate stimulatory effect on endothelial cell migration via PTPRZ1/ανβ3/cell surface NCL (Mikelis et al., 2009; Koutsioumpa et al., 2013) but an inhibitory effect through VEGFR2 (Lamprou et al., 2020). The figure was created with BioRender.com.
In line with a balancing effect on angiogenesis,, PTN has had moderate effects on in vivo animal models of angiogenesis, such as the rabbit cornea, the chorioallantoic membrane of the chick embryo, the ischemic myocardium in rats, a model of acute posterior muscle ischemia, slow-twitch soleus and fast-twitch extensor digitorum longus muscles, and numerous tumor models (revised in Papadimitriou et al., 2009; 2016). When expressed by monocytes, it has been shown to lead to a decrease in the expression of monocyte markers and an increase in endothelial cell markers, inducing differentiation of monocytes into functional endothelial cells (Sharifi et al., 2006). A role played by PTN in angiogenesis is also supported by the expression of PTN and its receptors PTPRZ1, ALK, SDC1 and SDC3 in mesenchymal cells, fetal macrophages, and fetal vessels, in line with increasing levels of PTN in maternal bloodstream as pregnancy progresses (Ball et al., 2009). PTN has been also suggested as a promising factor for inducing angiogenesis during aging based on the observation that it restores the age-related attenuation of angiogenesis in the aortic ring model (Besse et al., 2013).
Most discussion of the role of PTPRZ1 in angiogenesis has been based on its function as a PTN and VEGFA receptor, as mentioned above. We have recently shown that PTPRZ1 knockout mice show enhanced angiogenesis in several organs, such as lung, heart and the retina, and microvascular endothelial cells isolated from the lungs of these animals have enhanced angiogenic properties (Ntenekou et al., 2020; Katraki-Pavlou et al., unpublished).
Conclusions
PTN is a highly conserved, ubiquitously expressed protein that seems to play a notable role in the development and maturation of the nervous system. Data from several other organs show that it is also involved in epithelial-mesenchymal interactions during development and is expressed by endothelial cells, regulating angiogenesis. Due to the high degree of homology with MK, the one compensates for the other in several but not all cases, and this adds to the complexity of studying PTN. Another point of complexity is the increasing number of receptors that have been identified as mediating the effects of PTN in several different types of cells and organs, forcing researchers to try to clarify the interactions and the crosstalk of the different receptors/pathways involved in each case. Moreover, the PTN receptors identified to date are not specific for PTN and mediate the effects of several other molecules besides PTN and MK (revised in Pantazaka and Papadimitriou, 2014; Papadimitriou et al., 2016), suggesting that PTN may regulate the effects of other growth factors, as has been shown for VEGFA in endothelial cells (Koutsioumpa et al., 2015; Poimenidi et al., 2016). Although PTN does not seem to be indispensable for survival, it seems to play important roles in several pathological conditions, affecting pathways that are deranged in pathological settings, and this is still an open field to study. Meticulous in vitro work combined with high throughput assays and loss- or gain-of-function in vivo studies will shed light on many unidentified aspects related to the role of PTN in development, angiogenesis, and pathologies.
Acknowledgements
Eleni Mourkogianni and Despoina Ntenekou are recipients of PhD scholarships financed by the State Scholarship Foundation in Greece (IKY) (Operational Program “Human Resources Development – Education and Lifelong Learning”, Partnership Agreement 2014-2020).
Abbreviations
ALK, anaplastic lymphoma kinase ; CAH, carbonic anhydrase domain ; cAMP, Cyclic adenosine monophosphate ; c-Src, proto-oncogene tyrosine-protein kinase cellular sarcoma ; ERK1/2, extracellular signal-regulated kinases ; FNIII, fibronectin type III domain ; GR, glycine-rich domain ; HARP, heparin affin-regulatory peptide ; HB-GAM, heparin binding growth-associated molecule ; HBGF-8, heparin binding growth factor-8 ; HBNF, heparin binding neurotrophic factor ; HSCs, hematopoietic stem cells ; MAM, meprin A-5, μ domain ; MK, midkine ; NCL, nucleolin ; NK cells, natural killer cells ; OSF-1, osteoblast specific factor ; PTN, pleiotrophin ; PTPRZ1, receptor protein tyrosine phosphatase zeta 1 ; RAS, rat sarcoma virus pathway ; SDC1, syndecan-1 ; SDC2, syndecan-2 ; SDC3, syndecan-3 ; SDC4, syndecan-4 ; TSR-1, thrombospondin type I repeat ; VEGFA, vascular endothelial growth factor A ; VEGFR2, vascular endothelial growth factor receptor 2 ;References
Achour A., Laaroubi D., Caruelle D., Barritault D., Courty J., (2001). The angiogenic factor heparin affin regulatory peptide (HARP) induces proliferation of human peripheral blood mononuclear cells. Cellular and molecular biology (Noisy-le-Grand, France) 47: OL73-OL77.
Achour A., M'Bika J. P., Baudouin F., Caruelle D., Courty J. (2008). Pleiotrophin induces expression of inflammatory cytokines in peripheral blood mononuclear cells. Biochimie 90: 1791-1795.
Asahina K., Sato H., Yamasaki C., Kataoka M., Shiokawa M., Katayama S., Tateno C., Yoshizato K. (2002). Pleiotrophin/Heparin-Binding Growth-Associated Molecule as a Mitogen of Rat Hepatocytes and Its Role in Regeneration and Development of Liver. The American Journal of Pathology 160: 2191-2205.
Ball M., Carmody M., Wynne F., Dockery P., Aigner A., Cameron I., Higgins J., Smith S.D., Aplin J.D., Moore T. (2009). Expression of Pleiotrophin and its Receptors in Human Placenta Suggests Roles in Trophoblast Life Cycle and Angiogenesis. Placenta 30: 649-653.
Bany B. M., Schultz G. A. (2001). Pleiotrophin Messenger Ribonucleic Acid Levels Increase in Mouse Endometrial Stromal Cells During Decidualization. Endocrinology 142: 955-958.
Basille-Dugay M., Hamza M. M., Tassery C., Parent B., Raoult E., Bénard M., Raisman-Vozari R., Vaudry D., Burel D. C. (2013). Spatio-temporal characterization of the pleiotrophinergic system in mouse cerebellum: Evidence for its key role during ontogenesis. Experimental Neurology 247: 537-551.
Bertram S., Roll L., Reinhard J., Groß K., Dazert S., Faissner A., Volkenstein S. (2019). Pleiotrophin increases neurite length and number of spiral ganglion neurons in vitro. Experimental Brain Research 237: 2983-2993.
Besse S., Comte R., Fréchault S., Courty J., Joël L., Delbé J. (2013). Pleiotrophin promotes capillary-like sprouting from senescent aortic rings. Cytokine 62: 44-47.
Camerino G. M., Pierno S., Liantonio A., De Bellis M., Cannone M., Sblendorio V., Conte E., Mele A., Tricarico D., Tavella S., Ruggiu A., Cancedda R., Ohira Y., Danieli-Betto D., Ciciliot S., Germinario E., Sandonà D., Betto R., Camerino D. C., Desaphy J.F. (2013). Effects of Pleiotrophin Overexpression on Mouse Skeletal Muscles in Normal Loading and in Actual and Simulated Microgravity. PLoS ONE 8: e72028.
Canoll P. D., Barnea G., Levy J. B., Sap J., Ehrlich M., Silvennoinen O., Schlessinger J., Musacchio J.M. (1993). The expression of a novel receptor-type tyrosine phosphatase suggests a role in morphogenesis and plasticity of the nervous system. Developmental Brain Research 75: 293-298.
Caruelle D., Mazouzi Z., Husmann I., Delbé J., Duchesnay A., Gautron J., Martelly I., Courty J. (2004). Upregulation of HARP during in vitro myogenesis and rat soleus muscle regeneration. Journal of Muscle Research and Cell Motility 25: 45-53.
Chen H.W. (2004). Dynamic changes of gene expression profiles during postnatal development of the heart in mice. Heart 90: 927-934.
Cohen S., Shoshana O., Zelman-Toister E., Maharshak N., Binsky-Ehrenreich I., Gordin M., Hazan-Halevy I., Herishanu Y., Shvidel L., Haran M., Leng L., Bucala R., Harroch S., Shachar I. (2012). The Cytokine Midkine and Its Receptor RPTPζ Regulate B Cell Survival in a Pathway Induced by CD74. The Journal of Immunology 188: 259-269.
Cui R., Lwigale P. (2019). Expression of the heparin-binding growth factors Midkine and pleiotrophin during ocular development. Gene Expression Patterns 32: 28-37.
Delbé J., Vacherot F., Laaroubi K., Barritault D., Courty J. (1995). Effect of herparin on bovine epithelial lens cell proliferation induced by heparin affin regulatory peptide. Journal of Cellular Physiology 164: 47-54.
Du C., Wang L., Li Y., Xiao W., Guo Q., Chen F., Tan X. (2014). Elevated expression of pleiotrophin in lymphocytic leukemia CD19 + B cells . APMIS 122: 905-913.
Eill G. J., Sinha A., Morawski M., Viapiano M. S., Matthews R. T. (2020). The protein tyrosine phosphatase RPTPζ/phosphacan is critical for perineuronal net structure. Journal of Biological Chemistry 295: 955-968.
Elahouel R., Blanc C., Carpentier G., Frechault S., Cascone I., Destouches D., Delbé J., Courty J., Hamma-Kourbali Y. (2015). Pleiotrophin Exerts Its Migration and Invasion Effect through the Neuropilin-1 Pathway. Neoplasia 17: 613-624.
Erlandsen H., Ames J. E., Tamkenath A., Mamaeva O., Stidham K., Wilson M. E., Perez-Pinera P., Deuel T. F., MacDougall M. (2012). Pleiotrophin Expression during Odontogenesis. Journal of Histochemistry & Cytochemistry 60: 366-375.
Feng W., Nguyen H., Shen D., Deng H., Jiang Z., Podolnikova N., Ugarova T., Wang X. (2021). Structural Characterization of the Interaction between the α M I-Domain of the Integrin Mac-1 (α M β 2 ) and the Cytokine Pleiotrophin . Biochemistry 60: 182-193.
Fu B., Zhou Y., Ni X., Tong X., Xu X., Dong Z., Sun R., Tian Z., Wei H. (2017). Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 47: 1100-1113.e6.
Fujikawa A., Chow J. P. H., Shimizu H., Fukada M., Suzuki R., Noda M. (2007). Tyrosine Phosphorylation of ErbB4 is Enhanced by PSD95 and Repressed by Protein Tyrosine Phosphatase Receptor Type Z. Journal of Biochemistry 142: 343-350.
Fujiwara K., Maliza R., Tofrizal A., Batchuluun K., Ramadhani D., Tsukada T., Azuma M., Horiguchi K., Kikuchi M., Yashiro T. (2014). In situ hybridization analysis of the temporospatial expression of the midkine/pleiotrophin family in rat embryonic pituitary gland. Cell and Tissue Research 357: 337-344.
González-Castillo C., Ortuño-Sahagún D., Guzmán-Brambila C., Pallás M., Rojas-Mayorquín A. E. (2015). Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus. Frontiers in Cellular Neuroscience 8: 843.
Guyot N., Labas V., Harichaux G., Chessé M., Poirier J.C., Nys Y., Réhault-Godbert S. (2016). Proteomic analysis of egg white heparin-binding proteins: towards the identification of natural antibacterial molecules. Scientific Reports 6: 27974.
Hamza M. M., Rey S. A., Hilber P., Arabo A., Collin T., Vaudry D., Burel D. (2016). Early Disruption of Extracellular Pleiotrophin Distribution Alters Cerebellar Neuronal Circuit Development and Function. Molecular Neurobiology 53: 5203-5216.
Harroch S., Furtado G. C., Brueck W., Rosenbluth J., Lafaille J., Chao M., Buxbaum J. D., Schlessinger J. (2002). A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nature Genetics 32: 411-414.
Héroult M., Bernard-Pierrot I., Delbé J., Hamma-Kourbali Y., Katsoris P., Barritault D., Papadimitriou E., Plouet J., Courty J. (2004). Heparin affin regulatory peptide binds to vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. Oncogene 23: 1745-1753.
Herradon G., Ramos-Alvarez M. P., Gramage E. (2019). Connecting Metainflammation and Neuroinflammation Through the PTN-MK-RPTPβ/ζ Axis: Relevance in Therapeutic Development. Frontiers in Pharmacology 10: 377.
Himburg H. A., Muramoto G. G., Daher P., Meadows S. K., Russell J. L., Doan P., Chi J.T., Salter A. B., Lento W. E., Reya T., Chao N. J., Chute J. P. (2010). Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nature Medicine 16: 475-482.
Himburg H. A., Termini C. M., Schlussel L., Kan J., Li M., Zhao L., Fang T., Sasine J. P., Chang V. Y., Chute J. P. (2018). Distinct Bone Marrow Sources of Pleiotrophin Control Hematopoietic Stem Cell Maintenance and Regeneration. Cell Stem Cell 23: 370-381.e5.
Himburg H. A., Yan X., Doan P. L., Quarmyne M., Micewicz E., McBride W., Chao N. J., Slamon D. J., Chute J. P. (2014). Pleiotrophin mediates hematopoietic regeneration via activation of RAS. Journal of Clinical Investigation 124: 4753-4758.
Istvanffy R., Kröger M., Eckl C., Gitzelmann S., Vilne B., Bock F., Graf S., Schiemann M., Keller U. B., Peschel C., Oostendorp R. A. J. (2011). Stromal pleiotrophin regulates repopulation behavior of hematopoietic stem cells. Blood 118: 2712-2722.
Ito K., Yanagida A., Okada K., Yamazaki Y., Nakauchi H., Kamiya A. (2014). Mesenchymal progenitor cells in mouse foetal liver regulate differentiation and proliferation of hepatoblasts. Liver International 34: 1378-1390.
Jin L., Gao F., Zhang L., Wang C., Hu L., Fan Z., Xia D. (2020). Pleiotropin enhances the osteo/dentinogenic differentiation potential of dental pulp stem cells. Connective Tissue Research 62: 495-507.
Jin L., Jianghai C., Juan L., Hao K. (2009). Pleiotrophin and peripheral nerve injury. Neurosurgical Review 32: 387-393.
Jubb A. M., Strickland L. A., Liu S. D., Mak J., Schmidt M., Koeppen H. (2012). Neuropilin-1 expression in cancer and development. The Journal of Pathology 226: 50-60.
Kalamatianos T., Denekou D., Stranjalis G., Papadimitriou E. (2018). Anaplastic Lymphoma Kinase in Glioblastoma: Detection/Diagnostic Methods and Therapeutic Options. Recent Patents on Anti-Cancer Drug Discovery 13: 209-223.
Kim Y.H., Cho K.A., Lee H.J., Park M., Shin S.J., Park J.W., Woo S.Y., Ryu K.H. (2020). Conditioned Medium from Human Tonsil-Derived Mesenchymal Stem Cells Enhances Bone Marrow Engraftment via Endothelial Cell Restoration by Pleiotrophin. Cells 9: 221.
Koutsioumpa M., Drosou G., Mikelis C., Theochari K., Vourtsis D., Katsoris P., Giannopoulou E., Courty J., Petrou C., Magafa V., Cordopatis P., Papadimitriou E. (2012). Pleiotrophin expression and role in physiological angiogenesis in vivo: potential involvement of nucleolin. Vascular Cell 4: 4.
Koutsioumpa M., Papadimitriou E. (2014). Cell Surface Nucleolin as a Target for Anti-Cancer Therapies. Recent Patents on Anti-Cancer Drug Discovery 9: 137-152.
Koutsioumpa M., Poimenidi E., Pantazaka E., Theodoropoulou C., Skoura A., Megalooikonomou V., Kieffer N., Courty J., Mizumoto S., Sugahara K., Papadimitriou E. (2015). Receptor protein tyrosine phosphatase beta/zeta is a functional binding partner for vascular endothelial growth factor. Molecular Cancer 14: 19.
Koutsioumpa M., Polytarchou C., Courty J., Zhang Y., Kieffer N., Mikelis C., Skandalis S. S., Hellman U., Iliopoulos D., Papadimitriou E. (2013). Interplay between αvβ3 Integrin and Nucleolin Regulates Human Endothelial and Glioma Cell Migration. Journal of Biological Chemistry 288: 343-354.
Kuboyama K., Fujikawa A., Suzuki R., Tanga N., Noda M. (2016). Role of Chondroitin Sulfate (CS) Modification in the Regulation of Protein-tyrosine Phosphatase Receptor Type Z (PTPRZ) Activity. Journal of Biological Chemistry 291: 18117-18128.
Lampropoulou E., Logoviti I., Koutsioumpa M., Hatziapostolou M., Polytarchou C., Skandalis S. S., Hellman U., Fousteris M., Nikolaropoulos S., Choleva E., Lamprou M., Skoura A., Megalooikonomou V., Papadimitriou E. (2018). Cyclin-dependent kinase 5 mediates pleiotrophin-induced endothelial cell migration. Scientific Reports 8: 5893.
Lamprou M., Kaspiris A., Panagiotopoulos E., Giannoudis P. V., Papadimitriou E. (2014). The role of pleiotrophin in bone repair. Injury 45: 1816-1823.
Lamprou M., Kastana P., Kofina F., Tzoupis H., Barmpoutsi S., Sajib M. S., Koutsioumpa M., Poimenidi E., Zompra A. A., Tassopoulos D., Choleva E., Tselios T., Mikelis C. M., Papadimitriou E. (2020). Pleiotrophin selectively binds to vascular endothelial growth factor receptor 2 and inhibits or stimulates cell migration depending on ανβ3 integrin expression. Angiogenesis 23: 621-636.
Landgraf P., Wahle P., Pape H.C., Gundelfinger E. D., Kreutz M. R. (2008). The Survival-promoting Peptide Y-P30 Enhances Binding of Pleiotrophin to Syndecan-2 and -3 and Supports Its Neuritogenic Activity. Journal of Biological Chemistry 283: 25036-25045.
Ledoux D., Caruelle D., Sabourin J.C., Liu J., Crepin M., Barritault D., Courty J. (1997). Cellular Distribution of the Angiogenic Factor Heparin Affin Regulatory Peptide (HARP) mRNA and Protein in the Human Mammary Gland. Journal of Histochemistry & Cytochemistry 45: 1239-1245.
Levy J.B., Canoll P.D., Silvennoinen O., Barnea G., Morse B., Honegger A.M., Huang J.T., Cannizzaro L.A., Park S.H., Druck T. (1993). The cloning of a receptor-type protein tyrosine phosphatase expressed in the central nervous system. Journal of Biological Chemistry 268: 10573-10581.
Li F., Tian F., Wang L., Williamson I. K., Sharifi B. G., Shah P. K. (2010). Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN‐γ/JAK/STAT1 signaling is critical for the expression of PTN in macrophages. The FASEB Journal 24: 810-822.
Li Y.S., Gurrieri M., Deuel T. F. (1992). Pleiotrophin gene expression is highly restricted and is regulated by platelet-derived growth factor. Biochemical and Biophysical Research Communications 184: 427-432.
Maartens A. P., Brown N. H. (2015). Anchors and Signals. In Cellular Adhesion in Development and Disease. (Ed. Yap A. S., ) Elsevier.
Margolis R. K., Rauch U., Maurel P., Margolis R. U., (1996). Neurocan and phosphacan: two major nervous tissue-specific chondroitin sulfate proteoglycans. Perspectives on developmental neurobiology 3: 273-290.
Masuda H., Tsujimura A., Yoshioka M., Arai Y., Kuboki Y., Mukai T., Nakamura T., Tsuji H., Nakagawa M., Hashimoto-Gotoh T. (1997). Bone Mass Loss Due to Estrogen Deficiency Is Compensated in Transgenic Mice Overexpressing Human Osteoblast Stimulating Factor-1. Biochemical and Biophysical Research Communications 238: 528-533.
McClain C. R., Sim F. J., Goldman S. A. (2012). Pleiotrophin Suppression of Receptor Protein Tyrosine Phosphatase- / Maintains the Self-Renewal Competence of Fetal Human Oligodendrocyte Progenitor Cells. Journal of Neuroscience 32: 15066-15075.
Miao J., Wang F., Wang R., Zeng J., Zheng C., Zhuang G., (2019). Pleiotrophin regulates functional heterogeneity of microglia cells in EAE animal models of multiple sclerosis by activating CCr-7/CD206 molecules and functional cytokines. American journal of translational research 11: 2013-2027.
Michelotti G. A., Tucker A., Swiderska-Syn M., Machado M. V., Choi S. S., Kruger L., Soderblom E., Thompson J. W., Mayer-Salman M., Himburg H. A., Moylan C. A., Guy C. D., Garman K. S., Premont R. T., Chute J. P., Diehl A. M. (2016). Pleiotrophin regulates the ductular reaction by controlling the migration of cells in liver progenitor niches. Gut 65: 683-692.
Mikelis C., Lamprou M., Koutsioumpa M., Koutsioubas A. G., Spyranti Z., Zompra A. A., Spiliopoulos N., Vradis A. A., Katsoris P., Spyroulias G. A., Cordopatis P., Courty J., Papadimitriou E. (2011). A peptide corresponding to the C-terminal region of pleiotrophin inhibits angiogenesis in vivo and in vitro. Journal of Cellular Biochemistry 112: 1532-1543.
Mikelis C., Sfaelou E., Koutsioumpa M., Kieffer N., Papadimitriou E. (2009). Integrin α v β 3 is a pleiotrophin receptor required for pleiotrophin‐induced endothelial cell migration through receptor protein tyrosine phosphatase β/ζ . The FASEB Journal 23: 1459-1469.
Milhiet P.E., Vacherot F., Caruelle J.P., Barritault D., Caruelle D., Courty J. (1998). Upregulation of the angiogenic factor heparin affin regulatory peptide by progesterone in rat uterus. Journal of Endocrinology 158: 389-399.
Mitsiadis T.A., Salmivirta M., Muramatsu T., Muramatsu H., Rauvala H., Lehtonen E., Jalkanen M., Thesleff I. (1995). Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121: 37-51.
Mitsiadis T. A., Caton J., De Bari C., Bluteau G. (2008). The large functional spectrum of the heparin-binding cytokines MK and HB-GAM in continuously growing organs: The rodent incisor as a model. Developmental Biology 320: 256-266.
Mourlevat S., Debeir T., Ferrario J. E., Delbe J., Caruelle D., Lejeune O., Depienne C., Courty J., Raisman-Vozari R., Ruberg M. (2005). Pleiotrophin mediates the neurotrophic effect of cyclic AMP on dopaminergic neurons: Analysis of suppression-subtracted cDNA libraries and confirmation in vitro. Experimental Neurology 194: 243-254.
Muñoz-Soriano V., Ruiz C., Pérez-Alonso M., Mlodzik M., Paricio N. (2013). Nemo regulates cell dynamics and represses the expression of miple, a midkine/pleiotrophin cytokine, during ommatidial rotation. Developmental Biology 377: 113-125.
Muramatsu H., Zou P., Kurosawa N., Ichihara-Tanaka K., Maruyama K., Inoh K., Sakai T., Chen L., Sato M., Muramatsu T. (2006). Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes to Cells 11: 1405-1417.
Ntenekou D., Kastana P., Hatziapostolou M., Polytarchou C., Marazioti A., Nikou S., Herradon G., Papadaki E., Stathopoulos G., Mikelis C. M., Papadimitriou E., (2020). Anaplastic Lymphoma Kinase inhibition as an effective treatment strategy against the enhanced lung carcinogenesis and cancer angiogenesis related to decreased PTPRZ1 expression. European Respiratory Journal 56: -3939.
Orr B., Vanpoucke G., Grace O. C., Smith L., Anderson R. A., Riddick A. C.P., Franco O. E., Hayward S. W., Thomson A. A. (2011). Expression of pleiotrophin in the prostate is androgen regulated and it functions as an autocrine regulator of mesenchyme and cancer associated fibroblasts and as a paracrine regulator of epithelia. The Prostate 71: 305-317.
Palmieri D., Mura M., Mambrini S., Palombo D. (2015). Effects of Pleiotrophin on endothelial and inflammatory cells: Pro-angiogenic and anti-inflammatory properties and potential role for vascular bio-prosthesis endothelialization. Advances in Medical Sciences 60: 287-293.
Pantazaka E., Papadimitriou E. (2014). Chondroitin sulfate-cell membrane effectors as regulators of growth factor-mediated vascular and cancer cell migration. Biochimica et Biophysica Acta (BBA) - General Subjects 1840: 2643-2650.
Papadimitriou E., Mikelis C., Lampropoulou E., Koutsioumpa M., Theochari K., Tsirmoula S., Theodoropoulou C., Lamprou M., Sfaelou E., Vourtsis D., Boudouris P. (2009). Roles of pleiotrophin in tumor growth and angiogenesis. European Cytokine Network 20: 180-190.
Papadimitriou E., Pantazaka E., Castana P., Tsalios T., Polyzos A., Beis D. (2016). Pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta as regulators of angiogenesis and cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1866: 252-265.
Papadimitriou E., Polykratis A., Hatziapostolou M., Parthymou A., Polytarchou C., Mikelis C. (2004). Heparin Affin Regulatory Peptide: A New Target for Tumour Therapy?. Current Cancer Drug Targets 4: 471-482.
Paveliev M., Fenrich K. K., Kislin M., Kuja-Panula J., Kulesskiy E., Varjosalo M., Kajander T., Mugantseva E., Ahonen-Bishopp A., Khiroug L., Kulesskaya N., Rougon G., Rauvala H. (2016). HB-GAM (pleiotrophin) reverses inhibition of neural regeneration by the CNS extracellular matrix. Scientific Reports 6: 33916.
Poimenidi E., Theodoropoulou C., Koutsioumpa M., Skondra L., Droggiti E., van den Broek M., Koolwijk P., Papadimitriou E. (2016). Vascular endothelial growth factor A (VEGF-A) decreases expression and secretion of pleiotrophin in a VEGF receptor-independent manner. Vascular Pharmacology 80: 11-19.
Roger J., Brajeul V., Thomasseau S., Hienola A., Sahel J.A., Guillonneau X., Goureau O. (2006). Involvement of Pleiotrophin in CNTF-mediated differentiation of the late retinal progenitor cells. Developmental Biology 298: 527-539.
Rosenfield S. M., Bowden E. T., Cohen-Missner S., Gibby K. A., Ory V., Henke R. T., Riegel A. T., Wellstein A. (2012). Pleiotrophin (PTN) Expression and Function and in the Mouse Mammary Gland and Mammary Epithelial Cells. PLoS ONE 7: e47876.
Sakurai H., Bush K. T., Nigam S. K. (2001). Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development 128: 3283-3293.
Sato H., Funahashi M., Kristensen D. B., Tateno C., Yoshizato K. (1999). Pleiotrophin as a Swiss 3T3 Cell-Derived Potent Mitogen for Adult Rat Hepatocytes. Experimental Cell Research 246: 152-164.
Sharifi B. G., Zeng Z., Wang L., Song L., Chen H., Qin M., Sierra-Honigmann M. R., Wachsmann-Hogiu S., Shah P. K. (2006). Pleiotrophin Induces Transdifferentiation of Monocytes Into Functional Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology 26: 1273-1280.
Shen D., Podolnikova N. P., Yakubenko V. P., Ardell C. L., Balabiyev A., Ugarova T. P., Wang X. (2017). Pleiotrophin, a multifunctional cytokine and growth factor, induces leukocyte responses through the integrin Mac-1. Journal of Biological Chemistry 292: 18848-18861.
Sone M., Muramatsu H., Muramatsu T., Nakashima T. (2011). Morphological observation of the stria vascularis in midkine and pleiotrophin knockout mice. Auris Nasus Larynx 38: 41-45.
Svensson S. L., Pasupuleti M., Walse B., Malmsten M., Mörgelin M., Sjögren C., Olin A. I., Collin M., Schmidtchen A., Palmer R., Egesten A. (2010). Midkine and Pleiotrophin Have Bactericidal Properties. Journal of Biological Chemistry 285: 16105-16115.
Tang C., Wang M., Wang P., Wang L., Wu Q., Guo W. (2019). Neural Stem Cells Behave as a Functional Niche for the Maturation of Newborn Neurons through the Secretion of PTN. Neuron 101: 32-44.e6.
Tanga N., Kuboyama K., Kishimoto A., Kihara M., Kiyonari H., Watanabe T., Fujikawa A., Noda M. (2019). Behavioral and neurological analyses of adult mice carrying null and distinct loss-of-receptor function mutations in protein tyrosine phosphatase receptor type Z (PTPRZ). PLOS ONE 14: e0217880.
Tavella S., Ruggiu A., Giuliani A., Brun F., Canciani B., Manescu A., Marozzi K., Cilli M., Costa D., Liu Y., Piccardi F., Tasso R., Tromba G., Rustichelli F., Cancedda R. (2012). Bone Turnover in Wild Type and Pleiotrophin-Transgenic Mice Housed for Three Months in the International Space Station (ISS). PLoS ONE 7: e33179.
Theodorakopoulou O., Hatziapostolou M., Papadimitriou E., (2006). ATRA's inhibitory effect on prostate cancer cell growth involves HARP expression. The FEBS journal 273: S86 (PP-35).
Vazin T., Becker K. G., Chen J., Spivak C. E., Lupica C. R., Zhang Y., Worden L., Freed W. J. (2009). A Novel Combination of Factors, Termed SPIE, which Promotes Dopaminergic Neuron Differentiation from Human Embryonic Stem Cells. PLoS ONE 4: e6606.
Wanaka A., Carroll S. L., Milbrandt J. (1993). Developmentally regulated expression of pleiotrophin, a novel heparin binding growth factor, in the nervous system of the rat. Developmental Brain Research 72: 133-144.
Wang K., Yin Y.H., Yang Z.Q., Yu H.F., Wang Y.S., Guo B., Yue Z.P. (2019). Hmgb3 Induces the Differentiation of Uterine Stromal Cells Through Targeting Ptn. Reproductive Sciences 26: 891-899.
Wang W., Wan L., Chen Z., Jin X., Li D. (2020). Myofibroblasts control the proliferation of fetal hepatoblasts and their differentiated cholangiocytes during the hepatoblast-to-cholangiocyte transition. Biochemical and Biophysical Research Communications 522: 845-851.
Weng T., Gao L., Bhaskaran M., Guo Y., Gou D., Narayanaperumal J., Chintagari N. R., Zhang K., Liu L. (2009). Pleiotrophin Regulates Lung Epithelial Cell Proliferation and Differentiation during Fetal Lung Development via β-Catenin and Dlk1. Journal of Biological Chemistry 284: 28021-28032.
Winkler C., Yao S. (2014). The midkine family of growth factors: diverse roles in nervous system formation and maintenance. British Journal of Pharmacology 171: 905-912.
Xi G., Demambro V. E., D’Costa S., Xia S. K., Cox Z. C., Rosen C. J., Clemmons D. R. (2020). Estrogen Stimulation of Pleiotrophin Enhances Osteoblast Differentiation and Maintains Bone Mass in IGFBP-2 Null Mice. Endocrinology 161: bqz007.
Xia Z., Ouyang D., Li Q., Li M., Zou Q., Li L., Yi W., Zhou E. (2019). The Expression, Functions, Interactions and Prognostic Values of PTPRZ1: A Review and Bioinformatic Analysis. Journal of Cancer 10: 1663-1674.
Yanagisawa H., Komuta Y., Kawano H., Toyoda M., Sango K. (2010). Pleiotrophin induces neurite outgrowth and up-regulates growth-associated protein (GAP)-43 mRNA through the ALK/GSK3β/β-catenin signaling in developing mouse neurons. Neuroscience Research 66: 111-116.
Yang S., Xue D., Wu B., Sun H., Li X., Dong F., Li W., Ji F., Zhou D. (2013). Pleiotrophin is involved in the amniotic epithelial cell-induced differentiation of human umbilical cord blood-derived mesenchymal stem cells into dopaminergic neuron-like cells. Neuroscience Letters 539: 86-91.
Yu H.F., Tao R., Yang Z.Q., Wang K., Yue Z.P., Guo B. (2018). Ptn functions downstream of C/EBPβ to mediate the effects of cAMP on uterine stromal cell differentiation through targeting Hand2 in response to progesterone. Journal of Cellular Physiology 233: 1612-1626.
Zhang L., Xia D., Wang C., Gao F., Hu L., Li J., Jin L. (2021). Pleiotrophin attenuates the senescence of dental pulp stem cells. Oral Diseases In press: .
Zhang N., Yeh H.J., Zhong R., Li Y.S., Deuel T. F. (1999). A dominant-negative pleiotrophin mutant introduced by homologous recombination leads to germ-cell apoptosis in male mice. Proceedings of the National Academy of Sciences 96: 6734-6738.
Zhou Y., Fu B., Xu X., Zhang J., Tong X., Wang Y., Dong Z., Zhang X., Shen N., Zhai Y., Kong X., Sun R., Tian Z., Wei H. (2020). PBX1 expression in uterine natural killer cells drives fetal growth. Science Translational Medicine 12: eaax1798.
Zou P., Muramatsu H., Sone M., Hayashi H., Nakashima T., Muramatsu T. (2006). Mice doubly deficient in the midkine and pleiotrophin genes exhibit deficits in the expression of β-tectorin gene and in auditory response. Laboratory Investigation 86: 645-653.