Int. J. Dev. Biol. 67: 65 - 78 (2023)
Insights into the role of the Wnt signaling pathway in the regeneration of animal model systems
Review | Published: 31 October 2023
Abstract
Regeneration enables the regrowth and restoration of missing body parts. It is a common phenomenon among animals. However, only some species exhibit remarkable regeneration capabilities and can regenerate organs such as limbs, lenses or hearts. Regeneration has been widely studied, thereby giving rise to new fields, such as regenerative medicine. Furthermore, regeneration has the potential to be applied to the human body. However, the molecular mechanisms governing this process should be elucidated first. Recent advancements in research methods have led to the identification of numerous signaling pathways involved in regeneration. One of them, the Wnt transduction pathway, is an ancient and evolutionarily conserved pathway that plays an important role in both embryonic development and regeneration. The Wnt pathway plays an important role during the regeneration process, as it is implicated in cell fate determination, cell migration, cell polarity and adult cell homeostasis. To date, two major Wnt pathways have been identified: the canonical (β-catenin dependent) pathway and the non-canonical pathway. The latter pathway can be further divided into planar cell polarity, the Wnt/Ca2+ pathway and the JNK pathway. In this review, we summarize the current state of knowledge regarding the Wnt signaling pathway and its role in regeneration, with a particular emphasis on key model species.
Keywords
regeneration, Wnt/β-catenin, signalling pathway, model organisms
Introduction
Regeneration is a common phenomenon in the animal kingdom that enables the restoration of missing body parts after injury or amputation; as a result, damaged structures are perfectly or near-perfectly replaced (Gilbert, 2000). Although some form of regeneration exists in almost all species, remarkable regenerative capabilities are observed only in a few taxa, such as cnidarians, crustaceans and salamanders. Humans have very poor regenerative abilities, and only a few types of tissue can regenerate. Nevertheless, people have been fascinated with this process since the 18th century. In recent years, there have been considerable advancements in the field of regenerative medicine, which aims to restore tissues, organs or even entire body parts. To achieve this, the first step should be full elucidating the molecular components underlying the regeneration process. In this review, we attempt to compile existing knowledge regarding a small part of the regeneration process.
The process of regeneration varies widely in terms of complexity, underlying mechanisms and magnitude (Tsonis, 2000). Four major modes can be distinguished: morphallaxis, compensatory regeneration, stem cell-mediated regeneration and epimorphosis. Morphallaxis, which is observed in Hydra, is repatterning of existing tissues. Compensatory regeneration occurs when differentiated cells divide but maintain their functions (neither dedifferentiation nor stem cells are involved). For example, in the mammalian liver, stem cell-mediated regeneration allows for regrowth of certain tissues, such as blood cells from hematopoietic stem cells in the bone marrow. Epimorphosis enables the formation of a new structure from an undifferentiated mass of cells that later redifferentiates; this process is typical in amphibian limb regeneration (Iismaa et al., 2018).
In brief, epimorphic regeneration is accomplished by cell dedifferentiation and the formation of a regeneration blastema that proliferates and redifferentiates into new body parts (Fig. 1B). Generally, the regeneration process can be divided into three phases: wound healing, dedifferentiation and redevelopment. Within a few hours following amputation or injury, the wound is sealed by the rapid migration of epithelial cells that form the wound epidermis (no scar) (Brockes, 1997). In the next few days, all the cells undergo dedifferentiation and lose their characteristics. This cell mass is known as the regeneration blastema. In addition, the wound epidermis thickens and forms the apical epidermal cap (AEC). Growth of the blastema depends on the presence of AECs, nerves and ion currents (Altizer et al., 2002). It elongates for the next several days through mitotic activity. During the second phase, which is unique to limb regeneration, spatial and temporal patterns of gene expression differ from those observed in development; then, in the third phase, the expression of genes shows similarities with the developing limbs (Gardiner et al., 2002). Although gene functions are conserved during development and regeneration, there are still many differences in expression patterns.
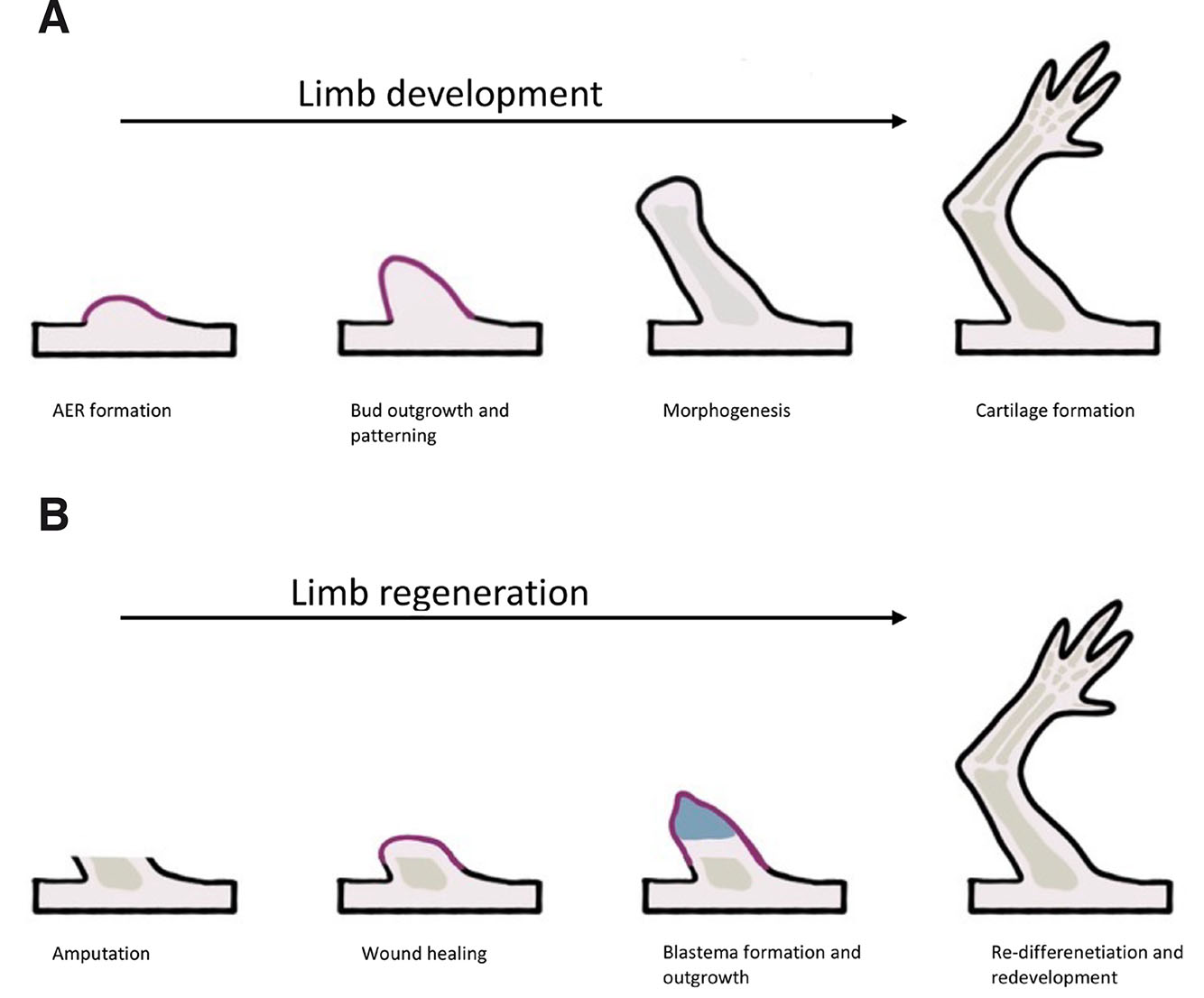
Fig. 1. Comparison of limb development (A) and limb regeneration (B).
Limb development starts with bud formation, followed by apical ectodermal ridge (AER) formation. The next phase is bud outgrowth and patterning. Later, morphogenesis starts, followed by chondrocyte condensation, and finishing with cartilage formation. Limb regeneration starts with wound healing, then the blastema forms and cells proliferate. At the end, re-differentiation and redevelopment is observed.
The development of sophisticated research tools previously led to the identification of the main components taking part in the regeneration of species with high regeneration potential, such as planarians, newts, and zebrafish. These studies revealed evolutionarily conserved signaling pathways involved in the process, including the Wnt (Clevers et al., 2014), Notch (Raya et al., 2003), Hedgehog (Hh) (Torok et al., 1998), BPM (Molina et al., 2007), and JAK/STAT pathways (Herrera and Bach 2019). Although there are many other pathways that play a considerable role in regeneration, this review will focus on the Wnt pathway, which has been identified to be essential for the regeneration of Hydra, Planaria, newts, zebrafish, Drosophila and even mouse or human liver. The highly conserved Wnt pathway has a critical function during development and regeneration. Although Wnt genes are extant in the earliest metazoans, such as sponges, placozoans and ctenophores, they have not been described in any unicellular eukaryotes (Holstein et al., 2011). Recent research advancements have helped to better understand its components, signals and consequences resulting from its perturbations.
In this review, we characterize the main components of the Wnt signaling pathway and summarize the current state of knowledge regarding the role that the Wnt transduction pathway plays in the regeneration of vertebrates. Finally, we identify gaps in knowledge and indicate possible directions for future research.
Wnt signaling
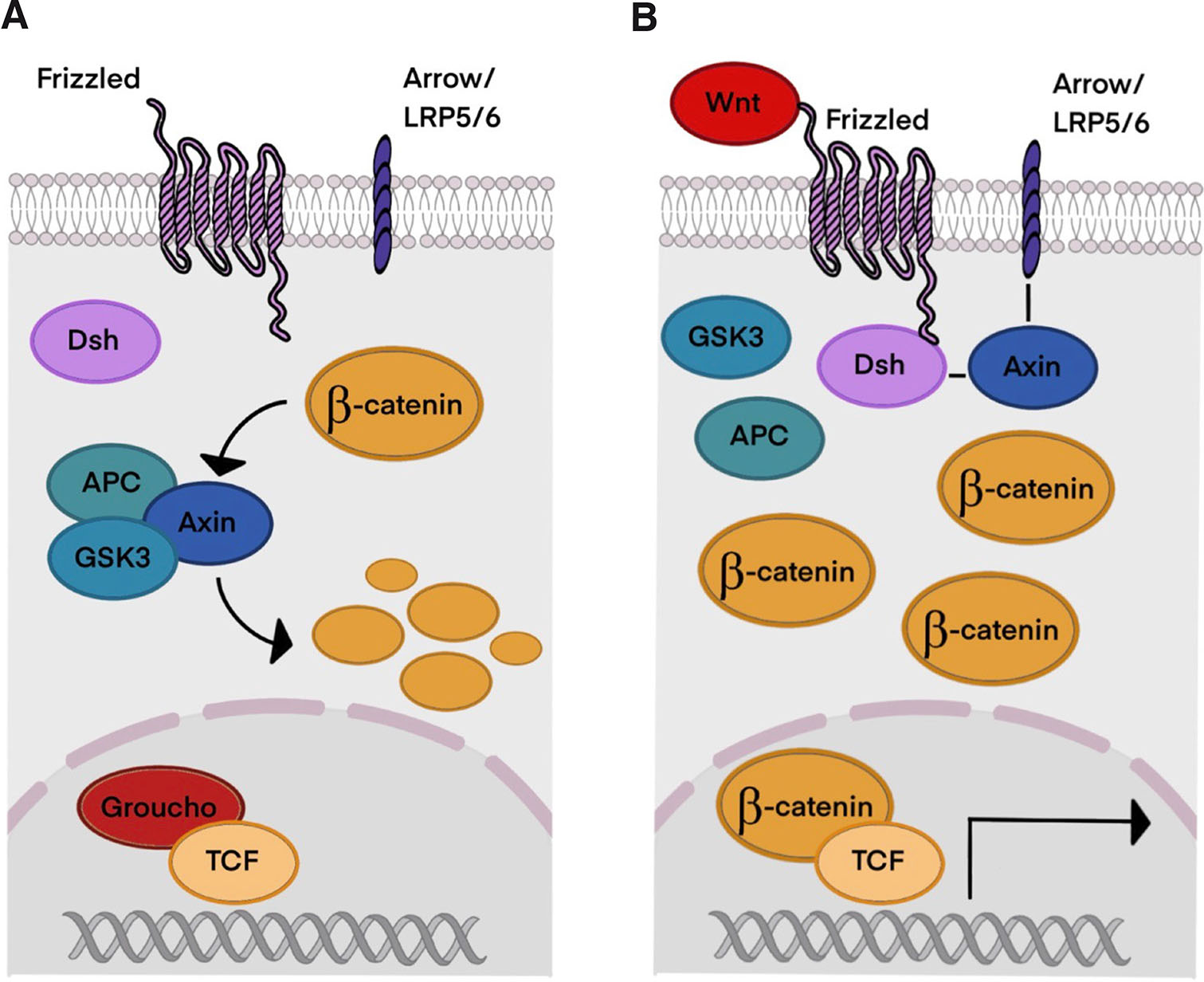
Wnt genes are characterized by their structure, and they encode palmitoylated proteins with a characteristic cysteine pattern. It has been shown that cysteine is critical for their function, while palmitate is essential for signaling. In the Wnt/β-catenin pathway, Wnts bind to the cysteine-rich domain of the seven-transmembrane-span protein Frizzled (Fz). Additionally, Wnt signaling also requires a molecule from the LRP family (arrow in Drosophila, LRP5 or LRP6 in vertebrates). Dishevelled (Dsh), a cytoplasmic protein, transduces Wnt signals through direct binding between Dsh and Fz. Wnt signaling leads to phosphorylation of Dsh. LRP may also interact with a cytoplasmic component of the Wnt pathway: Wnts induce phosphorylation of LRP, allowing it to dock Axin. Wnt binding to Fz and LRP enhances the direct interaction between Axin and Dsh, modifying the protein complex that regulates β-catenin concentration in the cell (Huelsken and Behrens, 2002).
In the absence of Wnt signaling (Fig. 2A), β-catenin is phosphorylated by the kinases and glycogen synthase kinase 3 (GSK3). This interaction is facilitated by the scaffolding proteins Axin and anaphase-promoting complex (APC), which together form a β-catenin degradation complex. Phosphorylated β-catenin is then ubiquitinated and degraded by the proteasome. Without Wnt signaling in the nucleus, TCF acts as a repressor of Wnt target genes by forming a complex with Groucho. Additionally, the Wnt pathway may be suppressed by multiple inhibitors, of which Dickkopf (Dkk) is one of the most potent, as it binds to LRP and other transmembrane molecules, which makes it unavailable for Wnt reception.
Fig. 2. The Wnt/β-catenin signaling pathway.
(A) Without Wnt signaling β-catenin concentration remains low because of its degradation and ubiquitination. (B) When Wnt signaling is active, β-catenin accumulates in the cell and nucleus, initiating transcription of Wnt target genes. Abbreviations: LRP5/6, Low density lipoprotein receptor-related protein 5/6; Dsh, Disheveled; APC, adenomatous polyposis coli protein; GSK3, glycogen synthase kinase 3; TCF, T cell factor.
Activation of the Wnt pathway prevents phosphorylation of β-catenin and hence its degradation, leading to the disruption of the degradation complex and accumulation of β-catenin. When Wnt ligand binds to its receptor, it induces a conformational modification of the receptor, which leads to phosphorylation by protein kinases and inhibition of GSK3 (Clevers et al., 2014). Nuclear accumulation of β-catenin causes conversion of the TCF repressor complex into a transcriptional activator complex. Groucho is replaced with CBP, which may bind β-catenin/TCF as a coactivator (Fig. 2B). In summary, β-catenin forms a complex with the LEF/TCF transcription factor that results in transcriptional activation of Wnt target genes. Wnt target genes control a wide range of biological processes, and they are involved in embryonic and larval patterning and homeostasis in adults. Even loss of a single Wnt gene can create dramatic phenotypes (Logan and Nusse 2004). Disruption of this pathway can lead to degenerative diseases and cancer in humans (Katoh 2005; Yin et al., 2016). Although in some cases Wnt signals may be transduced independently of β-catenin and although Frizzled and Dishevelled play crucial roles, this review is concentrated on the β-catenin-mediated pathway.
Wnt signaling pathway in regeneration
Hydra
Hydra is a freshwater cnidarian with high regeneration potential, which is why it is ubiquitously used as a model species in many laboratories. Hydra can regenerate the foot, basal and apical head via morphallactic (without cell proliferation) or epimorphic modes. In comparison with vertebrates, due to its simple anatomy, it is relatively easy to study; consequently, there is much available data, including data on the Wnt signaling pathway (Mehta and Singh 2019).
In Cnidaria, 13 Wnt families have been identified, eleven of which were found in Hydra and include genes involved in both canonical and non-canonical pathways (Lengfeld et al., 2009a). During Hydra embryogenesis, most Wnt genes are expressed, while during asexual reproduction, all of them are expressed in the initial stages of bud formation. Their expression ratio varies between ectoderm and endoderm for each gene. Most of the genes are strongly expressed in the ectoderm, and then their expression shifts to the endoderm.
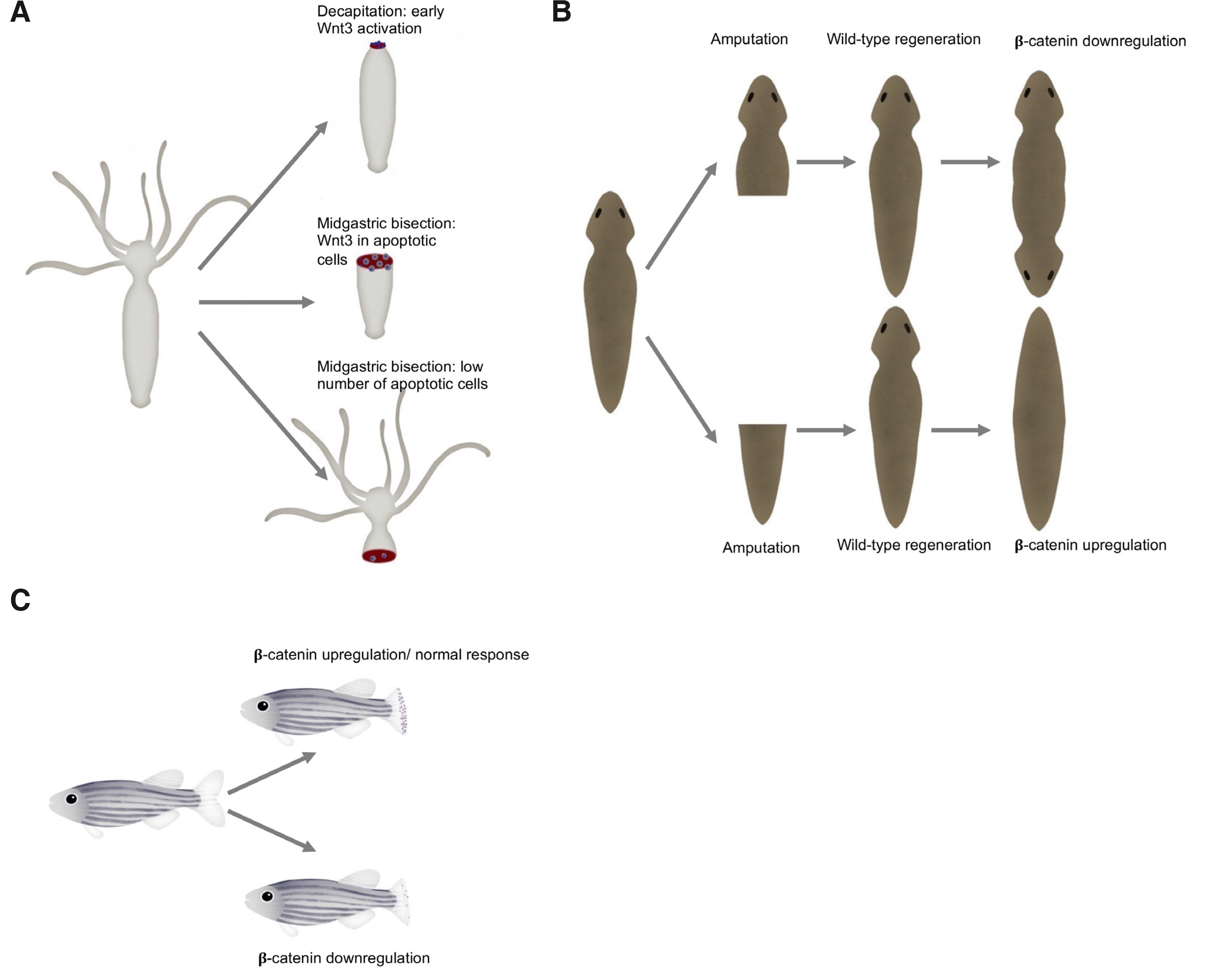
Numerous studies have shown that Wnt signaling is pivotal for Hydra head regeneration (Philipp et al., 2009). Cells close to the amputation plane undergo apoptosis. During the short time window, apoptotic cells are filled with Wnt3a, and it is likely that it is already present in dying cells. Then, Wnt3 released by apoptotic cells activates the β-catenin pathway. Moreover, the course of the regeneration process in Hydra varies depending on the amputation level. Following decapitation, direct redevelopment is induced as a result of Wnt3 signaling from epithelial cells (Fig. 3A). In the case of mid-gastric amputation, Wnt3 signaling is initiated in interstitial cells, and activation in epithelial cells occurs later (3 h post-amputation in comparison to 1.5 h post-amputation after decapitation) (Galliot and Chera 2010). Silencing Wnt3 or β-catenin inhibits head regeneration (Chera et al., 2009). β-Catenin is required for both head and foot regeneration. On the other hand, overexpression of β-catenin results in regeneration of extra heads and feet (Gufler et al., 2018).
Fig. 3. The Wnt/β-catenin signaling components are required in the regeneration of animal model systems.
(A) Decapitation of Hydra leads to fast Wnt3 activation, followed by a cascade of events resulting in regeneration (upper right). Comparatively, expression of Wnt3 in epithelial cells is delayed in the case of mid-gastric bisection (middle right). During foot regeneration there is a low apoptotic cell activity (lower right). (B) Following amputation, wild-type planarian regenerates missing structures (middle). In the absence of β-catenin signaling, an animal regenerates a head instead of a tail resulting in a two-headed animal (upper right corner). In the other case, when β-catenin is upregulated, it leads to the animal with two tails - without a head (lower right corner). (C) During zebrafish regeneration, Wnt/β-catenin signaling is activated, and cell proliferation occurs (upper right). When the Wnt/β-catenin signaling pathway is suppressed cell proliferation decreases and regeneration fails (lower right).
The remaining Wnt genes are activated sequentially. Following decapitation, Wnt1 and Wnt16 are upregulated at 3 hours post-amputation (hpa), and then Wnt9, Wnt10a and Wnt7 start at 6 hpa. In the case of mid-gastric bisection, Wnt7, Wnt9, Wnt10a and Wnt16 are expressed at 12 hpa and Wnt1 at 24 hpa (Lengfeld et al., 2009a). Furthermore, head regeneration from the mid-gastric level depends upon two waves of Wnt3 signaling. Although the course of the Wnt signaling pathway in Hydra regeneration is quite well documented, there are still some unanswered questions. For example, how apoptosis directs Wnt3 release and how interstitial Wnt3 signaling leads to Wnt3 expression in the endodermal epithelial cells.
Based on functional analysis, research indicated that eight Wnt genes are involved in the formation and maintenance of the head organizer (Hobmayer et al., 2000), such as Wnt1, -3, -7, -11 and probably Wnt2, which plays a role in the early stages of axis formation. As mentioned before, Wnt3 acts as a primary ligand; it not only induces head formation but is also crucial for axis formation. In contrast, Wnt5 acts as a putative inhibitor of the Wnt/β-catenin pathway, repressing head growth. It remains unclear whether Wnt5 acts on the canonical, non-canonical or both pathways, even though different studies have indicated that Wnt5 is involved in the non-canonical pathway (PhilippPhilipp et al., 2009). Another inhibitor, Sp5, represses Wnt3 expression a few hours after bisection, preventing ectopic head formation but providing enough time to instruct head formation. Interestingly, Sp5 most likely autorepresses when reaching high intracellular levels; however, more evidence should be acquired (Vogg et al., 2019).
As stated above, there are various non-canonical pathways. Their role in the regeneration process has not been studied as extensively as the canonical pathway. Nevertheless, Philipp et al., (2008) have taken a first step toward that direction. They showed that Wnt/β-catenin signaling is required for the activation of the non-canonical pathways. The JNK pathway is involved in lateral cell intercalation. Inhibition of the JNK pathway stops tentacle formation, which could be due to morphogenetic tissue movements. Interestingly, it does not influence positional information.
In other species, hierarchical dependency of the genes involved in various signaling pathways has been observed. In the case of Hydra, Notch signaling is required for the activation of Wnt3 and β-catenin, and its inhibition represses Wnt3 and β-catenin expression; therefore, the head organizer does not form. This raises a question: how is the Wnt pathway related to other pathways during regeneration? Research on Hydra provides useful information about animal regeneration and the role of the Wnt pathway in that process. Additionally, previous studies indicate the significance of the evolutionarily conserved Wnt pathway, suggesting that molecular mechanisms in Hydra regeneration might have applications in other species.
Planaria
Planaria (Platyhelminthes) are freshwater free-living flatworms with bilateral symmetry and a nervous system. They have astonishing regenerative potential, as they can regrow any part of their body. They regenerate via cell proliferation (with blastema formation) and morphallaxis (Reddien and Sánchez Alvarado, 2004). The Wnt signaling pathway has been identified in Planaria as one of the most important pathways during the regeneration process. To date, nine Wnt genes have been found. It seems that the Wnt/β-catenin pathway determines head-tail polarity during regeneration. Intriguingly, the β-catenin anterior-posterior specification system is present in adult tissue. Knockdown of β-catenin results in regeneration of the animal with two heads (with no tail) (Fig. 3); moreover, in that case, the animal may grow numerous sideways facing heads (Petersen and Reddien, 2009b). In contrast, when β-catenin is upregulated, it may result in regeneration of posterior structures. Moreover, β-catenin silencing in uncut adult animals triggered the transformation of a tail into a head. Hence, β-catenin is required for the regeneration of posterior structures; it acts as a switch to specify and maintain anteroposterior identity (Gurley et al., 2008). It is possible that the secret behind planarian abilities to regenerate lies in the application of embryonic signaling profiles during adult tissue homeostasis (Tanaka and Weidinger, 2008). A similar situation takes place in the absence of Evi (Evenness, also known as Wntless): its depletion caused the growth of posterior heads. However, Evi is also necessary for the proper regeneration of the brain and ventral brain cords in Planaria (Adell et al., 2009). Interestingly, Wnt signaling is pivotal for antero-posterior brain patterning in various planarian species (Kobayashi et al., 2007).
WntP-1 is one of the genes required for tail formation; typically, it is expressed posteriorly. Surprisingly, at the beginning of regeneration, it is expressed anteriorly and posteriorly in all kinds of wounds, indicating its role in the process. Another gene, WntP-2, is activated afterward in posterior wounds, as it requires the expression of WntP-1 and β-catenin (de Robertis, 2010). Both WntP-1 and WntP-2 have a role in polarity, which seems independent and instructive for tail formation. It is possible that neoblasts are active after Wnt-mediated polarity to implement further tail formation (Petersen and Reddien, 2009a). WntP-3 and WntP-11 are also expressed posteriorly; however, expression of WntP2-1 is restricted anteriorly in heads. These data suggest that the AP axis in Planaria is controlled by anterior Wnt inhibition and posterior Wnt activation (Petersen and Reddien, 2009b). Petersen and Reddien (2009a) found that none of the Wnt genes result in posterior head regeneration, likely due to redundancy among these genes. Nonetheless, double knockdown of WntP-1 and WntP-2 boosted the rate of posterior head development (de Robertis, 2010).
As mentioned in the Hydra paragraph, there is a certain hierarchy of transduction pathways. For example, in the case of Planaria, an early regenerative response is activated by Hedgehog (Hh) signaling acting upstream of Wnt (de Robertis, 2010). Experiments activating the Hh pathway led to the overexpression of WntP-1 and hence regeneration of tails instead of heads.
Research on Planaria contributed substantially to our understanding of the Wnt signaling pathway’s role in the wounding response, regeneration and stem cell proliferation. Among others, tissue identity determination is a considerable function of Wnt proteins.
Zebrafish
Zebra fish (Danio rerio) is a teleost freshwater fish native to South Asia. As a lower vertebrate, it constitutes a perfect model species for regeneration studies since it has the ability to regrow numerous tissues, such as fins, retina, pancreas, liver, kidney, spinal cord, brain and even heart (Table 1) (Gemberling et al., 2013). Moreover, considering that many human genes have zebrafish orthologs (Howe et al., 2013), new knowledge regarding the mechanisms of zebrafish regeneration gives hope for applying zebrafish to the human body in the future (Poss et al., 2003). Wnt signaling is required for the regeneration of various tissues in zebrafish. For example, during retinal regeneration, a canonical pathway is necessary for the proliferation of Müller glia (Meyers et al., 2012). During bone regeneration, epithelial to mesenchymal transformation of osteoblasts depends on Wnt/β-catenin signaling, which is necessary to generate proliferative pre-osteoblasts (Stewart et al., 2014).
Table 1
Regenerating organs and body parts in described organisms
| Body parts/organs/ tissues | Hydra | Planaria | Zebrafish | Xenopus | Axolotl |
|---|---|---|---|---|---|
| Head | Lengfeld et al., 2009 | Petersen and Reddien, 2009a | |||
| Tail | Petersen and Reddien, 2009a | Gemberling et al., 2013 | Lin and Slack 2008 | Brockes and Kumar 2008 | |
| Limbs/fins/ feet | Lengfeld et al., 2009 | Gemberling et al., 2013 | Lin and Slack 2008 | Brockes and Kumar 2008 | |
| Heart | Liao et al., 2017 | Cano Martínez et al., 2010 | |||
| Eyes (retina) | Gemberling et al., 2013 | Beck et al., 2009 | Suetsugu-Maki et al., 2012 | ||
| Muscles | Gemberling et al., 2013 | Lin et al., 2012 | McCusker and Gardiner 2011 | ||
| Bones | Gemberling et al., 2013 | Lin and Slack 2008 | McCusker and Gardiner 2011 | ||
| Spine | Gemberling et al., 2013 | Edwards-Faret et al., 2017 | Sabin et al., 2019 | ||
| Liver | Gemberling et al., 2013 | Ohashi et al., 2021 | |||
| Kidney | Gemberling et al., 2013 | Krneta-Stankic et al., 2017 | |||
| Pancreas | Gemberling et al., 2013 | ||||
| Brain | Gemberling et al., 2013 | Lee-Liu et al., 2017 | Roy and Gatien, 2008 |
As human hearts have no ability to regenerate by themselves, knowledge about regeneration among other species may be very valuable for regenerative medicine, such as zebrafish, which can regrow up to 20% of their cardiac muscle (Plackett 2021). Moreover, unlike other regenerating organisms, teleost fish can regenerate hearts throughout their life. They constitute a good model for regeneration studies, as some aspects of their heart physiology are similar to those of mammals; however, these fish hearts only have two chambers, which makes them easier to work with. Following heart injury, transcriptional responses occur within a few days, and many genes expressed during development are upregulated. Nevertheless, the role of the Wnt pathway has yet to be thoroughly investigated. Just a few years ago, researchers only suspected that the Wnt pathway takes part in heart regeneration; however, tools available at the time did not allow verification (Ozhan and Weidinger 2015). Fortunately, since then, significant technological advances have been made, and the role of the Wnt pathway in cardiac regeneration has been extensively reviewed by Li et al., (2022). They state that Wnt and Wnt antagonists are involved in various processes during cardiac injury repair; furthermore, non-canonical or Wnt-independent processes contribute to cardiac repair. Heart injury in zebrafish results in the expression of Wnt inhibitors such as Dkk1 and Dkk3 and secreted Frizzled-related protein 1 (sFrp1) and sFrp2 (Peng et al., 2021). Wnt inhibition is beneficial for cardiac recovery, as it supports cardiomyocyte dedifferentiation and proliferation and prevents scarring (Zhao et al., 2019). Developing tools allowing for Wnt antagonist employment without affecting other pathways will be crucial for treating human diseases.
Another impressive ability that regenerative medicine would like to apply to the human body involves zebrafish’s potential to regenerate the spinal cord. In contrast to cardiac injury, during spinal cord repair, the Wnt/β-catenin pathway is pivotal for fish recovery, specifically for axon regrowth and functional recovery. It was found that overexpression of Wnt antagonists such as Dkk1 inhibits processes including locomotory recovery, axon regeneration, and glial bridge formation. Furthermore, spinal cord transection leads to the upregulation of Wnt/β-catenin in non-neural cells. It is essential as it controls the composition of the lesion site extracellular matrix, and it induces expression of col12a1a/b and Collagen XII: a promoter of axonal regeneration. Interestingly, inhibition of Wnt signaling impacts col12a1a/b in a delayed manner, thereby sustaining its expression and resulting in successful regeneration (Wehner et al., 2015, 2017). Additionally, Strand et al., (2016) demonstrated that the role of this pathway is conserved in larval and adult zebrafish, indicating that using developing fish for spinal cord injury research might have advantages over adult fish.
Zebrafish fin regeneration
The first implication of Wnt signaling in zebrafish regeneration was made by Poss et al., (2000), who observed the expression of lef1, a Wnt target gene. Initial research on zebrafish was not focused on the members of the Wnt family but on the target genes. Except for lef1, msxb (marker of blastema formation) and other genes were also downregulated by Dkk overexpression, (Kawakami et al., 2006) and their silencing led to regeneration failure. As in other animals described above, in epimorphic regeneration of zebrafish, Wnt/β-catenin signaling plays a crucial role, and its signals can be detected even 3 h following the injury. Ligand activating the canonical pathway, wnt10 is expressed very early during regeneration. It has been demonstrated that the same gene activates the Wnt pathway during limb development. Activation of Wnt/β-catenin signaling may be a conserved trait, as a similar pattern was observed during zebrafish heart regeneration.
Ligand expression does not have to result in activation of transcription; however, it leads to the expression of Wnt target genes (Stoick-Cooper et al., 2007). This leads to the expression of genes from the fibroblast-like growth factor (FGF) family that, together with the canonical pathway, regulate blastemal proliferation. After blastema formation, the non-canonical pathway is activated by the expression of Wnt5 genes and is maintained throughout the process (Poss et al., 2000).
Expression of Wnt inhibitors in transgenic fish after wound healing but before blastema formation results in complete regeneration failure. Subsequent experiments expressing Dkk during the outgrowth phase proved that Wnt/β-catenin signaling is required not only for blastema formation but also for its maintenance. On the other hand, overexpression of Wnt8 led to a significant increase in blastema mesenchyme proliferation; however, it did not influence fin length. Intriguingly, fish with the mutated copy of Axin (a potent inhibitor of the Wnt pathway) regenerated significantly longer fins than wild-type fish. This aspect of Wnt/β-catenin signaling in regeneration was reported for the first time, and it sheds new light on its function. As mentioned above, after the canonical and non-canonical pathways are activated, they have opposing roles in the process. Wnt5b activates the non-canonical pathway; when this gene is overexpressed, proliferation is reduced, resulting in regeneration failure. Moreover, it produces similar phenotypes as in the case of Dkk overexpression. The lack of this gene’s expression did not cause a patterning effect or inappropriate growth, suggesting that it acts in a negative feedback loop to modulate Wnt/β-catenin signaling.
In previous chapters, we mentioned the hierarchy of various pathways. Work on zebrafish indicated that Wnt/β-catenin acts upstream of FGF and is necessary to maintain FGF signaling (Stoick-Cooper et al., 2007). Later studies implied that Wnt/β-catenin signaling affects regeneration in an indirect way, as it regulates other key pathways, including the Hh, BMP, retinoic acid (RA), Igf, Notch and Activin pathways (Wehner et al., 2014). In summary, zebrafish injury activates the expression of Wnt ligands (such as Wnt10a), which initiate Wnt/β-catenin signaling. This leads to the expression of FGF genes, which together with Wnts prompt blastema formation and thus regeneration. In the meantime, β-catenin-independent signaling is triggered, which regulates β-catenin signaling. It is likely that these two pathways form a loop that guarantees accurate level, duration, and location of β-catenin signaling. Wnt and FGF signaling are assumed to be the main pathways that govern epimorphic fin regeneration in zebrafish (Tal et al., 2010).
Xenopus
Xenopus laevis is another ubiquitously used model organism in numerous studies worldwide. It has a remarkable regeneration capacity as a tadpole (Table 1), but its ability to regenerate significantly decreases after metamorphosis (Muneoka et al., 1986). After limb injury, young post-metamorphosis adults can regenerate only spike-like structures. Even at later stages when regeneration capacity is partially attenuated, β-catenin can promote regeneration.
As in many other species, in a dose-dependent manner, Wnt signaling is necessary for AP patterning during Xenopus gastrulation, and it also constitutes a transforming morphogen to form the central nervous system (Kiecker and Niehrs, 2001). Although both β-catenin and non-canonical pathways are involved in cardiogenesis, little is known about their involvement in heart regeneration (Hoppler and Conlon 2020).
In recent years, the role of Wnt/β-catenin signaling in the regeneration process has been widely researched. Wnt/β-catenin signaling is essential for tadpole early-stage limb regeneration, especially during apical epithelial thickening and blastema formation (Yokoyama et al., 2007). Without it, initiation of tadpole limb regeneration will fail, while β-catenin can promote tail outgrowth (Lin and Slack, 2008). On the other hand, at the later stages of regeneration, Wnt signaling is still involved but not needed, as tadpoles with the heat-shocked expressed Dkk still managed to regenerate (at least partially!). According to Yokoyama et al., (2007), even though many ligands activate β-catenin signaling, only Wnt3a activates the pathway in both developing and regenerating limbs. Its expression pattern suggests that it contributes to the formation of AECs. Similar expression patterns were observed in both tadpoles and froglets (young-metamorphosed adults). Thus, it could be assumed that the absence of Wnt signaling during regeneration of these two stages will have similar effects. As previously explained, the lack of Wnt signaling in tadpoles will result in regeneration failure; however, the situation is slightly different in the case of froglets.
Previous research established that limb regeneration requires the presence of nerves (Suzuki et al., 2005). Surprisingly, even when Wnt signaling is blocked, froglets can still regenerate spike structures. Yokoyama et al., (2011) reported that nerve signals can functionally substitute for Wnt/β-catenin signaling during froglet regeneration. Nerve signals may involve members of other families, such as FGF, compensating for impaired Wnt signaling. Consequently, they can stimulate blastema formation. On the other hand, Wnt signaling can rescue defects caused by partial innervation. It is conspicuous that while inhibition of Wnt/β-catenin signaling is not sufficient to block regeneration, total denervation will entirely hinder this process. These facts support the hypothesis that nerve signals are more pivotal for the initiation of froglet limb regeneration because nerve signals can activate more target genes involved in regeneration than Wnt/β-catenin signaling. This paper confirms that nerve signals and Wnt/β-catenin have overlapping functions in the initiation of the regeneration process. To our knowledge, no prior studies have examined the mutual implications of nerve and Wnt/β-catenin signaling. It is clear that nerves play a role in regeneration; however, more studies are needed to understand the complete correlation between nerve signaling, Wnt and other pathways. To apply these data to the practical aspects of regenerative medicine, it is particularly recommended to focus future investigations on other model species.
Another fascinating aspect of regeneration in Xenopus is the role of the non-canonical pathway. Tail regeneration requires Wnt5a, which is mediated by the Wnt/JNK pathway. Studies have shown that Wnt/JNK plays a role in the distal specification of the regenerating tail. Sugiura et al., (2009) deduced that Wnt/β-catenin and Wnt/JNK are required to form the distal epidermis. Nevertheless, as described in the above chapter, there is strong evidence that canonical and non-canonical pathways antagonize each other (Stoick-Cooper et al., 2007). For that reason, future research should examine whether an antagonistic relationship between these pathways occurs in the case of Xenopus tail regeneration.
Resembling zebrafish, during Xenopus regeneration, Wnt/β-catenin signaling acts upstream of FGF, which has a noteworthy implication (Slack et al., 2008)). Suppression of the Wnt pathway stops the expression of some Fgf genes in the blastema (Ng et al., 2002), although others imply that they are not directly affected (Yokoyama et al., 2007). In comparison with Wnt signaling, FGF signaling occurs quite late and is supposedly necessary for the late outgrowth stage. In contrast, the BMP pathway is upstream of both Wnt and FGF and is required for their maintenance (Lin and Slack, 2008). Another factor influencing the Wnt pathway and hence Xenopus tail regeneration is reactive oxygen species (ROS). ROS inhibition is followed by a decrease in Wnt/β-catenin signaling, which leads to a reduction in cell proliferation and compromises tail regeneration (Love et al., 2013). Even so, there are still gaps in knowledge regarding elements that impact the Wnt pathway.
In recent years, most discussions regarding Xenopus regeneration have focused solely on the tail and limb, yet Xenopus can regenerate other tissues and organs. Xenopus is a well-established and vital model for eye development and regeneration studies, as its eye structure is similar to that of humans. Additionally, Xenopus can regenerate both the retina and lens, even after metamorphosis (Tseng, 2017). As mentioned, tail and limb regeneration in Xenopus rely on epimorphosis However, in eye regeneration, another process can be distinguished: transdifferentiation of the cornea to the lens. Wnt has been shown to be one of the pathways involved in lens regeneration, and several of its components were significantly upregulated in that process (Day and Beck, 2011). Malloch et al., (2009) suggested that Wnt, among other pathways such as BMP, Hedgehog, FGF, and TGF-beta signaling, plays an important role in regeneration. Although the Wnt pathway in retinal development has been extensively studied, its detailed role in Xenopus has yet to be elucidated.
Lin et al., (2012) documented that Wnt and FGF signals are compulsory for spinal cord and muscle regeneration. Generally, different types of tissues undergo regeneration independently; therefore, they require specific signaling. Nevertheless, there seems to be a relationship in the regeneration of these tissues. Although regeneration might proceed even in the absence of the spinal cord, inhibition of that process reduces the volume of the regenerated muscles and affects a notochord. Whereas attenuation of the Wnt and Fgf signals in muscles may reduce their size, it will not block spinal cord regeneration. As in many other organisms or tissue types, overexpression of Dkk1 caused reduction or complete inhibition of spinal cord regeneration, which resulted in tail regeneration failure (Lin et al., 2012). In addition to these exciting insights, little is known about the role of Wnt signaling in the regeneration of other tissue types.
Urodele amphibians
Urodeles: Newts and salamanders are the only tetrapod vertebrates that can fully regenerate injured limbs throughout their life cycle. They also have the ability to regenerate tails, lenses, retina, spinal cord and heart tissue (Table 1) (Brockes and Kumar 2008). Hence, they offer a functional model for studying molecular and cellular regeneration mechanisms. Analysis of amphibian regeneration is the focus of regenerative biology with the anticipation that once we understand them, we will be able to replicate it in mammalian limbs (Stocum and Cameron, 2011). One of the most popular urodele model species used in regenerative studies is Ambystoma mexicanum, commonly known as an axolotl. As axolotl limbs are anatomically similar to those of humans, knowledge of their regeneration can provide valuable information for regenerative medicine (Haas and Whited, 2017).
Axolotl hearts have two atria and an avascular ventricle. After heart injury, they can replace lost tissues, starting with clot-preventing blood loss, followed by fibrin and collagen deposits. Axolotls can functionally and structurally regenerate within 60-90 days, and the response is related to the formation of fibrotic scars, replaced by new cardiomyocytes (Vivien et al., 2016). They can also regenerate their spinal cord via ependymoradial glial cell proliferation and differentiation (Khyeam et al., 2021). Although heart and spinal cord regeneration in urodele amphibians is very impressive, there is little knowledge regarding the role of the Wnt pathway in these processes.
Nevertheless, they are absorbing material for analysis of the Wnt pathway: not only does its genome have orthologs representing all Wnt classes, but it also has a complete set of vertebrate Wnt genes (Nowoshilow et al., 2018). It has been well established that the Wnt signaling pathway can not only promote axolotl limb regeneration but is also necessary in both adults and embryos. Reduction of Wnt signaling can alter AEC formation, which causes defects in the regeneration process (Kawakami et al., 2006). Although axolotls are highly regenerative and possess all Wnt genes, the expression patterns and function during regeneration have been characterized for only a few of them.
There have been many reports suggesting that Wnt functions in specifying the AP axis, while data regarding the DV axis remain limited (Mehta and Singh, 2019). It has been demonstrated that in amniotes, Wnt7a expression is restricted to the dorsal limb bud, and its inhibition leads to ventralization of the dorsal limb mesoderm, indicating that Wnt7a plays a role in specifying the DV axis (Cygan et al., 1997). However, in axolotl regeneration, Wnt7a is expressed in the epithelium and mesenchyme of the blastema in a diffuse manner, inferring that it has a different function than in amniotes (Shimokawa et al., 2013). It seems that although AP patterning is similar in amphibians and amniotes, there are major differences in DV patterning (Christen and Slack, 1998). Therefore, this information highlights the need for more detailed functional analysis of Wnt7 and the mechanisms governing the formation of the DV axis.
Wnt5a and Wnt5b were detected in regenerating limb blastemas. Wnt5a was expressed during late dedifferentiation (prior blastema formation) and the early digit stage. Wnt5b expression was observed in the mesenchyme of a medium bud when blastema had already formed. Overexpression of Wnt5a inhibited dedifferentiation by diminishing the expression of genes related to that process. As in other described species, these genes are expressed distally, where they inhibit Wnt/β-catenin signaling, allowing for the differentiation of the distal limb structures (Ghosh et al., 2008). A similar pattern can be observed when the Wnt pathway is blocked by the expression of Axin or Dkk (Kawakami et al., 2006).
As can be presumed based on the previous data, Wnt/β-catenin signaling does not work in a uniform way across the entire regeneration process. It has been reported that a lack of Wnt/β-catenin signaling may inhibit regeneration; however, little is known about the opposite situation. What happens when Wnt/β-catenin is chemically activated? It has been shown that in some cases, overexpression of Wnt genes enhances regeneration (Stoick-Cooper et al., 2007); however, it may have various effects depending on the regeneration phase. Wischin et al., (2017) investigated how chemical activation of this pathway influences the key regeneration stages. Treatment at the early stage of wound healing completely blocked regeneration, and at the later stage, it resulted in denervated phenotypes, thereby inhibiting regeneration. Treatment applied after blastema formation caused skeletal malfunctions, but it did not influence cell proliferation. This study illustrates that Wnt/β-catenin signaling controls skeletal morphogenesis, which is consistent with the processes occurring during limb development (Tamamura et al., 2005).
It has been noted that in other species, such as zebrafish, Wnt signaling is necessary in the late wound healing stage but before blastema formation. Studies on axolotl embryos demonstrated that during tail regeneration, Wnt signaling is relatively delayed, and it is not required for early wound healing (Ponomareva et al., 2015). It seems that there is a lack of data regarding details of the regeneration early stages and a more detailed screen would be useful in order to evaluate the exact role of the Wnt signaling. It has been established that the early phase of regeneration is significant for the further course of this process, but more research is needed to determine the involvement of particular Wnt genes.
Most models of axolotl regeneration have studied the limb and tail; however, as stated before, urodele amphibians exhibit the ability to regenerate a wider range of tissues. At present, there are no data regarding the role of the Wnt pathway in these processes. Even so, axolotls are not the only animals that were analyzed with an emphasis on Wnt signaling. Newts are another well-investigated group that includes numerous species with the ability to regenerate (Caubit et al., 1997). Except for tail and limb research, there is evidence that the Wnt pathway is involved in lens regeneration. According to earlier research, initiation of lens regeneration occurs only in the dorsal iris. Wnt/β-catenin pathway initiates the lens developmental program in pigmented epithelium. There are many Wnt genes taking part in newt development, but only two of them were detected during regeneration: Wnt2b (canonical pathway) in the dorsal half of the iris, and Wnt5a (non-canonical pathway) in both the dorsal and ventral parts of the iris (Hayashi et al., 2006). These data support not only that lens development from the dorsal iris is dependent on Wnt signals but also that there is a variation in Wnt signaling in the regeneration of different tissues or organs. Remarkably, the addition of exogenous Wnt3a accelerates lens regeneration and promotes the development of larger lenses. A similar event takes place after the addition of Wnt5a, although the effect was weaker than in the case of Wnt3a. Based on the patterns of Wnt gene expression, it can be inferred that Wnt signaling is required for acquiring positional information.
Mammals and birds
Mammals have very limited regenerative capacity, but they have the ability to regenerate some tissues, such as bones, skeletal muscles or skin. Adult mice and humans can regenerate the distal tip of the terminal phalanges. Unfortunately, their limbs do not regenerate after amputation at a more proximal level. Currently, modern medicine offers solutions such as replants or bionic appendages. Nevertheless, scientists continuously explore mammalian regeneration, and there is considerable evidence supporting the significance of the Wnt/β-catenin transduction pathway. The role of the Wnt pathway in development has been well documented, and it has been established that it is involved in numerous processes (Vainio et al., 1999; Stump et al., 2003; Zhang et al., 2011; el Wakil and Lalli, 2011; Buikema et al., 2013). In mice, Wnt/β-catenin participates in liver regeneration, as β-catenin promotes hepatocyte proliferation (Li et al., 2019) and supports cochlear regeneration (Samarajeewa et al., 2018).
It has been shown that during mouse heart development, Hippo and Wnt pathway interactions restrict cardiomyocyte proliferation and regulate heart size (Heallen et al., 2011). Following myocardial infarction, reduced levels of β-catenin or overexpression of Wnt11 improved the recovery of cardiomyocyte function (Hoppler and Conlon 2020).
In different systems, Müller glial cells have the potential to become progenitor cells: various pathways contribute to the formation of Müller glia-derived progenitor cells (MGPCs) and neuronal regeneration in the retina. In the zebrafish retina, the Wnt pathway activates the formation of MGPCs and neuronal regeneration. During retina development in chicks, Wnt signaling maintains an undifferentiated state of neural stem cells in peripheral regions of the retina; β-catenin must be downregulated to allow retinal regeneration from ciliary marginal tissues at the peripheral edge of the developing neural retina. In healthy retinas, the Wnt pathway is not active, and nuclear β-catenin is absent in Müller glia. In the case of retinal damage, as in the zebrafish model, the Wnt pathway is activated in Müller glia and MGPCs, while Wnt inhibition results in a reduced number of proliferating MGPCs (Osakada et al., 2007). Gallina et al., (2016) noted that there might be significant differences between how Wnt signaling regulates MGPC formation in mammals, birds and fish. The authors’ research showed complex relations among different pathways and how they can vary. One example involves Ascl1, which promotes neurogenesis in the developing retina. In the chick retina, inhibition of Wnt signaling following NMDA treatment results in an ascl1a increase and does not affect cell specification. In zebrafish, ascl1a is expressed within four hours after injury and works downstream of Wnt signaling during reprogramming of Müller glia into MGPCs. On the other hand, ascl1a is not upregulated in rodent retinas, indicating the presence of factors or signaling pathways that override changes in ASCl1a, preventing neuronal differentiation (potentially Jak/Stat, BMP or Notch). In summary, Wnt signaling stimulates proliferation during MGPCs formation in the chick retina without influencing the differentiation of the progeny produced by MGPCs (Gallina et al., 2016).
Deer antlers constitute one of the most impressive regeneration examples in mammals. β-catenin regulates antler progenitor cell survival and fate (Mount et al., 2006). Another function of Wnt signaling in mammals is to sustain tissue renewal; this pathway is responsible for continuous tissue replenishment and maintenance over a lifetime (Clevers et al., 2014). Generally, Wnt signaling is activated during injuries of various mammalian organs, such as lungs, kidneys or skin, and this process has been reviewed in detail by Bastakoty and Young (2016). They reported that although the Wnt/β-catenin pathway obviously contributes to regeneration, its complexity and numerous factors (timing, cell type, target) make it challenging to apply in therapeutics.
Discussion
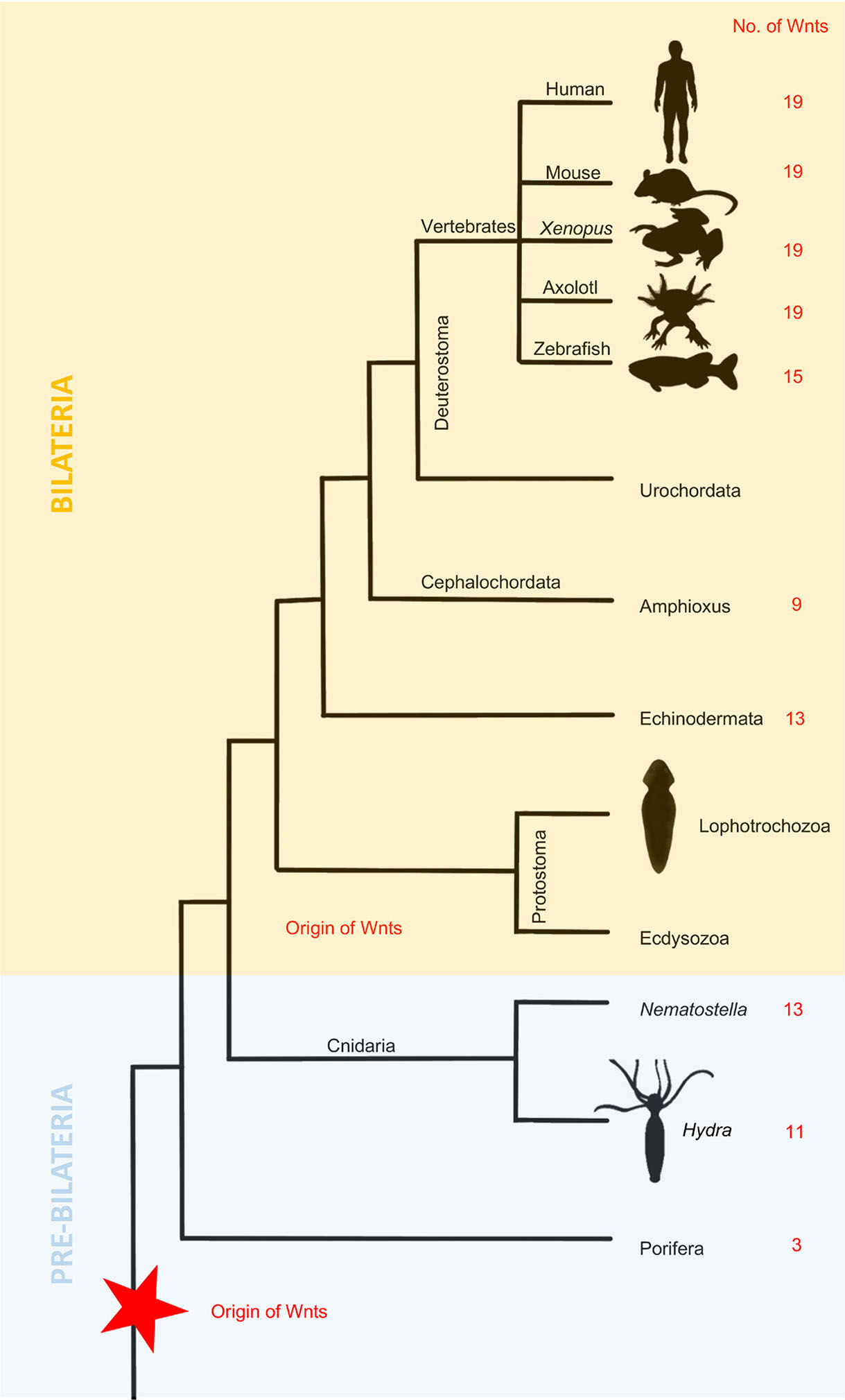
Phylogenetic data suggest that the Wnt signaling pathway present in bilaterians first appeared earlier in metazoan evolution (Lengfeld et al., 2009). The number of Wnt genes ranges from 11 to 19 in deuterostomes and from 4 to 9 among protostomes (Fig. 4) due to the loss of family members during their evolution (Guder et al., 2006). Wnt is an evolutionarily conserved pathway, and its function is also conserved during regeneration. As shown above, Wnt signaling plays a critical role in almost every studied regeneration system, including cnidarians, fish, frogs, and salamanders. As shown in Table 2, the role of many Wnt genes in regeneration has yet to be described. There are also differences among various species. It is clear that the function of more genes has been elucidated in animals with relatively simple body structures, such as Hydra. Interestingly, these species have fewer Wnt genes, most likely lost during evolution. Although some stages of limb regeneration seem equivalent to limb development, there are still significant discrepancies. Wnt gradients occurring during regeneration are not the same as those in early embryos. Therefore, we cannot say that hierarchical development is entirely consistent with regeneration.
Table 2
Summary of Wnt genes and their function in various model species
| Hydra | Planaria | Zebrafish | Xenopus | Axolotl | |
|---|---|---|---|---|---|
| Wnt1 | bud formation (Lengfeld et al., 2009) | head formation (de Robertis, 2010) | ✓ | ✓ | ✓ |
| Wnt2 | axis formation (Lengfeld et al., 2009) | AP positional information (Petersen and Reddien, 2009a) | ✓ | ✓ | ✓ |
| Wnt2B | ✓ | ✓ | ✓ | ✓ | |
| Wnt3 | primary ligand (Lengfeld et al., 2009) | AP positional information (Petersen and Reddien, 2009b) | ✓ | ✓ | ✓ |
| Wnt3A | primary ligand (Lengfeld et al., 2009) | outgrowth in basal layer of wound epidermis (Poss et al., 2000) | blastema formation (Yokoyama et al., 2007) | ✓ | |
| Wnt4 | ✓ | ✓ | (Sugiura et al., 2009) | ✓ | |
| Wnt4B | ligand required for β-catenin reporter activity (Strand et al., 2016) | ||||
| Wnt5A | inhibition of the canonical pathway (Philipp et al., 2009) | patterning mediolateral axis (Gurley et al., 2008) | modulation of the regeneration rate (Stoick-Cooper et al., 2007) | regenerative outgrowth; activation of JNK pathway (Sugiura et al., 2009) | inhibition of the canonical pathway (Ghosh et al., 2008) |
| Wnt5B | modulation of the regeneration rate (Stoick-Cooper et al., 2007) | ✓ | ✓ | ||
| Wnt6 | ✓ | ✓ | ✓ | ||
| Wnt7A | bud formation (Lengfeld et al., 2009) | ✓ | ✓ | ✓ | specification of the DV axis (Shimokawa et al., 2013) |
| Wnt7B | ✓ | ✓ | |||
| Wnt7C | ✓ | ||||
| Wnt8A | Lengfeld et al., 2009 | cell proliferation (Stoick-Cooper et al., 2007) | (Sugiura et al., 2009) | ✓ | |
| Wnt8B | ✓ | ✓ ligand | ✓ | ||
| Wnt9A | bud formation (Lengfeld et al., 2009) | ✓ | |||
| Wnt9B | ✓ | ✓ | |||
| Wnt10A | bud formation (Lengfeld et al., 2009) | activation of the canonical pathway (Stoick-Cooper et al., 2007) | ✓ ligand | ✓ | |
| Wnt10B | ✓ | ✓ | ✓ | ||
| Wnt11 | bud formation (Lengfeld et al., 2009) | AP positional information, patterning the posterior midline (Petersen and Reddien, 2009b) | ✓ | (Sugiura et al., 2009) | ✓ |
| Wnt16 | bud formation (Lengfeld et al., 2009) | ✓ | ✓ |
The ✓ symbol indicates that a gene was detected in a particular species; however, whether it is involved in regeneration or the function it plays remains unknown. Second column (P) indicates whether a particular gene acts via canonical (C) or non-canonical (NC) pathway, although in some cases there are discrepancies between different authors (Croce and McClay, 2008; Ackers and Malgor, 2018).
Bilaterians can be divided into deuterostomes and protostomes (Ecdysozoa and Lophotrochozoa). Although there are major differences between these phyla, it seems that Wnt/β-catenin signaling specifies the anteroposterior axis in all of them, suggesting that it might be an ancient trait present in the ancestor of all bilaterians (Fig. 4). A theory has been derived promoting that whole-body regeneration is probably an ancestral trait lost in most animal lineages, while structural regeneration might be de novo adaptation (Slack, 2017). The ancestral role of Wnt signaling is the specification and regulation of posterior aspects of the AP axis. There is a high resemblance in the expression of Wnt genes and their antagonists among many deuterostome species concerning AP polarity (Petersen and Reddien, 2009a). One example is an evolutionarily ancient β-catenin protein that in many metazoan species acts as a molecular switch responsible for polarity specification (Gurley et al., 2008). Its critical function is deeply conserved, as it controls AP polarity in species such as C. elegans (Ackley, 2014), Drosophila (Siegfried and Perrimon, 1994), Gryllus bimaculatus (Nakamura et al., 2007) and many others.
The Wnt/β-catenin transduction pathway is evolutionarily conserved, and it plays a similar role in the regeneration of various animals; moreover, similar molecular mechanisms underline the regeneration of anatomically distinct species. Notwithstanding, the involvement of dedifferentiation and stem cell activation in the generation of progenitor cells during regeneration differs between organisms (Stoick-Cooper et al., 2007). Part of Wnt/β-catenin signaling in dedifferentiation and cell activation in blastema remains vague. Numerous data presented in this paper have shown that in various systems, Wnt signaling may be activated at different stages (initial or later part of the process), and little is known regarding what controls the activation of Wnt signaling. For example, Kawakami et al., (2006) indicated that the primary role of Wnt signaling resides at the early stages of regeneration, such as AEC formation (by controlling numerous transcription factors). Yokoyama et al., (2007) stated that the Wnt/β-catenin pathway is essential during early stages, but it is not required after blastema formation. Some genes are expressed later during the outgrowth phase (Poss et al., 2000). There might also be functional differences between Wnt genes in various species (Table 1); therefore, more studies are needed to compare their function in different animals as well as within the same species but for different tissues or organs. What is clear, however, is that Wnt/β-catenin signaling plays a main role in many regenerative processes. It is well established that in the regeneration of vertebrates, the Wnt pathway is crucial in the organization of cell polarity. A further, potentially open question regards the role of the β-catenin-independent pathway and its role in cell fate determination.
Some of the data released in recent years are contradictory to older information, especially regarding gene function. For instance, some of the genes taking part in zebrafish tail regeneration (Stoick-Cooper et al., 2007) were not detected in similar experiments before, and the function of some genes was not assigned correctly. At first, it was thought that Wnt5 stimulates blastema formation (Poss et al., 2000, 2003), whereas currently, we know that Wnt5 operates in the opposite way to β-catenin signaling by reducing proliferation (Stoick-Cooper et al., 2007). We believe that such a discrepancy results from the emergence of new, more precise research techniques, and we can assume that more detailed knowledge will also appear in the future. Furthermore, with the advancement of complex research tools (genome sequencing, transgenics, CRISPR, etc.) there are completely new perspectives awaiting in the field of regeneration.
We believe that experiments on organisms that constitute model systems in evolutionary studies could significantly explain some of the issues raised in this article. One such animal is amphioxus, cephalochordate, which represents the earliest evolutionary lineage of chordates (Liang et al., 2019; Bertrand et al., 2021). Although some of the amphioxus Wnt genes were characterized (not described here as it exceeds the scope of this paper), it is evident that there are still many aspects that can be revealed thanks to the position of amphioxus in the evolutionary tree (Somorjai et al., 2012, 2018; Somorjai, 2017). Therefore, we would like to encourage scientists to explore possibilities for unconventional research materials, for instance, among non-model species, as they can also be a useful source of information.
Data emerge daily; however, we still need a full view of how the Wnt pathway interacts with others. For example, while some findings suggest that Wnt acts upstream of numerous other pathways (Wehner et al., 2014), others state something completely opposite (Blum and Begemann, 2012). Accordingly, there is an obvious need for more detailed work regarding this topic.
Finally, there is no doubt that Wnt/β-catenin signaling regulates key signaling pathways involved in regeneration, and this pathway is required regardless of age and developmental stage. Scientists currently extensively study its application in cancer research (Barker and Clevers, 2006; Kahn, 2014) and regenerative medicine (de Santis et al., 2018).
It is currently being developed in the direction of stem cell therapies and ex vivo organs. It is an exciting approach, as traditional transplants have many limitations, such as insufficient suitable donors and post-transplant complications such as graft failures. Therefore, a novel technology such as ex vivo organs based on stem cell therapy might offer an alternative solution (Luijmes et al., 2022). There have been attempts to perform transplants of bioengineered organ scaffolds in animal systems (Wagner et al., 2013). Wnt/β-catenin signaling has been proven to have numerous applications in development and regeneration; hence, scientists working in this field naturally pay much attention to it (Majidinia et al., 2018). In an experiment where ex vivo liver was cultured in the presence of Wnt-3A, embryonic cultures were proliferating with minimal apoptosis. The study showed that Wnt enhances stem cell proliferation, which supports hepatocyte transdifferentiation and promotes biliary survival (Hussain et al., 2004). Gentile and Garcovich (2019) reported that stem cells had been used in therapies supporting hair regrowth and contributing to hair follicular regeneration. They discovered that the Wnt pathway is one of the key factors stimulating hair growth through cellular proliferation and suppression of apoptotic cues. It seems that activation of the Wnt pathway is a promising instrument to support stem cell renewal, tissue homeostasis and repair, as well as preventing aging. Nevertheless, safety issues should also be considered, as stem cells rely on various interactions and crosstalk of different pathways; therefore, Wnt activation may lead to dysregulation of mechanisms that keep stem cells inactive, especially since Wnt overactivation can lead to cancer. Ultimately, Wnt activators are still in the early stages of research, and more data are needed. Additionally, Wnt antagonists can be applied to promote stem cell expansion in vivo as well as ex vivo (Bonnet et al., 2021).
In summary, recent findings provide an optimistic perspective for the future of regenerative medicine, as one day, knowledge about the Wnt transduction pathway might contribute to the application of human tissue regeneration. The rapid development of sophisticated tools and increasing understanding of the Wnt pathway provide promising opportunities. Despite the challenges, we believe it is just a matter of time before therapies using the Wnt/β-catenin pathway will be applied. Further investigation of the Wnt pathway’s role in regeneration is necessary.
Acknowledgements
We thank Stefan Thresh for his valuable and constructive suggestions on this manuscript as well as language editing and proofreading.
Declarations
Funding
This work was supported by the Graduate independent research projects of Ocean University of China (202161020), the National Key Research and Development Program of China (2017YFC1404402), the Special Scientific Research Funds for Central Non-profit Institutes, the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (20603022020001).
Conflicts of interest
The authors declare no competing interests.
Ethical approval
This review was performed using previously published results, without additional experimentation involving human participants or animals.
References
Ackers I., Malgor R. (2018). Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diabetes and Vascular Disease Research 15: 3-13.
Ackley B. D. (2014). Wnt‐signaling and planar cell polarity genes regulate axon guidance along the anteroposterior axis in C. elegans . Developmental Neurobiology 74: 781-796.
Adell T., Salò E., Boutros M., Bartscherer K. (2009). Smed-Evi/Wntless is required for β-catenin-dependent and-independent processes during planarian regeneration. Development 136: 905-910.
Altizer A. M., Stewart S. G., Albertson B. K., Borgens R. B. (2002). Skin flaps inhibit both the current of injury at the amputation surface and regeneration of that limb in newts. Journal of Experimental Zoology 293: 467-477.
Barker N., Clevers H. (2006). Mining the Wnt pathway for cancer therapeutics. Nature Reviews Drug Discovery 5: 997-1014.
Bastakoty D., Young P. P. (2016). Wnt/β‐catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. The FASEB Journal 30: 3271-3284.
Beck C. W., Izpisúa Belmonte J. C., Christen B. (2009). Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms . Developmental Dynamics 238: 1226-1248.
Bertrand S., Carvalho J. E., Dauga D., Matentzoglu N., Daric V., Yu J.K., Schubert M., Escrivá H. (2021). The Ontology of the Amphioxus Anatomy and Life Cycle (AMPHX). Frontiers in Cell and Developmental Biology 9: 668025.
Blum N., Begemann G. (2012). Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 139: 107-116.
Bonnet C., Brahmbhatt A., Deng S. X., Zheng J. J. (2021). Wnt signaling activation: targets and therapeutic opportunities for stem cell therapy and regenerative medicine. RSC Chemical Biology 2: 1144-1157.
Brockes J. P. (1997). Amphibian Limb Regeneration: Rebuilding a Complex Structure. Science 276: 81-87.
Brockes J. P., Kumar A. (2008). Comparative Aspects of Animal Regeneration. Annual Review of Cell and Developmental Biology 24: 525-549.
Buikema J. W., Mady A. S., Mittal N. V., Atmanli A., Caron L., Doevendans P. A., Sluijter J. P. G., Domian I. J. (2013). Wnt/β-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development 140: 4165-4176.
Cano-Martínez A., Vargas-gonzález A., Guarner-lans V., Prado-zayago E., León-oleda M., Nieto-lima B., (2010). Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Archivos de cardiologia de Mexico 80: 79-86.
Caubit X., Nicolas S., Le Parco Y. (1997). Possible roles for Wnt genes in growth and axial patterning during regeneration of the tail in urodele amphibians. Developmental Dynamics 210: 1-10.
Chera S., Ghila L., Dobretz K., Wenger Y., Bauer C., Buzgariu W., Martinou J.C., Galliot B. (2009). Apoptotic Cells Provide an Unexpected Source of Wnt3 Signaling to Drive Hydra Head Regeneration. Developmental Cell 17: 279-289.
Christen B., Slack J. M. W. (1998). All limbs are not the same. Nature 395: 230-231.
Clevers H., Loh K. M., Nusse R. (2014). An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346: 54.
Croce J. C., McClay D. R. (2008). Evolution of the Wnt Pathways. In Wnt Signaling. (Ed. Vincan Elizabeth) Humana Press, Totowa, NJ.
Cygan J. A., Johnson R. L., McMahon A. P. (1997). Novel regulatory interactions revealed by studies of murine limb pattern in Wnt-7a and En-1 mutants . Development 124: 5021-5032.
Day R. C., Beck C. W. (2011). Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC Developmental Biology 11: 54.
De Robertis E. M. (2010). Wnt Signaling in Axial Patterning and Regeneration: Lessons from Planaria. Science Signaling 3: pe21.
De Santis M., Di Matteo B., Chisari E., Cincinelli G., Angele P., Lattermann C., Filardo G., Vitale N. D., Selmi C., Kon E. (2018). The Role of Wnt Pathway in the Pathogenesis of OA and Its Potential Therapeutic Implications in the Field of Regenerative Medicine. BioMed Research International 2018: 1-8.
Edwards-Faret G., Muñoz R., Méndez-Olivos E. E., Lee-Liu D., Tapia V. S., Larraín J. (2017). Spinal cord regeneration in Xenopus laevis. Nature Protocols 12: 372-389.
El Wakil A., Lalli E. (2011). The Wnt/beta-catenin pathway in adrenocortical development and cancer. Molecular and Cellular Endocrinology 332: 32-37.
Gallina D., Palazzo I., Steffenson L., Todd L., Fischer A. J. (2016). Wnt/β‐catenin‐signaling and the formation of M üller glia‐derived progenitors in the chick retina . Developmental Neurobiology 76: 983-1002.
Galliot B., Chera S. (2010). The Hydra model: disclosing an apoptosis-driven generator of Wnt-based regeneration. Trends in Cell Biology 20: 514-523.
Gardiner D. M., Endo T., Bryant S. V., (2002). The molecular basis of amphibian limb regeneration: integrating the old with the new. Cell Dev Biol 13: 243-249.
Gemberling M., Bailey T. J., Hyde D. R., Poss K. D. (2013). The zebrafish as a model for complex tissue regeneration. Trends in Genetics 29: 611-620.
Gentile P., Garcovich S. (2019). Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 8: 466.
Ghosh S., Roy S., Séguin C., Bryant S. V., Gardiner D. M. (2008). Analysis of the expression and function of Wnt‐5a and Wnt‐5b in developing and regenerating axolotl ( Ambystoma mexicanum ) limbs . Development, Growth & Differentiation 50: 289-297.
Gilbert S. F., (2000). Developmental Biology. Sinaeur Associates, Sunderland.
Guder C., Philipp I., Lengfeld T., Watanabe H., Hobmayer B., Holstein T. W. (2006). The Wnt code: cnidarians signal the way. Oncogene 25: 7450-7460.
Gufler S., Artes B., Bielen H., Krainer I., Eder M.K., Falschlunger J., Bollmann A., Ostermann T., Valovka T., Hartl M., Bister K., Technau U., Hobmayer B. (2018). β-Catenin acts in a position-independent regeneration response in the simple eumetazoan Hydra. Developmental Biology 433: 310-323.
Gurley K. A., Rink J. C., Alvarado A. S. (2008). β-Catenin Defines Head Versus Tail Identity During Planarian Regeneration and Homeostasis. Science 319: 323-327.
Haas B. J., Whited J. L. (2017). Advances in Decoding Axolotl Limb Regeneration. Trends in Genetics 33: 553-565.
Hayashi T., Mizuno N., Takada R., Takada S., Kondoh H. (2006). Determinative role of Wnt signals in dorsal iris-derived lens regeneration in newt eye. Mechanisms of Development 123: 793-800.
Herrera S. C., Bach E. A. (2019). JAK/STAT signaling in stem cells and regeneration: from Drosophila to vertebrates . Development 146: dev167643.
Hobmayer B., Rentzsch F., Kuhn K., Happel C. M., von Laue C. C., Snyder P., Rothbächer U., Holstein T. W. (2000). WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407: 186-189.
Holstein T. W., Watanabe H., Özbek S. (2011). Signaling Pathways and Axis Formation in the Lower Metazoa. In Growth Factors in Development. Academic Press Inc..
Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L., McLaren S., Sealy I., Caccamo M., Churcher C., Scott C., Barrett J. C., Koch R., Rauch G.J., White S., Chow W., Kilian B., Quintais L. T., Guerra-Assunção J. A., Zhou Y., Gu Y., Yen J., Vogel J.H., Eyre T., Redmond S., Banerjee R., Chi J., Fu B., Langley E., Maguire S. F., Laird G. K., Lloyd D., Kenyon E., Donaldson S., Sehra H., Almeida-King J., Loveland J., Trevanion S., Jones M., Quail M., Willey D., Hunt A., Burton J., Sims S., McLay K., Plumb B., Davis J., Clee C., Oliver K., Clark R., Riddle C., Elliott D., Threadgold G., Harden G., Ware D., Begum S., Mortimore B., Kerry G., Heath P., Phillimore B., Tracey A., Corby N., Dunn M., Johnson C., Wood J., Clark S., Pelan S., Griffiths G., Smith M., Glithero R., Howden P., Barker N., Lloyd C., Stevens C., Harley J., Holt K., Panagiotidis G., Lovell J., Beasley H., Henderson C., Gordon D., Auger K., Wright D., Collins J., Raisen C., Dyer L., Leung K., Robertson L., Ambridge K., Leongamornlert D., McGuire S., Gilderthorp R., Griffiths C., Manthravadi D., Nichol S., Barker G., Whitehead S., Kay M., Brown J., Murnane C., Gray E., Humphries M., Sycamore N., Barker D., Saunders D., Wallis J., Babbage A., Hammond S., Mashreghi-Mohammadi M., Barr L., Martin S., Wray P., Ellington A., Matthews N., Ellwood M., Woodmansey R., Clark G., Cooper J. D., Tromans A., Grafham D., Skuce C., Pandian R., Andrews R., Harrison E., Kimberley A., Garnett J., Fosker N., Hall R., Garner P., Kelly D., Bird C., Palmer S., Gehring I., Berger A., Dooley C. M., Ersan-Ürün Z., Eser C., Geiger H., Geisler M., Karotki L., Kirn A., Konantz J., Konantz M., Oberländer M., Rudolph-Geiger S., Teucke M., Lanz C., Raddatz G., Osoegawa K., Zhu B., Rapp A., Widaa S., Langford C., Yang F., Schuster S. C., Carter N. P., Harrow J., Ning Z., Herrero J., Searle S. M. J., Enright A., Geisler R., Plasterk R. H. A., Lee C., Westerfield M., de Jong P. J., Zon L. I., Postlethwait J. H., Nüsslein-Volhard C., Hubbard T. J. P., Crollius H. R., Rogers J., Stemple D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498-503.
Huelsken J., Behrens J. (2002). The Wnt signalling pathway. Journal of Cell Science 115: 3977-3978.
Hussain S. Z., Sneddon T., Tan X., Micsenyi A., Michalopoulos G. K., Monga S. P.S. (2004). Wnt impacts growth and differentiation in ex vivo liver development. Experimental Cell Research 292: 157-169.
Iismaa S. E., Kaidonis X., Nicks A. M., Bogush N., Kikuchi K., Naqvi N., Harvey R. P., Husain A., Graham R. M. (2018). Comparative regenerative mechanisms across different mammalian tissues. npj Regenerative Medicine 3: 1-20.
Kahn M. (2014). Can we safely target the WNT pathway?. Nature Reviews Drug Discovery 13: 513-532.
Katoh M. (2005). WNT/PCP signaling pathway and human cancer (Review). Oncology Reports 14: 1583-1588.
Kawakami Y., Rodriguez Esteban C., Raya M., Kawakami H., Martí M., Dubova I., Izpisúa Belmonte J. C. (2006). Wnt/β-catenin signaling regulates vertebrate limb regeneration. Genes & Development 20: 3232-3237.
Khyeam S., Lee S., Huang G. N. (2021). Genetic, epigenetic, and post‐transcriptional basis of divergent tissue regenerative capacities among vertebrates. Advanced Genetics 2: e10042.
Kiecker C., Niehrs C. (2001). A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus . Development 128: 4189-4201.
Kobayashi C., Saito Y., Ogawa K., Agata K. (2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Developmental Biology 306: 714-724.
Krneta-Stankic V., DeLay B. D., Miller R. K. (2017). Xenopus: leaping forward in kidney organogenesis. Pediatric Nephrology 32: 547-555.
Lee-Liu D., Méndez-Olivos E. E., Muñoz R., Larraín J. (2017). The African clawed frog Xenopus laevis : A model organism to study regeneration of the central nervous system. Neuroscience Letters 652: 82-93.
Lengfeld T., Watanabe H., Simakov O., Lindgens D., Gee L., Law L., Schmidt H. A., Özbek S., Bode H., Holstein T. W. (2009). Multiple Wnts are involved in Hydra organizer formation and regeneration. Developmental Biology 330: 186-199.
Li D., Sun J., Zhong T. P. (2022). Wnt Signaling in Heart Development and Regeneration. Current Cardiology Reports 24: 1425-1438.
Li N., Kong M., Zeng S., Hao C., Li M., Li L., Xu Z., Zhu M., Xu Y. (2019). Brahma related gene 1 (Brg1) contributes to liver regeneration by epigenetically activating the Wnt/β‐catenin pathway in mice. The FASEB Journal 33: 327-338.
Liang Y., Rathnayake D., Huang S., Pathirana A., Xu Q., Zhang S. (2019). BMP signaling is required for amphioxus tail regeneration. Development 146: dev166017.
Liao S., Dong W., Lv L., Guo H., Yang J., Zhao H., Huang R., Yuan Z., Chen Y., Feng S., Zheng X., Huang J., Huang W., Qi X., Cai D. (2017). Heart regeneration in adult Xenopus tropicalis after apical resection. Cell & Bioscience 7: 70.
Lin G., Chen Y., Slack J. M.W. (2012). Transgenic Analysis of Signaling Pathways Required for Xenopus Tadpole Spinal Cord and Muscle Regeneration . The Anatomical Record 295: 1532-1540.
Lin G., Slack J. M.W. (2008). Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Developmental Biology 316: 323-335.
Logan C. Y., Nusse R. (2004). THE WNT SIGNALING PATHWAY IN DEVELOPMENT AND DISEASE. Annual Review of Cell and Developmental Biology 20: 781-810.
Love N. R., Chen Y., Ishibashi S., Kritsiligkou P., Lea R., Koh Y., Gallop J. L., Dorey K., Amaya E. (2013). Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nature Cell Biology 15: 222-228.
Luijmes S. H., Verstegen M. M.A., Hoogduijn M. J., Seghers L., Minnee R. C., Mahtab E. A.F., Taverne Y. J.H.J., Reinders M. E.J., van der Laan L. J.W., de Jonge J. (2022). The current status of stem cell-based therapies during ex vivo graft perfusion: An integrated review of four organs. American Journal of Transplantation 22: 2723-2739.
Majidinia M., Aghazadeh J., Jahanban‐Esfahlani R., Yousefi B. (2018). The roles of Wnt/β‐catenin pathway in tissue development and regenerative medicine. Journal of Cellular Physiology 233: 5598-5612.
Malloch E. L., Perry K. J., Fukui L., Johnson V. R., Wever J., Beck C. W., King M. W., Henry J. J. (2009). Gene expression profiles of lens regeneration and development in Xenopus laevis . Developmental Dynamics 238: 2340-2356.
McCusker C., Gardiner D. M. (2011). The Axolotl Model for Regeneration and Aging Research: A Mini-Review. Gerontology 57: 565-571.
Mehta A. S., Singh A. (2019). Insights into regeneration tool box: An animal model approach. Developmental Biology 453: 111-129.
Meyers J. R., Hu L., Moses A., Kaboli K., Papandrea A., Raymond P. A. (2012). β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Development 7: 30.
Molina M. D., Saló E., Cebrià F. (2007). The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Developmental Biology 311: 79-94.
Mount J. G., Muzylak M., Allen S., Althnaian T., McGonnell I. M., Price J. S. (2006). Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Developmental Dynamics 235: 1390-1399.
Muneoka K., Holler‐Dinsmore G., Bryant S. V. (1986). Intrinsic control of regenerative loss in Xenopus laevis limbs . Journal of Experimental Zoology 240: 47-54.
Nakamura T., Mito T., Tanaka Y., Bando T., Ohuchi H., Noji S. (2007). Involvement of canonical Wnt/Wingless signaling in the determination of the positional values within the leg segment of the cricket Gryllus bimaculatus . Development, Growth & Differentiation 49: 79-88.
Ng J. K., Kawakami Y., Büscher D., Raya A., Itoh T., Koth C. M., Esteban C. R., Rodríguez-León J., Garrity D. M., Fishman M. C., Belmonte J. C. I. (2002). The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10 . Development 129: 5161-5170.
Nowoshilow S., Schloissnig S., Fei J.F., Dahl A., Pang A. W. C., Pippel M., Winkler S., Hastie A. R., Young G., Roscito J. G., Falcon F., Knapp D., Powell S., Cruz A., Cao H., Habermann B., Hiller M., Tanaka E. M., Myers E. W. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nature 554: 50-55.
Ohashi A., Saito N., Kashimoto R., Furukawa S., Yamamoto S., Satoh A. (2021). Axolotl liver regeneration is accomplished via compensatory congestion mechanisms regulated by ERK signaling after partial hepatectomy. Developmental Dynamics 250: 838-851.
Osakada F., Ooto S., Akagi T., Mandai M., Akaike A., Takahashi M. (2007). Wnt Signaling Promotes Regeneration in the Retina of Adult Mammals. The Journal of Neuroscience 27: 4210-4219.
Ozhan G., Weidinger G. (2015). Wnt/β-catenin signaling in heart regeneration. Cell Regeneration 4: 4:3.
Petersen C. P., Reddien P. W. (2009a). A wound-induced Wnt expression program controls planarian regeneration polarity. Proceedings of the National Academy of Sciences 106: 17061-17066.
Petersen C. P., Reddien P. W. (2009b). Wnt Signaling and the Polarity of the Primary Body Axis. Cell 139: 1056-1068.
Philipp I., Aufschnaiter R., Özbek S., Pontasch S., Jenewein M., Watanabe H., Rentzsch F., Holstein T. W., Hobmayer B. (2009). Wnt/β-Catenin and noncanonical Wnt signaling interact in tissue evagination in the simple eumetazoan Hydra . Proceedings of the National Academy of Sciences 106: 4290-4295.
Ponomareva L. V., Athippozhy A., Thorson J. S., Voss S. R. (2015). Using Ambystoma mexicanum (Mexican axolotl) embryos, chemical genetics, and microarray analysis to identify signaling pathways associated with tissue regeneration. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 178: 128-135.
Poss K. D., Keating M. T., Nechiporuk A. (2003). Tales of regeneration in zebrafish. Developmental Dynamics 226: 202-210.
Poss K. D., Shen J., Keating M. T. (2000). Induction of lef1 during zebrafish fin regeneration. Developmental Dynamics 219: 282-286.
Raya A., Koth C. M., Büscher D., Kawakami Y., Itoh T., Raya R. M., Sternik G., Tsai H.J., Rodríguez-Esteban C., Izpisúa-Belmonte J. C. (2003). Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proceedings of the National Academy of Sciences 100: 11889-11895.
Reddien P. W., Alvarado A. S. (2004). FUNDAMENTALS OF PLANARIAN REGENERATION. Annual Review of Cell and Developmental Biology 20: 725-757.
Roy S., Gatien S. (2008). Regeneration in axolotls: a model to aim for!. Experimental Gerontology 43: 968-973.
Sabin K. Z., Jiang P., Gearhart M. D., Stewart R., Echeverri K. (2019). AP-1cFos/JunB/miR-200a regulate the pro-regenerative glial cell response during axolotl spinal cord regeneration. Communications Biology 2: 91.
Samarajeewa A., Lenz D. R., Xie L., Chiang H., Kirchner R., Mulvaney J. F., Edge A. S. B., Dabdoub A. (2018). Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development 145: dev166579.
Shimokawa T., Yasutaka S., Kominami R., Shinohara H. (2013). Lmx-1b and Wnt-7a expression in axolotl limb during development and regeneration. Okajimas Folia Anatomica Japonica 89: 119-124.
Siegfried E., Perrimon N. (1994). Drosophila wingless: A paradigm for the function and mechanism of Wnt signaling. BioEssays 16: 395-404.
Slack J. M. W., Lin G., Chen Y. (2008). Molecular and Cellular Basis of Regeneration and Tissue Repair. Cellular and Molecular Life Sciences 65: 54-63.
Slack J. M.W. (2017). Animal regeneration: ancestral character or evolutionary novelty?. EMBO reports 18: 1497-1508.
Somorjai I. M. L., Martí-Solans J., Diaz-Gracia M., Nishida H., Imai K. S., Escrivà H., Cañestro C., Albalat R. (2018). Wnt evolution and function shuffling in liberal and conservative chordate genomes. Genome Biology 19: 98.
Somorjai I. M.L. (2017). Amphioxus regeneration: evolutionary and biomedical implications. The International Journal of Developmental Biology 61: 689-696.
Somorjai I. M.L., Escrivà H., Garcia-Fernàndez J. (2012). Amphioxus makes the cut—Again. Communicative & Integrative Biology 5: 499-502.
Stewart S., Gomez A. W., Armstrong B. E., Henner A., Stankunas K. (2014). Sequential and Opposing Activities of Wnt and BMP Coordinate Zebrafish Bone Regeneration. Cell Reports 6: 482-498.
Stocum D. L., Cameron J. A. (2011). Looking proximally and distally: 100 years of limb regeneration and beyond. Developmental Dynamics 240: 943-968.
Stoick-Cooper C. L., Weidinger G., Riehle K. J., Hubbert C., Major M. B., Fausto N., Moon R. T. (2007). Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134: 479-489.
Strand N. S., Hoi K. K., Phan T. M.T., Ray C. A., Berndt J. D., Moon R. T. (2016). Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochemical and Biophysical Research Communications 477: 952-956.
Stump R. J.W., Ang S., Chen Y., von Bahr T., Lovicu F. J., Pinson K., de Iongh R. U., Yamaguchi T. P., Sassoon D. A., McAvoy J. W. (2003). A role for Wnt/β-catenin signaling in lens epithelial differentiation. Developmental Biology 259: 48-61.
Suetsugu-Maki R., Maki N., Nakamura K., Sumanas S., Zhu J., Del Rio-Tsonis K., Tsonis P. A. (2012). Lens regeneration in axolotl: new evidence of developmental plasticity. BMC Biology 10: 2-9.
Sugiura T., Tazaki A., Ueno N., Watanabe K., Mochii M. (2009). Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mechanisms of Development 126: 56-67.
Suzuki M., Satoh A., Ide H., Tamura K. (2005). Nerve-dependent and -independent events in blastema formation during Xenopus froglet limb regeneration. Developmental Biology 286: 361-375.
Tal T. L., Franzosa J. A., Tanguay R. L. (2010). Molecular Signaling Networks That Choreograph Epimorphic Fin Regeneration in Zebrafish – A Mini-Review. Gerontology 56: 231-240.
Tamamura Y., Otani T., Kanatani N., Koyama E., Kitagaki J., Komori T., Yamada Y., Costantini F., Wakisaka S., Pacifici M., Iwamoto M., Enomoto-Iwamoto M. (2005). Developmental Regulation of Wnt/β-Catenin Signals Is Required for Growth Plate Assembly, Cartilage Integrity, and Endochondral Ossification. Journal of Biological Chemistry 280: 19185-19195.
Tanaka E. M., Weidinger G., (2008). Heads or tails: can Wnt tell which one is up?. Nat Cell Biol 10: 122-124.
Torok M. A., Gardiner D. M., Shubin N. H., Bryant S. V. (1998). Expression ofHoxDGenes in Developing and Regenerating Axolotl Limbs. Developmental Biology 200: 225-233.
Tseng A.S. (2017). Seeing the future: using Xenopus to understand eye regeneration . genesis 55: dvg.23003.
Tsonis P. A. (2000). Regeneration in Vertebrates. Developmental Biology 221: 273-284.
Vainio S., Heikkilä M., Kispert A., Chin N., McMahon A. P. (1999). Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405-409.
Vivien C. J., Hudson J. E., Porrello E. R. (2016). Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regenerative medicine 1: 16012.
Vogg M. C., Beccari L., Iglesias Ollé L., Rampon C., Vriz S., Perruchoud C., Wenger Y., Galliot B. (2019). An evolutionarily-conserved Wnt3/β-catenin/Sp5 feedback loop restricts head organizer activity in Hydra. Nature Communications 10: 312.
Wagner D. E., Bonvillain R. W., Jensen T., Girard E. D., Bunnell B. A., Finck C. M., Hoffman A. M., Weiss D. J. (2013). Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds . Respirology 18: 895-911.
Wehner D., Cizelsky W., Vasudevaro M. D., Özhan G., Haase C., Kagermeier-Schenk B., Röder A., Dorsky R. I., Moro E., Argenton F., Kühl M., Weidinger G. (2014). Wnt/β-Catenin Signaling Defines Organizing Centers that Orchestrate Growth and Differentiation of the Regenerating Zebrafish Caudal Fin. Cell Reports 6: 467-481.
Wehner D., Jahn C., Weidinger G. (2015). Use of the TetON System to Study Molecular Mechanisms of Zebrafish Regeneration. Journal of Visualized Experiments 100: e52756.
Wehner D., Tsarouchas T. M., Michael A., Haase C., Weidinger G., Reimer M. M., Becker T., Becker C. G. (2017). Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nature Communications 8: 126.
Wischin S., Castañeda-Patlán C., Robles-Flores M., Chimal-Monroy J. (2017). Chemical activation of Wnt/β-catenin signalling inhibits innervation and causes skeletal tissue malformations during axolotl limb regeneration. Mechanisms of Development 144: 182-190.
Yin D., Liu M., Fu H., Shu G., Zhou J., Qing X., Wu W. (2016). Solubility of Trimethoprim in Selected Pure Solvents and (Water + Ethanol/2-Propanol) Mixed-Solvent Systems. Journal of Chemical & Engineering Data 61: 404-411.
Yokoyama H., Maruoka T., Ochi H., Aruga A., Ohgo S., Ogino H., Tamura K. (2011). Different Requirement for Wnt/β-Catenin Signaling in Limb Regeneration of Larval and Adult Xenopus. PLoS ONE 6: e21721.
Yokoyama H., Ogino H., Stoick-Cooper C. L., Grainger R. M., Moon R. T. (2007). Wnt/β-catenin signaling has an essential role in the initiation of limb regeneration. Developmental Biology 306: 170-178.
Zhang L., Yang X., Yang S., Zhang J. (2011). The Wnt /β-catenin signaling pathway in the adult neurogenesis. European Journal of Neuroscience 33: 1-8.
Zhao L., Ben-Yair R., Burns C. E., Burns C. G. (2019). Endocardial Notch Signaling Promotes Cardiomyocyte Proliferation in the Regenerating Zebrafish Heart through Wnt Pathway Antagonism. Cell Reports 26: 546-554.e5.