Int. J. Dev. Biol. 69: 101 - 107 (2025)
TLR7 expression patterns in mouse eye development and adult ocular tissues
Open Access | Developmental Expression Pattern | Published: 13 June 2025
Abstract
Toll-Like Receptor 7 (TLR7) is recognized for its role in immune responses, particularly in detecting viral RNA. However, emerging evidence suggests that TLR7 may also contribute to ocular development. In this study, we assessed the expression pattern of TLR7 in various CD-1 mouse eye compartments during critical developmental stages, from embryonic day 12 to 16, as well as in adult tissues such as the cornea, pigmented epithelium, neural retina and lens. Our findings reveal a region-specific and time-dependent expression of TLR7, suggesting that it may play a role in the morphogenetic processes that shape the eye during intrauterine development.
Keywords
Toll-like receptors 7, eye, development
Introduction
In recent years, the development of animal models that mimic innate immune overactivation during pregnancy, such as through bacterial or viral infections (i.e., maternal immune activation), has allowed the scientific community to unravel the pathological consequences on offspring's neural development and their long-term effects (Rasile et al., 2022; Estes and McAllister, 2016; Knuesel et al., 2014). Moreover, these models have highlighted the physiological role that Toll-Like Receptors (TLRs) play in the morphogenesis of the mammalian nervous system, drawing parallels to their Drosophila ortholog, Toll. Indeed, several lines of evidence have demonstrated that TLRs orchestrate key stages of neurogenesis, guiding the proliferation, migration, differentiation, and maturation of neural progenitor cells (NPCs). Notably, in postnatal life, TLR activation assumes a vital role in modulating cognitive functions such as memory, learning, and behavior and dysregulation of these processes has been implicated in the pathogenesis of behavioral and psychiatric disorders (Abarca-Merlin et al., 2024). Furthermore, TLRs are also widely expressed in the eyes (Redfern and McDermott, 2010; Singh and Kumar, 2015; Di Zazzo et al., 2022), considered as an extension of the brain (London et al., 2013). In the eye, TLRs are aimed at patrolling the organ to promptly detect potential invading pathogens and, at the same time, avoiding exaggerated inflammatory processes that could compromise visual capacity (Kawai and Akira, 2010; Redfern and McDermott, 2010), creating a delicate balance between necessary immune responses and the maintenance of the ocular immune privilege. Nonetheless, this equilibrium can occasionally be disrupted, potentially leading to the occurrence of eye pathologies (Redfern and McDermott, 2010). For instance, TLRs alterations have been reported in aberrant innate immune responses such as Idiopathic Orbital Inflammation as well as retinal degeneration (Kohno et al., 2013; Wladis et al., 2012). Given the intricate relationship between TLRs and the eyes, TLR7 remains one of the least explored members in this field.
TLR7 is an endosomal receptor that recognizes single-stranded RNA (ssRNA) especially derived from viruses (Fitzgerald and Kagan, 2020) and, in adults, is prominently expressed by plasmacytoid dendritic cells and B lymphocytes, that orchestrate anti-viral immune response (Kawai et al., 2024). Beyond its well-established immune functions, it has been described that TLR7 plays a pivotal role in neuronal activity (Hung et al., 2018a; Hung et al., 2018b; Kaul et al., 2012) and in the embryogenesis of the central and peripheral nervous system (Arnaboldi et al., 2020; Hung et al., 2018a; Hung et al., 2018b; Barak et al., 2014; Anthoney et al., 2018; Kaul et al., 2012).
Recently, TLR7 has been implicated in ocular development. Offspring of mothers injected at embryonic day 12.5 with a TLR7/8 agonist, show delays in developmental milestones such as birth weight and eye opening, which occur 5 days later than in controls (Sheng and Tobet, 2024). Although TLR7 is known to be expressed at different levels of the ocular surface in adults, particularly in the superficial structures such as the cornea, conjunctiva, and eyelid of both humans and rodents (Redfern and McDermott, 2010; Singh and Kumar, 2015; Di Zazzo et al., 2022), a comprehensive evaluation of TLRs expression across the entire eye is missing.
In the present study, we assessed the spatiotemporal appearance of TLR7 in various embryonic murine eye compartments and observed that this receptor is already present at early stages of the development in the cornea, pigmented epithelium, neural retina, and lens in proliferating and differentiating cells. At a later stage, TLR7 distribution remains dynamically regulated, indicating its involvement in both proliferative and differentiative processes. By adulthood, its expression stabilizes and becomes more compartmentalized, particularly in the posterior segment of this organ, suggesting a transition from a developmental role to functions associated with tissue homeostasis and immune surveillance in the mature eye. A better comprehension of the involvement of TLR7 in eye morphogenesis can represent a fundamental step not only for understanding its potential roles beyond immunity but also can contribute to advancements in ophthalmic medicine paving the way for future possible therapeutic interventions targeting this receptor.
Results
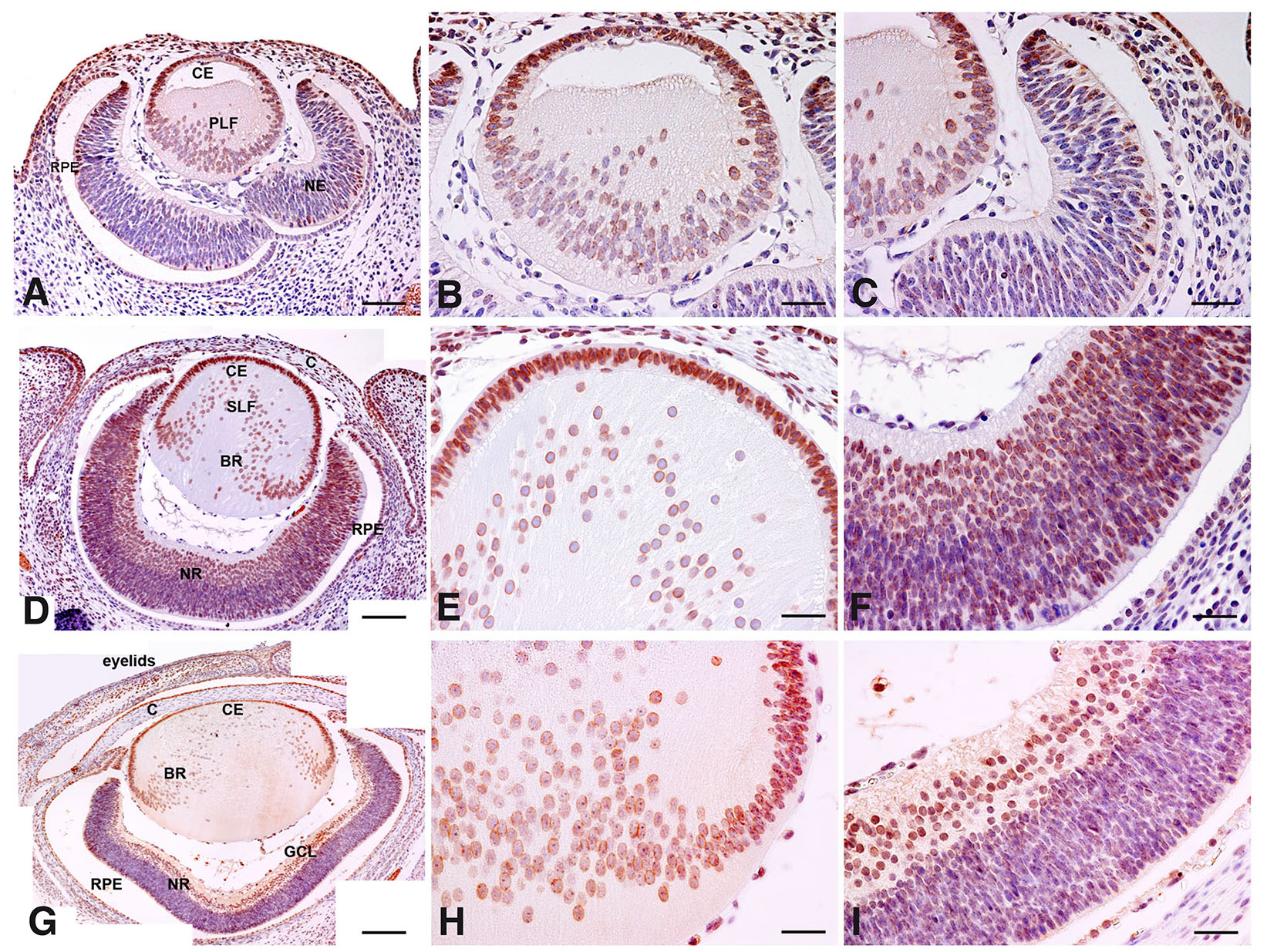
Immunolocalization performed on paraffin-embedded tissue revealed that TLR7 is expressed in different developing eye compartments. At E12, TLR7 expression was already detectable in the crystalline lens, the region of the future cornea and in the retina (Fig. 1A). Strong immunostaining was present in the region of the ciliary body and the anterior part of the eye (Fig. 1A). The crystalline lens was also positive for TLR7, showing strong perinuclear immunostaining visible in the anterior cuboidal epithelium (CE) up to the region of the bow where joining secondary fibers (SLF) were also labelled (Fig. 1B). Perinuclear staining was present in primary lens fibers (PLF), which are in the process of filling the cavity of lens vesicle at this stage. Lastly, labelled cells were present in the region overlying the lens vesicle, where the corneal layers are maturing (Fig. 1B). Immunoreactivity was observed in some cells of both retinal pigmented epithelium (RPE) and neural retina (Fig. 1C). In the latter, labelled cells were mostly present in the ventricular zone, where mitotic elements appear, and in the subventricular zone (Fig. 1C). The cornea could not be identified before E14.
Fig. 1. Immunohistochemical evaluation of the spatial distribution of Toll-Like Receptor 7 (TLR7) in E12, E14 and E16 mouse embryo eye - coronal section of the head.
Immunohistochemical analysis of TLR7 expression in the developing eye of mouse conceptuses at different developmental stages (E12, E14 and E16). (A,D,G) Low magnifications of the stained sections collected at E12, E14 and E16 eye respectively. Scale bar: 30 µm. (B,E,H) High magnifications of the stained sections collected at E12, E14 and E16 of the lens region, respectively. (C,F,I) High magnifications of the stained sections collected at E12, E14 and E16 of the retinal region, respectively. Scale bar: 15 µm. BR (bow region), C (cornea), CE (cuboidal epithelium), GCL (ganglion cell layer), NE (neuroepithelium), NR (neural retina), PLF (primary lens fibers), RPE (retinal pigmented epithelium), SLF (secondary lens fibers).
At E14, in the crystalline lens, almost filled by primary lens fibers, both the CE and the lens fibers showed a perinuclear staining. The superficial layer, now distinguishable in an organized cornea, was still positive (Fig. 1 D,E). Moreover, in both RPE and neural retina, where the ganglion cell layer (GCL) was now visible, the intensity of the TLR7 staining and the number of stained cells had increased (Fig. 1 D,F).
At E16, there was a decrease in TLR7 staining intensity in comparison to previous stages (Fig. 1G), particularly appreciable in the secondary lens fibers (Fig. 1 G,H). However, the labelling patterns are not different from previous stages, except for the cornea layers where the stroma and the epithelium were found negative and positive for TLR7 expression, respectively (Fig. 1G). The conjunctiva, now properly formed, began to appear positive (Fig. 1G), and the different layers of the retina were not yet recognizable (Fig. 1I).
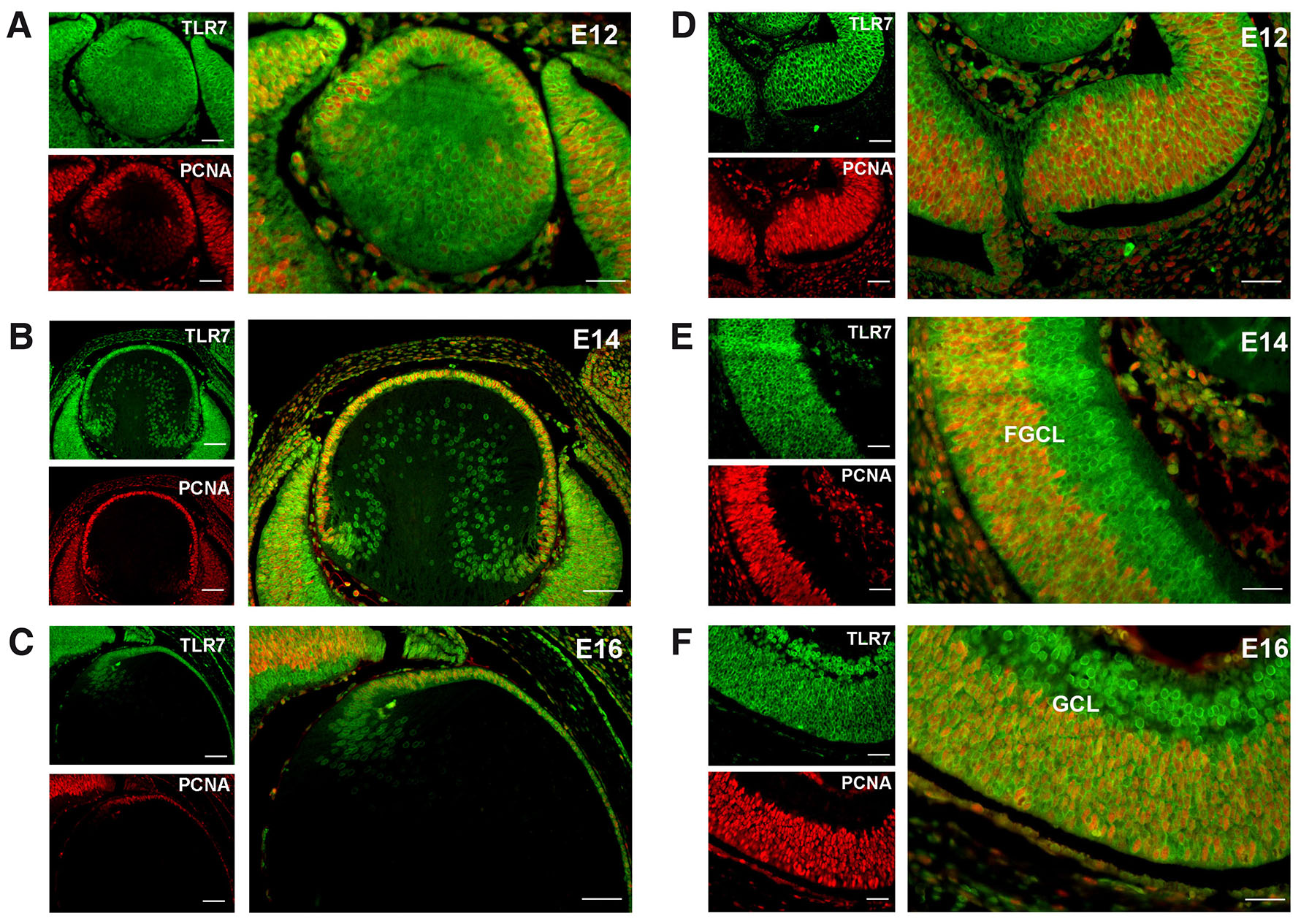
To determine if TLR7 expression is associated with the proliferative status of cells in the developing eye, we performed double immunofluorescence analysis for TLR7 and PCNA. At E12 and E14, in the lens, both the CE and the lens fibers were labelled for TLR7 while only the CE was found positive for PCNA (Fig. 2 A,B). At E16 most of the cells of the lens were still positive for both TLR7 and PCNA but showed regional differences. While in CE the colocalization is complete, along the bow region (BR) – secondary fibers – only the positivity for TLR7 was visible (Fig. 2C). Considering the neural retina at both E12 and E14 embryos, we could recognize three different zones: a ventricular zone and a basal zone, immunoreactive for TLR7 but not for PCNA, and an intermedium zone where TLR7 and PCNA colocalize. The ventricular zone, rich in mitotic cells at these stages, does not appear PCNA-positive (Fig. 2 D,E). At E16, we could also appreciate a fully differentiated layer, not stained for PCNA, in the basal zone of the neural retina (i.e. the becoming ganglionic cells layer), while the other retina layers - evidently in a proliferative phase - showed positivity for PCNA (Fig. 2F). In all embryonic stages, it is possible to appreciate the TLR7 labelling in the anterior eye regions, particularly in the CE of the lens and in the peripheral area of the neural retina (NR), which are areas near the ciliary body, while no TLR7 staining was observable in the optic fibers (Fig. 2 A-F).
Fig. 2. Immunofluorescence analysis of TLR7 and Proliferating Cell Nuclear Antigen (PCNA) expression in the mouse embryo retina at different developmental stages.
Immunolocalization of TLR7 (green signal) and PCNA (red signal) in the neural retina of developing lens and retina of mouse conceptuses at different developmental stages. (A,D, E12; B,E, E14; C,F, E16). Scale bar: 15 µm.
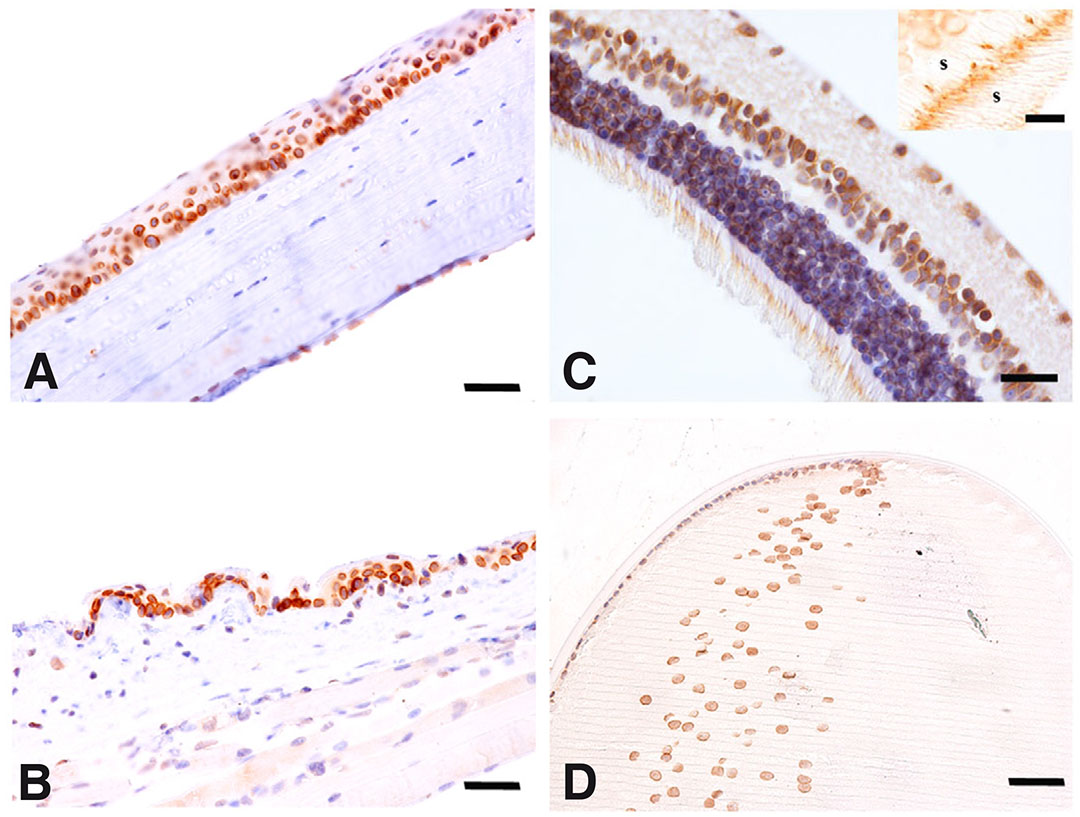
Finally, the expression of TLR7 was assessed in the eyes of 3-month-old mice. Proceeding along the anterior-to-posterior axis, in the cornea, TLR7 labelling was present in the epithelium, especially in basal cells. TLR7 was also detectable in the deep endothelial layer and the basal layer of the superficial squamous stratified epithelium (Fig. 3A). TLR7 was also observed in both bulbar and palpebral conjunctiva with a pattern similar to that observed in the cornea: presence of labelled cells beneath a layer of non-positive cells (Fig. 3B). In all the layers of the neural retina, TLR7 staining is visible in the perinuclear region (Fig. 3C). Of note, in the photoreceptors layer, TLR7 labelling was confined between the inner segment (IS) and the outer segment (OS) (Fig. 3C, inlet). In the lens, a very strong TLR7 staining was detectable in the secondary lens fibers, mainly localized in the perinuclear region (Fig. 3D). Collectively, TLR7 is expressed in all the adult eye compartments even in the posterior segment of these organs.
Fig. 3. Immunohistochemical evaluation of the spatial distribution of TLR7 in the adult mouse eye.
Immunohistochemical analysis of TLR7 in adult mouse eye. (A) Cornea, (B) conjunctiva, (C) neural retina and (D) lens. Scale bar: 15 µm. Inset in (C) shows the photoreceptor area. Bar: 20 µm. IS (inner segment of photoreceptor), OS (outer segment of photoreceptor).
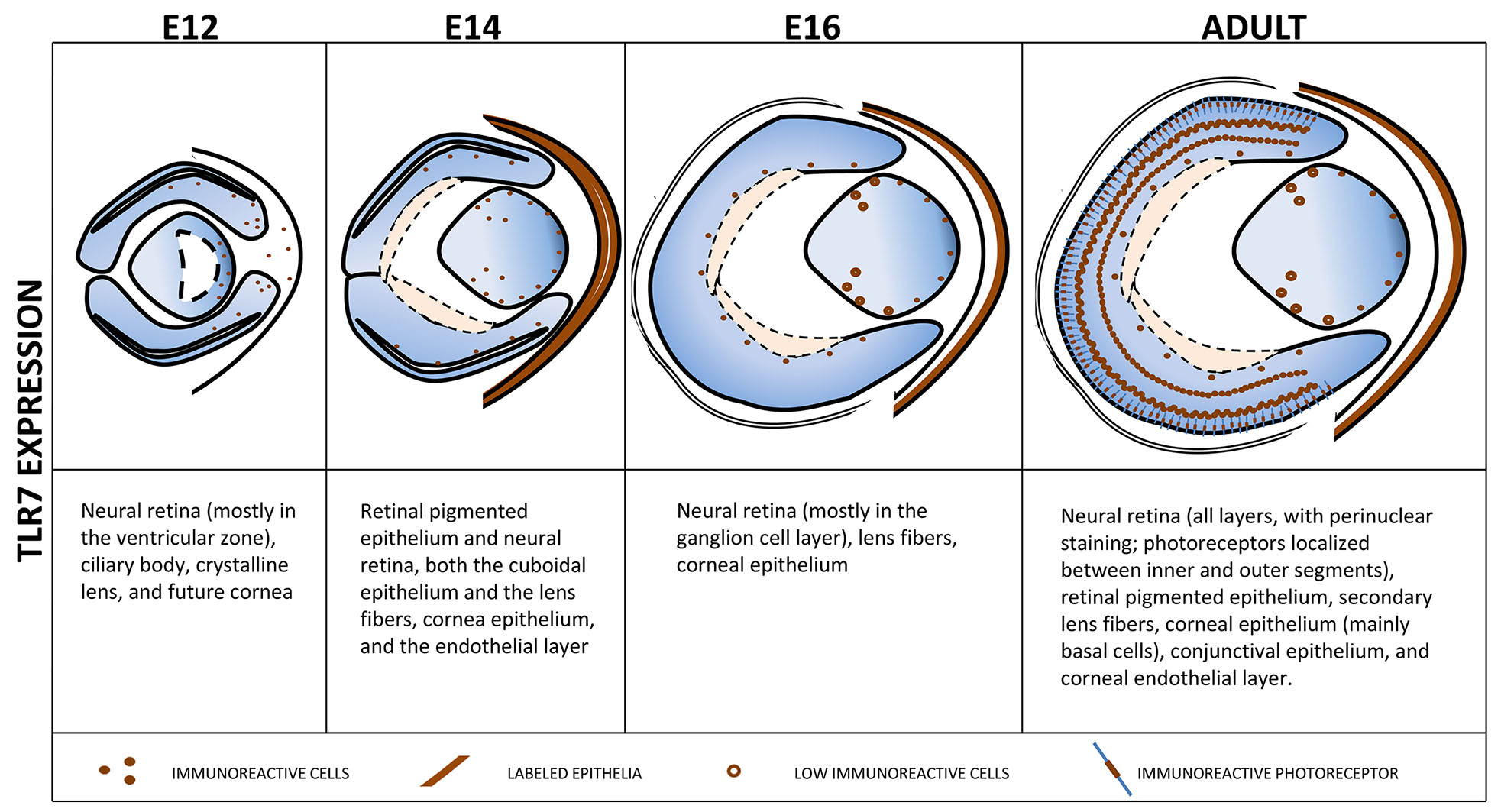
Overall, as showed in Fig. 4, TLR7 expression follows a dynamic pattern during eye development, with an initial increase in expression starting from E12 to E14, followed by a region-specific reduction at E16, particularly in differentiating structures such as the lens fibers. This temporal regulation may reflect the involvement of TLR7 in distinct phases of ocular morphogenesis, transitioning from early proliferative activity to later differentiation processes.
Fig. 4. Chart summarizing the spatial distribution of TLR7 expression in the eye at the different analyzed developmental stages of mouse embryos (E12, E14 and E16) and in adult mice.
Schematic representation of embryo and adult eye showing the localization of TLR7-positive cells during embryo development and in adulthood.
Discussion
In the present study, we analyzed the expression of TLR7 in both surface and deep mouse embryonic and adult eye structures. TLR7, like all other members of the TLR family, has always been associated with the promotion of an immune response against potential pathogens, even within the ocular environment (Redfern and McDermott, 2010). However, in the eye, the situation appears to be far more complex based on the distinctive distribution of TLR7 observed in adult mice. The presence of TLR-positive cells under a layer of TLR7-negative cells both in the cornea and conjunctiva of the adult eye suggests that the compartmentalization of this receptor may serve to reduce the possibility of interaction with microorganisms on the apical surface of the eye, as described for other body compartments (Le Noci et al., 2021). Therefore, it is possible to speculate that TLR7 may also play a role in maintaining a state of immunological tolerance within the eye, which, as is well known, is constantly exposed to environmental stimuli and to a commensal microbial community (Aragona et al., 2021; Peter et al., 2023). Indeed, both in the eyes and in other body regions, various mechanisms are exploited to prevent damages from an uncontrolled TLR stimulation and, under specific conditions, to preserve the immune privilege. TLRs exhibit the paradoxical ability to promote immunosuppression by triggering a complex array of negative feedback mechanisms designed to mitigate potentially harmful inflammatory responses (Fitzgerald and Kagan, 2020; Forward et al., 2010; Koga-Yamakawa et al., 2015). For instance, repeated activation of TLR7 can induce hyporesponsiveness to subsequent stimulations (Hayashi et al., 2009; Hayashi et al., 2012; Michaelis et al., 2019) and it has been reported that TLR7 activation suppressed the release of pro-inflammatory chemokines and reactive oxygen species induced by IL-1β stimulation in conjunctival epithelial cells (Wang et al., 2023).
In the embryo, we found that the expression pattern of TLR7 varies across different areas of the eye and stages of development, possibly suggesting a role of this receptor in biological processes not strictly related to the immune response. Indeed, TLR7 has been reported to be modulated during peripheral and central nervous system development (Kaul et al., 2012; Arnaboldi et al., 2020) and it is described as directly involved in neuronal morphogenesis and differentiation (Hung et al., 2018b; Liu et al., 2013). In line, TLR7 deficiency can result in neuron activity impairment (Hung et al., 2018a). Furthermore, mice prenatally exposed to maternal immune stress and TLR activation exhibit developmental delays in eye opening (Haida et al., 2019; Smolders et al., 2018) and recent reports have included TLR7 in these findings (Sheng and Tobet, 2024). Therefore, it might be plausible to speculate that TLR7 could have a direct role during the embryonic developmental phases of the eye, possibly activated by bacterial molecules derived from ocular or placental microbiota (Aagaard et al., 2014), or endogenous TLR ligands released during embryonic tissue rearrangement (Yu et al., 2010).
However, we identified the presence of TLR7 in both proliferating and non-proliferating retinal cells at all the analyzed stages (from E12 to E16). Moreover, TLR7 immunoreactivity is consistently present in the germinative zone of the lens, where proliferating lens epithelial cells are exclusively located (Yamamoto et al., 2008; Gan et al., 1995), as well as in the secondary lens fibers and anterior cuboidal epithelium. These observations may not support the notion that TLR7 could have a direct role in the proliferation or differentiation processes of the retina cells. In this regard, it has been reported that TLR7 Knock-Out (KO) mice have no visual deficits compared to wild-type (WT) animals (Hung et al., 2018a) and TLR7 KO mice affected by experimental diabetic retinopathy experienced reduced retinal damage characterized by an improved retinal functioning compared to the WT counterpart (Liao et al., 2017). Although these findings suggest that TLR7 may be mainly involved in controlling the ocular immune processes rather than playing an active role in the physiological function of the eyes. the data obtained using KO animals cannot allow to draw definitive conclusions because it has been observed that the genetic ablation of TLR7 determines the up-regulation of TLR8 as a compensatory mechanism (Hung et al., 2018b; Liu et al., 2013). Therefore, it may be possible to hypothesize that the contribution of TLR7 can be replaced by other receptors.
Conclusion
Collectively, the results presented here reveal that TLR7 can be detected in various compartments of the adult and embryonic mouse eye. This expression is evident in cells undergoing both proliferation and differentiation, and it continues to persist after birth. While the distribution of TLR7 in the adult ocular surface is suggestive of a physical barrier against environmental stimuli and commensal microbes (Aragona et al., 2021; Peter et al., 2023), the expression pattern in the embryo suggests that, beyond its known role in patrolling the eye environment, TLR7 may also play a role in the intricate morphogenetic processes of this complex organ. Nevertheless, further studies are needed to fully comprehend its involvement in eye embryogenesis.
Material and Methods
Animals
CD1 mice (Charles River Laboratories, Calco, Italy) were housed in a thermostatically maintained animal house (T = 22 ± 2°C; relative humidity 55 ± 5%) with a 12 h light cycle, free food access (Italiana Mangimi, Settimo Milanese, Italy) and water ad libitum. Matings were arranged by caging females with one male of proven fertility overnight. The morning with evident vaginal plugs was considered day 0 of gestation (E0). Pregnant mice were euthanized by CO2 inhalation and uteri were removed and kept in ice-cold phosphate-buffered saline (PBS) 0.1 M pH 7.4 for dissection, and collecting of conceptuses at different developmental stages (E12, E14, and E16). After sacrifice, eye bulbs were collected from 3-month-old adult mice and embryos and fixed by immersion in 4% paraformaldehyde in PBS (Arnaboldi et al., 2020; Sommariva et al., 2023). The animal protocol was approved by the Ministry of Health – Department for Veterinary Public Health, Nutrition and Food Safety Committee. Animals were treated humanely and in compliance with procedures that alleviate suffering.
Histology
After fixation, samples were dehydrated in an ascending scale of ethanols, cleared in xylene, and paraffin embedded. Tissue sections (4 µm) were obtained by a rotatory microtome and immunolocalization analysis were performed.
Immunohistochemistry (IHC)
As previously described (Arnaboldi et al., 2020; Sommariva et al., 2023), for IHC evaluations.
Deparaffinized sections were autoclaved for 6 min at 120°C in Na citrate buffer 0.01 M pH 6 for antigen retrieval, before the quenching of endogenous peroxidase activity via 0.3% H2O2 in PBS. Unspecific binding site saturation was performed for 30 min with a solution of 0.05 M Tris-HCl, 0.15 M NaCl, 0.1% gelatin, 0.5% ovalbumin, 0.05% Tween-20, and 0.2% fish gelatin. Sections were then incubated overnight at 4°C with rabbit anti-TLR7 antibody diluted 1:100 in Tris-Buffered Saline (Novus Biologicals, Centennial, CO, USA) followed by incubation with goat anti-rabbit serum (dilution 1:100 in Tris-Buffered Saline, Agilent, Santa Clara, CA, USA) and then with rabbit peroxidase anti-peroxidase (PAP) (dilution 1:100 in Tris-Buffered Saline, VWR International, Bridgeport, NJ, USA). The immunoreaction was visualized with the 3,3’-diaminobenzidine substrate (DAB) (Merck KGaA, Darmstadt, Germany) and samples were counterstained with hematoxylin, dehydrated and mounted with Entellan (Merck KGaA, Darmstadt, Germany). Negative controls were performed by replacing the primary antibody with non-immune rabbit serum. Moreover, additional controls were introduced to exclude the possibility of non-suppressed endogenous peroxidase activity by sequential omission of the secondary antibody and PAP complex or by incubation with DAB reagent alone. Immunohistochemical experiments were performed on at least ten paraffin sections for each sample.
Immunofluorescence (IF)
After antigen retrieval process as described already above, sections were processed with sodium borohydride in PBS to remove tissue autofluorescence (0.1%, three times, 10 min each at 4°C). After washing with PBS, nonspecific sites were blocked incubating slides for 30 min with 1:10 goat serum in PBS-BSA (PBS-bovine serum albumin) then with TLR7 primary antibody (dilution 1:100 in PBS-BSA) overnight at 4°C. Subsequently, sections were rinsed in PBS-BSA, and then incubated with FITC-conjugated goat anti-rabbit antibody (dilution 1:200 in TBS-BSA). Nonspecific sites were blocked with mouse IgG blocking reagent (Mouse on Mouse, MOM, Immunodetection Kit, Vector Laboratories, Newark, CA, USA) for 1 h at RT, followed by incubation with mouse anti-mouse Proliferating Cell Nuclear Antigen (PCNA) for 1 h at 37°C (diluted 1:100 in M.O.M. Kit diluent); Samples were then stained with biotinylated anti-mouse IgG for 10 min and with TRITC-conjugated streptavidin (dilution 1:200 in TBS-BSA, Jackson Laboratories; West Grove, PA) for 30 min at RT. Slides were finally mounted with Mowiol 4-88 (Calbiochem; La Jolla, CA). Controls were treated as described above.
Microscopy analysis
Sections were observed with a Nikon Eclipse 80i (Nikon, Tokyo, Japan) equipped with bright-field high-quality objectives (20X, Nikon, Plan Fluor, NA 0.5; 40X, Nikon, Plan Fluor, NA 1, oil immersion; 60X, Nikon, Plan Apo, NA 1.40, oil immersion). Appropriate filter sets were used to collect fluorophore emissions. Images were acquired by a digital camera (Nikon Digital Sight 5MC) and then elaborated by ImageJ (Schneider et al., 2012).
Acknowledgements
The authors acknowledge support from the University of Milan through the APC initiative.
References
Aagaard K., Ma J., Antony K. M., Ganu R., Petrosino J., Versalovic J., (2014). The placenta harbors a unique microbiome. Science translational medicine 6: 237ra65.
Abarca-Merlin D. M., Martínez-Durán J. A., Medina-Pérez J. D., Rodríguez-Santos G., Alvarez-Arellano L. (2024). From Immunity to Neurogenesis: Toll-like Receptors as Versatile Regulators in the Nervous System. International Journal of Molecular Sciences 25: 5711.
Anthoney N., Foldi I., Hidalgo A. (2018). Toll and Toll-like receptor signalling in development. Development 145: dev156018.
Aragona P., Baudouin C., Benitez del Castillo J. M., Messmer E., Barabino S., Merayo-Lloves J., Brignole-Baudouin F., Inferrera L., Rolando M., Mencucci R., Rescigno M., Bonini S., Labetoulle M. (2021). The ocular microbiome and microbiota and their effects on ocular surface pathophysiology and disorders. Survey of Ophthalmology 66: 907-925.
Arnaboldi F., Sommariva M., Opizzi E., Rasile M., Camelliti S., Busnelli M., Menegola E., Di Renzo F., Menon A., Barajon I. (2020). Expression of Toll-like receptors 4 and 7 in murine peripheral nervous system development. Annals of Anatomy - Anatomischer Anzeiger 231: 151526.
Barak B., Feldman N., Okun E. (2014). Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Frontiers in Neuroscience 8: 272.
Di Zazzo A., De Piano M., Coassin M., Mori T., Balzamino B. O., Micera A. (2022). Ocular surface toll like receptors in ageing. BMC Ophthalmology 22: 185.
Estes M. L., McAllister A. K. (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science 353: 772-777.
Fitzgerald K. A., Kagan J. C. (2020). Toll-like Receptors and the Control of Immunity. Cell 180: 1044-1066.
Forward N. A., Furlong S. J., Yang Y., Lin T.J., Hoskin D. W. (2010). Signaling through TLR7 enhances the immunosuppressive activity of murine CD4+CD25+ T regulatory cells. Journal of Leukocyte Biology 87: 117-125.
Gan L., van Setten G., Fagerholm P. (1995). Proliferating cell nuclear antigen: contradictory results regarding its presence in the lens. Graefe's Archive for Clinical and Experimental Ophthalmology 233: 792-794.
Haida O., Al Sagheer T., Balbous A., Francheteau M., Matas E., Soria F., Fernagut P. O., Jaber M. (2019). Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Translational Psychiatry 9: 124.
Hayashi T., Gray C. S., Chan M., Tawatao R. I., Ronacher L., McGargill M. A., Datta S. K., Carson D. A., Corr M. (2009). Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proceedings of the National Academy of Sciences 106: 2764-2769.
Hayashi T., Yao S., Crain B., Chan M., Tawatao R. I., Gray C., Vuong L., Lao F., Cottam H. B., Carson D. A., Corr M. (2012). Treatment of Autoimmune Inflammation by a TLR7 Ligand Regulating the Innate Immune System. PLoS ONE 7: e45860.
Hung Y.F., Chen C.Y., Li W.C., Wang T.F., Hsueh Y.P. (2018a). Tlr7 deletion alters expression profiles of genes related to neural function and regulates mouse behaviors and contextual memory. Brain, Behavior, and Immunity 72: 101-113.
Hung Y.F., Chen C.Y., Shih Y.C., Liu H.Y., Huang C.M., Hsueh Y.P. (2018b). Endosomal TLR3, TLR7, and TLR8 control neuronal morphology through different transcriptional programs. Journal of Cell Biology 217: 2727-2742.
Kaul D., Habbel P., Derkow K., Krüger C., Franzoni E., Wulczyn F. G., Bereswill S., Nitsch R., Schott E., Veh R., Naumann T., Lehnardt S. (2012). Expression of Toll-Like Receptors in the Developing Brain. PLoS ONE 7: e37767.
Kawai T., Akira S. (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology 11: 373-384.
Kawai T., Ikegawa M., Ori D., Akira S. (2024). Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 57: 649-673.
Knuesel I., Chicha L., Britschgi M., Schobel S. A., Bodmer M., Hellings J. A., Toovey S., Prinssen E. P. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology 10: 643-660.
Koga-Yamakawa E., Murata M., Dovedi S. J., Wilkinson R. W., Ota Y., Umehara H., Sugaru E., Hirose Y., Harada H., Jewsbury P. J., Yamamoto S., Robinson D. T., Li C. J. (2015). TLR7 tolerance is independent of the type I IFN pathway and leads to loss of anti-tumor efficacy in mice. Cancer Immunology, Immunotherapy 64: 1229-1239.
Kohno H., Chen Y., Kevany B. M., Pearlman E., Miyagi M., Maeda T., Palczewski K., Maeda A. (2013). Photoreceptor Proteins Initiate Microglial Activation via Toll-like Receptor 4 in Retinal Degeneration Mediated by All-trans-retinal. Journal of Biological Chemistry 288: 15326-15341.
Le Noci V., Bernardo G., Bianchi F., Tagliabue E., Sommariva M., Sfondrini L. (2021). Toll Like Receptors as Sensors of the Tumor Microbial Dysbiosis: Implications in Cancer Progression. Frontiers in Cell and Developmental Biology 9: 732192.
Liao Y.R., Li Z.J., Zeng P., Lan Y.Q. (2017). TLR7 deficiency contributes to attenuated diabetic retinopathy via inhibition of inflammatory response. Biochemical and Biophysical Research Communications 493: 1136-1142.
Liu H.Y., Hong Y.F., Huang C.M., Chen C.Y., Huang T.N., Hsueh Y.P. (2013). TLR7 Negatively Regulates Dendrite Outgrowth through the Myd88-c-Fos-IL-6 Pathway. Journal of Neuroscience 33: 11479-11493.
London A., Benhar I., Schwartz M. (2013). The retina as a window to the brain—from eye research to CNS disorders. Nature Reviews Neurology 9: 44-53.
Michaelis K. A., Norgard M. A., Levasseur P. R., Olson B., Burfeind K. G., Buenafe A. C., Zhu X., Jeng S., McWeeney S. K., Marks D. L. (2019). Persistent Toll-like receptor 7 stimulation induces behavioral and molecular innate immune tolerance. Brain, Behavior, and Immunity 82: 338-353.
Peter V. G., Morandi S. C., Herzog E. L., Zinkernagel M. S., Zysset-Burri D. C. (2023). Investigating the Ocular Surface Microbiome: What Can It Tell Us?. Clinical Ophthalmology Volume 17: 259-271.
Rasile M., Lauranzano E., Faggiani E., Ravanelli M. M., Colombo F. S., Mirabella F., Corradini I., Malosio M. L., Borreca A., Focchi E., Pozzi D., Giorgino T., Barajon I., Matteoli M. (2022). Maternal immune activation leads to defective brain–blood vessels and intracerebral hemorrhages in male offspring. The EMBO Journal 41: e111192.
Redfern R. L., McDermott A. M. (2010). Toll-like receptors in ocular surface disease. Experimental Eye Research 90: 679-687.
Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671-675.
Sheng J. A., Tobet S. A. (2024). Maternal immune activation with toll‐like receptor 7 agonist during mid‐gestation alters juvenile and adult developmental milestones and behavior. Journal of Neuroendocrinology 36: e13417.
Singh P. K., Kumar A. (2015). Retinal Photoreceptor Expresses Toll-Like Receptors (TLRs) and Elicits Innate Responses Following TLR Ligand and Bacterial Challenge. PLOS ONE 10: e0119541.
Smolders S., Notter T., Smolders S. M.T., Rigo J.M., Brône B. (2018). Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders. Brain, Behavior, and Immunity 73: 51-65.
Sommariva M., Busnelli M., Menegola E., Renzo F. D., Indino S., Menon A., Barajon I., Arnaboldi F. (2023). Immunostaining patterns reveal potential morphogenetic role of Toll-like receptors 4 and 7 in the development of mouse respiratory system, liver and pancreas. Anatomy & Cell Biology 56: 228-235.
Wang L., Li S., Cai K., Xiao Y., Ye L. (2023). TLR7 Agonists Modulate the Activation of Human Conjunctival Epithelial Cells Induced by IL-1β via the ERK1/2 Signaling Pathway. Inflammation 46: 1430-1444.
Wladis E. J., Iglesias B. V., Adam A. P., Nazeer T., Gosselin E. J. (2012). Toll-Like Receptors in Idiopathic Orbital Inflammation. Ophthalmic Plastic & Reconstructive Surgery 28: 273-276.
Yamamoto N., Majima K., Marunouchi T. (2008). A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Medical Molecular Morphology 41: 83-91.
Yu L., Wang L., Chen S. (2010). Endogenous toll‐like receptor ligands and their biological significance. Journal of Cellular and Molecular Medicine 14: 2592-2603.