Int. J. Dev. Biol. 69: 21 - 34 (2025)
Melastatin family Transient Receptor Potential channels support spermatogenesis in planarian flatworms
Open Access | Original Article | Published: 15 April 2025
Abstract
The Transient Receptor Potential superfamily of proteins (TRPs) form cation channels that are abundant in animal sensory systems. Amongst TRPs, the Melastatin-related family (TRPMs) is composed of members that respond to temperature, pH, sex hormones, and various other stimuli. Some TRPMs exhibit enriched expression in the gonads of vertebrate and invertebrate species, but their contributions to germline development remain to be determined. We identified twenty-one potential TRPMs in the planarian flatworm Schmidtea mediterranea and analyzed their anatomical distribution of expression by whole-mount in situ hybridization. Enriched expression of two TRPMs (Smed-TRPM-c and Smed-TRPM-l) was detected in testis, whereas eight TRPM genes had detectable expression in patterns representative of neuronal and/or sensory cell types. Functional analysis of TRPM homologs by RNA-interference (RNAi) revealed that disruption of normal levels of Smed-TRPM-c expression impaired sperm development, indicating a role for this receptor in supporting spermatogenesis. Smed-TRPM-l RNAi alone did not result in a detectable phenotype, but it did increase sperm development deficiencies when combined with Smed-TRPM-c RNAi. Fluorescence in situ hybridization revealed expression of Smed-TRPM-c in early spermatogenic cells within testes, suggesting cell-autonomous regulatory functions in germ cells for this gene. In addition, Smed-TRPM-c RNAi resulted in reduced numbers of presumptive germline stem cell clusters in asexual planarians, suggesting that Smed-TRPM-c supports the establishment, maintenance, and/or expansion of spermatogonial germline stem cells. While further research is needed to identify the factors that trigger Smed-TRPM-c activity, these findings reveal one of the few known examples for TRPM function in the direct regulation of sperm development.
Keywords
spermatogenesis, planarian, Transient Receptor Potential Channel Melastatin, TRPM
Introduction
Studies of animal reproduction are relevant for preservation of natural resources, raising livestock, forestry, fishery, and protection of crops from pests. Environmental influences, such as temperature, are known to alter endogenous endocrine pathways that regulate seasonal reproduction either directly by serving as a cue for activation developmental processes, or indirectly by altering metabolic pathways that influence fecundity (Chmura and Williams, 2022). As climate change continues to threaten the survival of hundreds of species, our need to understand how organisms sense changes in environmental conditions, as well as the molecular mechanisms behind their influence on mating, fertility, and fecundity is of outmost urgency.
Planarian flatworms are free-living members of the superphylum Lophotrochozoa that are best known for their regenerative abilities. Although fission and regeneration serve as means for asexual reproduction of some planarians, most species reproduce sexually as cross-fertilizing hermaphrodites (Hyman, 1951, Vila-Farré and Rink, 2018; Issigonis and Newmark, 2019). Planarians that live in habitats with fluctuating temperatures have been reported to switch reproductive modes seasonally (Kenk, 1935; Nodono and Matsumoto, 2022). Sexualization can also be induced experimentally in the laboratory (Kobayashi et al., 2004), and the signaling molecules that ignite or modulate sexual maturation are starting to be identified (Collins et al., 2010; Kobayashi et al., 2017; Rouhana et al., 2017; Nakagawa et al., 2018; Issigonis et al., 2023). Furthermore, reproductive structures regress in response to injury or starvation, and redevelop upon healing and growth, showing extreme developmental plasticity (Newmark et al., 2008).
Given their dynamic reproductive biology, planarians provide a great opportunity to learn about the molecular mechanisms behind external influence on sexual reproduction. The sexual organs and entire reproductive system of planarians, including the germline, develop post-embryonically from somatic pluripotent stem cells called neoblasts (Kobayashi et al., 1999; Wang et al., 2007). Planarians lack gonads at birth, but these develop during growth in individuals whose genetic composition and environmental conditions allow for sexual maturation. In the planarian Schmidtea mediterranea, germline stem cells are observed quickly after birth in dorsolateral testis primordia that extend from the tail to the area immediately posterior to the head (Wang et al., 2007). Germline stem cells in testis primordia are observed before ovarian counterparts appear, which corroborates with development of male gonads prior to development of ovaries during growth as sexual maturation occurs in this species (reviewed by Issigonis and Newmark, 2019).
Planarians continuously replenish, grow, and regenerate their entire anatomy through differentiation of neoblasts (Newmark and Sanchez Alvarado, 2002). Given that expression of genes can be disrupted systemically by supplementing food with double-stranded RNA (dsRNA) of target-specific sequence (Rouhana et al., 2013), interruption of continuous cellular turnover allows the discovery of genes required for development of tissues (including the germline). Systemic RNAi in planarians also allows for identification of genes required during sexual maturation and development of reproductive structures without interfering with embryonic development. This approach has uncovered novel genes required for germline development, as well as previously unknown roles in gametogenesis for genes characterized in other systems (Wang et al., 2010; Rouhana et al., 2017; Saberi et al., 2016; Khan and Newmark, 2022).
This study includes characterization of genes predicted to encode Transient Receptor Potential (TRP) channels of the Melastatin family (TRPM) in the planarian S. mediterranea. TRPMs mediate cellular responses to environmental and physiological cues that include hormones, changes in pH, and temperature (Diver et al., 2022; Zheng, 2013). Mammalian TRPMs that respond to changes in temperature are expressed in somatosensory neurons, where they play roles in noxious and innocuous temperature response (Kashio and Tominaga, 2022). However, some members of the TRPM family are also expressed during mammalian spermatogenesis, where they are thought to influence sperm development and function (Martínez-López et al., 2011; De Blas et al., 2009; Lee et al., 2003; Borowiec et al., 2016). Here we demonstrate that one of the TRPM homologs in S. mediterranea, Smed-TRPM-c, displays testis-specific expression and is required to maintain normal capacities of spermatogenesis, providing an avenue to study the contributions of TRPMs to regulation of sperm development in planarians. In asexual planarians, Smed-TRPM-c is required for normal maintenance of presumptive germline stem cells, suggesting a function in the earlier stages of spermatogenesis. Altogether, these results complement findings in mice to indicate that TRPMs may have an ancestral role during spermatogenesis and suggest that TRPMs may regulate spermatogenesis in response to changes in temperature.
Results
Identification of Transient Receptor Potential Melastatin (TRPM) family channels in Schmidtea mediterranea
To identify putative TRPMs in the planarian flatworm S. mediterranea, TBLASTN searches for homologs of human TRPM3 (NCBI GenBank ID NP_066003.3) were performed against transcriptomes of sexual S. mediterranea deposited in PlanMine (the planarian flatworm sequence repository; Brandl et al., 2016; Rozanski et al., 2019). Hits were further analyzed by reciprocal BLAST searches against annotated human proteins. Twenty-one non-redundant putative TRPM genes were identified (Supplementary Table S1). Analysis of identical sequences by BLASTN in reference transcriptomes of the asexual biotype of S. mediterranea revealed matches for all 21 putative TRPM genes (Supplementary Table S1), indicating that these are expressed at levels detectable by RNAseq in asexual planarians. Three of these genes are orthologs of TRPMs previously characterized in the planarian Dugesia japonica (Inoue et al., 2014). The genes represented by contigs dd_Smed_v6_17857_0_1 and dd_Smed_v6_26481_0_1 on PlanMine are orthologs of DjTRPMa and were therefore named Smed-TRPM-a1 and Smed-TRPM-a2, respectively (BLASTP E-values = 0.0 and 2.04 x 10-135). The gene represented by contig dd_Smed_v6_9288_0_1 was named Smed-TRPM-b, as it is orthologous to DjTRPMb (E-value = 0.0). The remaining 18 TRPM homologs in S. mediterranea were named Smed-TRPM-c to -t. The sequence of Smed-TRPM-a to -e, and four additional genes, more closely matched TRPM3 than any other human TRPM in reciprocal BLAST results, while others more closely matched human TRPM1, 2, 4, 5, 6, and 8 (Supplementary Table S1). These results indicate an expansion of TRPM genes to at least 21 homologs in S. mediterranea.
Smed-TRPM-c and Smed-TRPM-l are expressed in testis lobes of S. mediterranea
To determine the anatomical distribution of expression of planarian TRPM genes in S. mediterranea, whole-mount in situ hybridization (WMISH) analyses were performed using sexually mature planarian hermaphrodites. Using riboprobes that corresponded to ~500 bps of reference cDNA contig sequence (Supplementary File S1), Smed-TRPM-a1 expression was detected in cells concentrated at the head tip and further distributed throughout the planarian body (Fig. 1A), matching the expression of its ortholog DjTRPMa (Inoue et al., 2014) in thermosensory neurons. Smed-TRPM-a2 expression was only detected in the gut (Fig. 1B). Expression of Smed-TRPM-b, -f, -h, and -m was detected in the brain region (Fig. 1 C,G,I,N), with WMISH signal for some of these genes being limited to subsets of putative neurons (Fig. 1 G’,N’) or very faint (Fig. 1 C’,I’). Smed-TRPM-c expression was only detected in testis lobes, which are abundantly located from the tail to the region posterior to the head along the dorsolateral anatomy of S. mediterranea (Fig. 1 D,D’). Expression of Smed-TRPM-j and -n were observed along the periphery of the head tip (Fig. 1 K,O), whereas Smed-TRPM-r expression was enriched in subsets of cells that reside along the ventral nerve cords and brain (Fig. 1 S,S”). Smed-TRPM-e, -f, -o, and -s expression was detected through much of the gut (Fig. 1 F,G,P,T), while expression of Smed-TRPM-l was detected in the gut (Fig. 1M) and testis (Fig. 1M’). Expression of Smed-TRPM-c and Smed-TRPM-l in testes was validated through single and double fluorescence in situ hybridization (FISH), which revealed partial overlap in expression of these genes in the outer layer of testis lobes (Supplementary Fig. S1). Smed-TRPM-o was detected in the gut and pharynx (Fig. 1P). It is important noting that the planarian gut and copulatory apparatus tend to generate background signal in WMISH analyses using colorimetric development, therefore some of the signals from these structures were deemed inconclusive (Supplementary Table S1). The TRPM homologs with no detected or inconclusive expression include Smed-TRPM-d, -g, -i, -k, -p, -q, and -t (Fig. 1 E,H,J,L,Q,R,U).
Fig. 1. Analysis of expression of TRPMs in S. mediterranea reveals two homologs expressed in testes.
(A-U) Whole-mount colorimetric in situ hybridization analyses using partial sequence riboprobes (~500 nts) display distribution of expression of Smed-TRPM-a1 (TRPM-a1; A), Smed-TRPM-a2 (TRPM-a2; B), and Smed-TRPM-b to -t (TRPM-b to -t; C-U). Insets show magnified view of planarian testis lobes in the posterior dorsal region of planarians (D’ and M’), as well as sensory cells (A’ and G’), brain region (C’, G’, I’, N’), head periphery (K’ and O’), and cells along the posterior (S’) and anterior (S”) ventral nerve cords. Arrows point at the location of brain lobes in (I’), and arrowheads point at posterior ventral nerve cords in (S’ and S”). Scale bars, 1 mm.
Smed-TRPM-c is required to maintain normal levels of sperm development
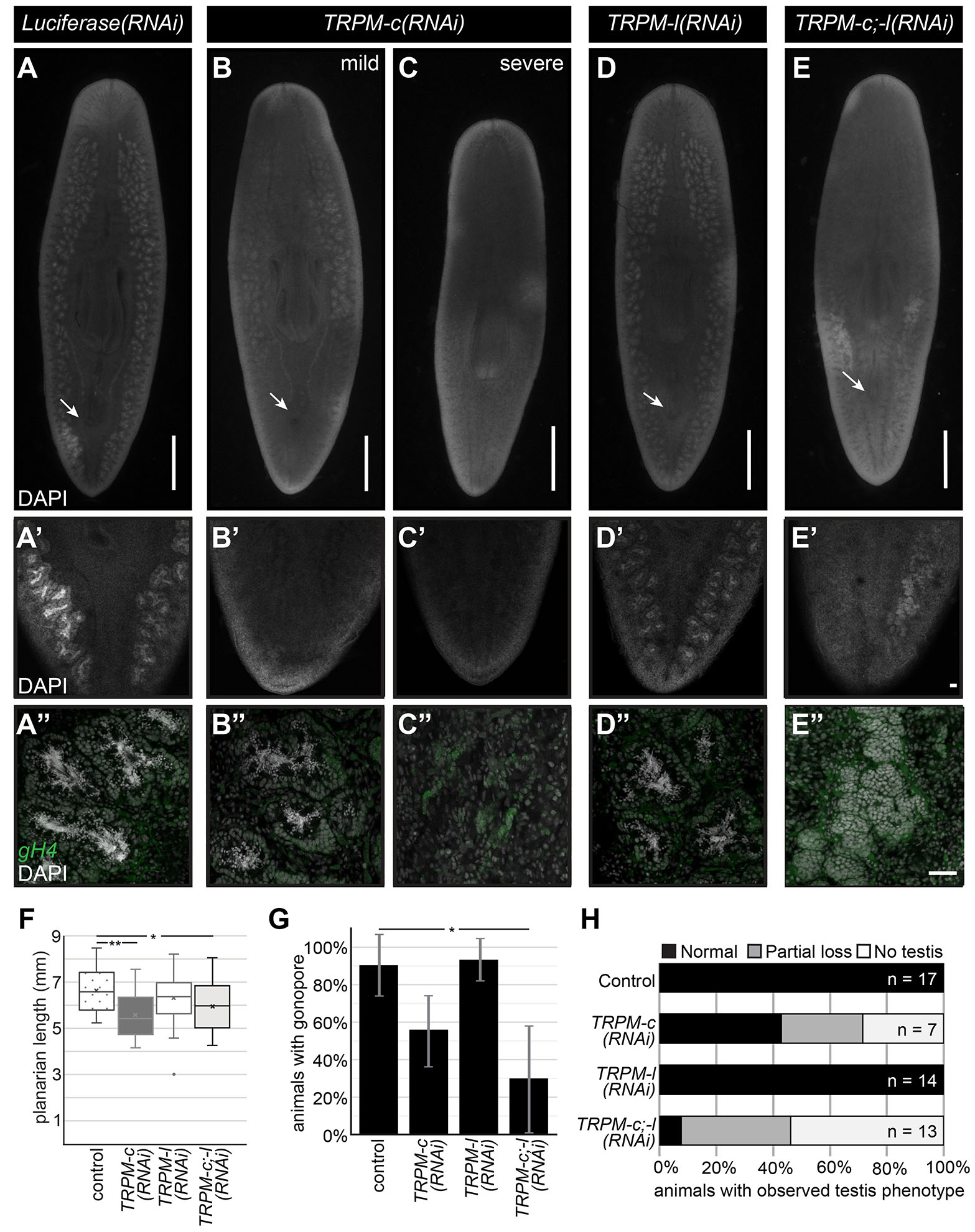
To test whether TRPMs expressed in planarian testes are required for sperm development, juvenile (sexually immature) planarian hermaphrodites were subjected to continuous RNAi treatments in groups targeting either Smed-TRPM-c (Smed-TRPM-c(RNAi)), Smed-TRPM-l (Smed-TRPM-l(RNAi)), or both Smed-TRPM-c and Smed-TRPM-l (Smed-TRPM-c;TRPM-l(RNAi)). Planarians were fed dsRNA corresponding to ~500 bps of gene-specific sequence (Supplementary File S1) mixed in their food twice per week. A control group was fed dsRNA with sequence that corresponds to a fragment of firefly Luciferase sequence, which does not interfere with planarian homeostasis or germline development (Magley and Rouhana, 2019; Lesko and Rouhana, 2020; Christman et al., 2021). After six weeks of continuous RNAi treatment, planarians grew and were fixed and stained with 4’,6-diamidino-2-phenylindole (DAPI) to visualize the anatomy and distribution of testis lobes. As expected, testis lobes were abundant along the dorsolateral anatomy of Luciferase(RNAi) (Fig. 2A), and the same was seen for Smed-TRPM-l(RNAi) planarians (Fig. 2D). On the other hand, testis lobes were partially or completely missing in regions of Smed-TRPM-c(RNAi) and Smed-TRPM-c;TRPM-l(RNAi) planarians (Fig. 2 B-C,E). The absence of testis lobes was particularly noticeable at the posterior end of these animals (Fig. 2 B’-C’,E’), which is a region where testis lobes are normally abundant (Fig. 2 A’,D’). Beyond distribution of testis lobes, analysis of sperm development by high magnification confocal microscopy revealed full progression of spermatogenesis and spermatozoa accumulation in the innermost region of testis lobes of Luciferase(RNAi) and Smed-TRPM-l(RNAi) planarians (Fig. 2 A”,D”). In contrast, Smed-TRPM-c(RNAi) and Smed-TRPM-c;TRPM-l(RNAi) planarians had testis lobes with disruptions at different stages of spermatogenesis. These included loss of spermatozoa (Fig. 2B”), loss of spermatids and/or spermatocytes (Fig. 2E”), and reduced spermatogonia (Fig. 2C”).
Fig. 2. Decreased testis development is observed upon Smed-TRPM-c knockdown and Smed-TRPM-c;TRPM-l double knockdown.
(A-E) DAPI signals from whole-mount staining of Luciferase(RNAi) (A-A”) and Smed-TRPM-l(RNAi) (D-D”) show normal distribution of testis lobes along the dorsolateral anatomy of sexual planarians (A,D). Accumulation of spermatozoa inside testis lobes can be observed in these same samples by confocal microscopy under 10X (A’, D’) and 20X (A”, D”) objectives. Parallel analysis of Smed-TRPM-c(RNAi) and Smed-TRPM-c;TRPM-l(RNAi) (TRPM-c;-l(RNAi)) show partial (B; E) to complete (C) loss of testis lobes and/or testis lobes producing spermatozoa (B”-C”, and E”). Germline stem cells and spermatogonia in testes are shown using germinal histone H4 (gH4) fluorescence in situ hybridization signal (green in A”-E”). Development of testis lobes observed in Luciferase(RNAi) and Smed-TRPM-l(RNAi) posterior regions (A’ and D’) was particularly reduced in Smed-TRPM-c(RNAi) and Smed-TRPM-c;TRPM-l(RNAi) samples (B’-C’, and E’). (F) Box and whisker plot showing average planarian length post-fixation. Mean is marked by an “X”. (G) Percentage of animals in each RNAi group displaying a fully developed copulatory apparatus at end of RNAi treatment. Graphs indicate averaged values from three different experiments with error bars representing standard deviation. (H) Quantification of testis development phenotypes (as in A-E) in animals of size comparable to sexually mature controls illustrate percentage of samples with normal testis development (black bar), as well as partial (gray bar) and complete (white bar) absence of testis in each group. Arrows indicate position of copulatory organ in (A-E). Scale bars, 1 mm (A-E) and 50 µm (E’ and E”). Statistical significance in (F) and (G) is indicated by one asterisk (*) in comparisons for which Student’s t-test p < 0.05, and by two asterisks (**) where p < 0.01.
Sexual maturation and development of reproductive structures are related to growth and somatic integrity (Kobayashi et al., 1999; Wang et al., 2007). Therefore, animal size and development of somatic reproductive structures were analyzed in Smed-TRPM-c, Smed-TRPM-l, and Smed-TRPM-c;TRPM-l knockdowns. Smed-TRPM-c(RNAi) displayed reduced average animal length when compared to the Luciferase(RNAi) control and Smed-TRPM-c(RNAi) groups (Fig. 2F). Additionally, about half of the Smed-TRPM-c(RNAi) as well as Smed-TRPM-c;TRPM-l(RNAi) planarians failed to develop copulatory structures (Fig. 2G), indicating compromised sexual maturation. To assess whether the reduced testis phenotype in Smed-TRPM-c(RNAi) resulted from size deficiencies, sperm development was compared between animals of equivalent size or larger than the smallest sexually mature planarian from the Luciferase(RNAi) group (i.e., exhibiting fully developed copulatory structures). Amongst these groups, Smed-TRPM-c(RNAi) planarians still displayed spermatogenesis defects not observed in the control or Smed-TRPM-l(RNAi) groups, and knockdown of Smed-TRPM-l in the Smed-TRPM-c;TRPM-l(RNAi) group seemed to exacerbate the loss of testis and sperm development (Fig. 2H). These results suggest that Smed-TRPM-c is required for testis development and/or spermatogenesis directly, and that the spermatogenesis phenotype of Smed-TRPM-c(RNAi) is not a consequence of growth defects. Interestingly, levels of Smed-TRPM-l RNA were more robustly decreased by RNAi than Smed-TRPM-c RNA levels (75% and 70% average decrease for Smed-TRPM-l 1-week into RNAi treatments for single and double target gene knockdown, respectively, vs. 20% and 15% for Smed-TRPM-c; Supplementary Fig. S2), which may explain the variability of phenotypic penetrance observed upon Smed-TRPM-c RNAi.
Smed-TRPM-c is preferentially expressed in developing sperm and required to maintain sperm production
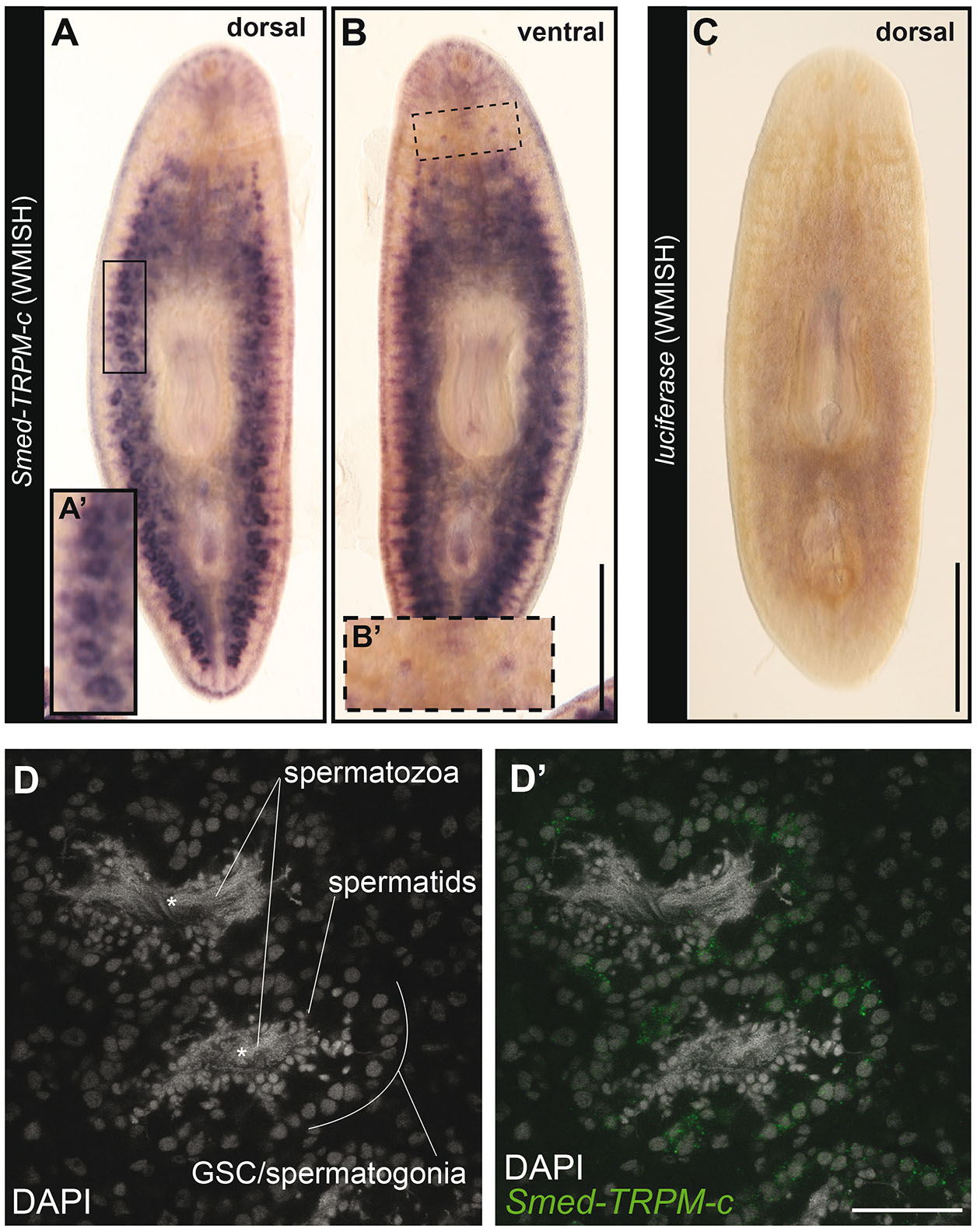
Given that disruption of Smed-TRPM-c expression alone resulted in phenotypic observations comparable to Smed-TRPM-c;TRPM-l double knockdown, further studies were carried out focusing on Smed-TRPM-c. The full cDNA sequence for Smed-TRPM-c was obtained by 3’ and 5’ rapid amplification of cDNA ends (RACE), followed by direct amplification, cloning, and sequencing of Smed-TRPM-c open reading frames. Expression of Smed-TRPM-c was analyzed using riboprobes generated from full-length ORF clones, which confirmed preferential expression in testis lobes (Fig. 3 A,A’; n = 8/8 planarians) and validated WMISH results observed using partial sequence (Fig. 1D). Interestingly, expression of Smed-TRPM-c was also detected in the ovaries in a fraction of our samples when using full-length riboprobes (Fig. 3 B,B’; n = 2/8 planarians). Non-specific signal from WMISH generated from parallel analyses using a riboprobes against firefly Luciferase as negative control was minimal (Fig. 3C), confirming the specificity of Smed-TRPM-c signals. Expression of Smed-TRPM-c was not conclusively detected outside of the gonads, although faint signals were observed in regions of the gut and head (Fig. 3 A,B). However, single cell RNAseq (scRNA-seq) data available for asexual planarians (Plass et al., 2018; Fincher et al., 2018), failed to support the notion that Smed-TRPM-c is expressed in the gut or in neurons (Supplementary Fig. S3). In fact, there is no corroboration regarding detection of expression of Smed-TRPM-c in any somatic cell type between different scRNA-seq studies (Plass et al., 2018; Fincher et al., 2018; Zeng et al., 2018), although one indicated comparably higher expression in glia than is other somatic tissues (Plass et al., 2018; Supplementary Fig. S3). These results indicate that Smed-TRPM-c is preferentially expressed in the testes.
Fig. 3. Smed-TRPM-c is preferentially expressed in testes.
(A-C) Whole-mount in situ hybridization analysis using full-length ORF probes in sexual planarians shows enriched expression of Smed-TRPM-c in testis lobes of sexual planarians (n = 8/8; A) and in the ventrally located ovaries (n = 2/8; B). Background signal generated from negative control Luciferase riboprobes is shown in (C). Insets show 2-fold magnified views of testis lobes (A’; full frame inset) and ovaries (B’; dashed frame). (D) Confocal section displaying the signal detection of Smed-TRPM-c fluorescence in situ hybridization, green (D’) in the outer cellular region of testis lobes. DAPI stain of nuclear DNA (gray; D and D’) show changes in cellular morphology during the progressive differentiation of spermatogenic cells from the outer to inner region of each lobe. Asterisks mark location of the innermost region of each testis lobe. Scale bar, 1 mm in A-C, and 50 µm in D’.
Fluorescent in situ hybridization was performed using full-length ORF riboprobes to further assess the cellular distribution of Smed-TRPM-c expression in testes. Planarian testis lobes are primarily populated by germ cells undergoing spermatogenesis (Wang et al., 2007; Newmark et al., 2008; Collins et al., 2010). Spermatogonial stem cells proliferate to form cysts that differentiate progressively as their location changes from periphery to center of each testis lobe (i.e., germline stem cells/spermatogonia in the outer region, and spermatids and spermatozoa in the innermost region of the lobe; Fig. 3D). Smed-TRPM-c expression was detected most abundantly in the periphery of the testis lobe, which is where male germline stem cells and spermatogonia are located (Fig. 3D’). These results indicate that Smed-TRPM-c is preferentially expressed during early stages of spermatogenesis.
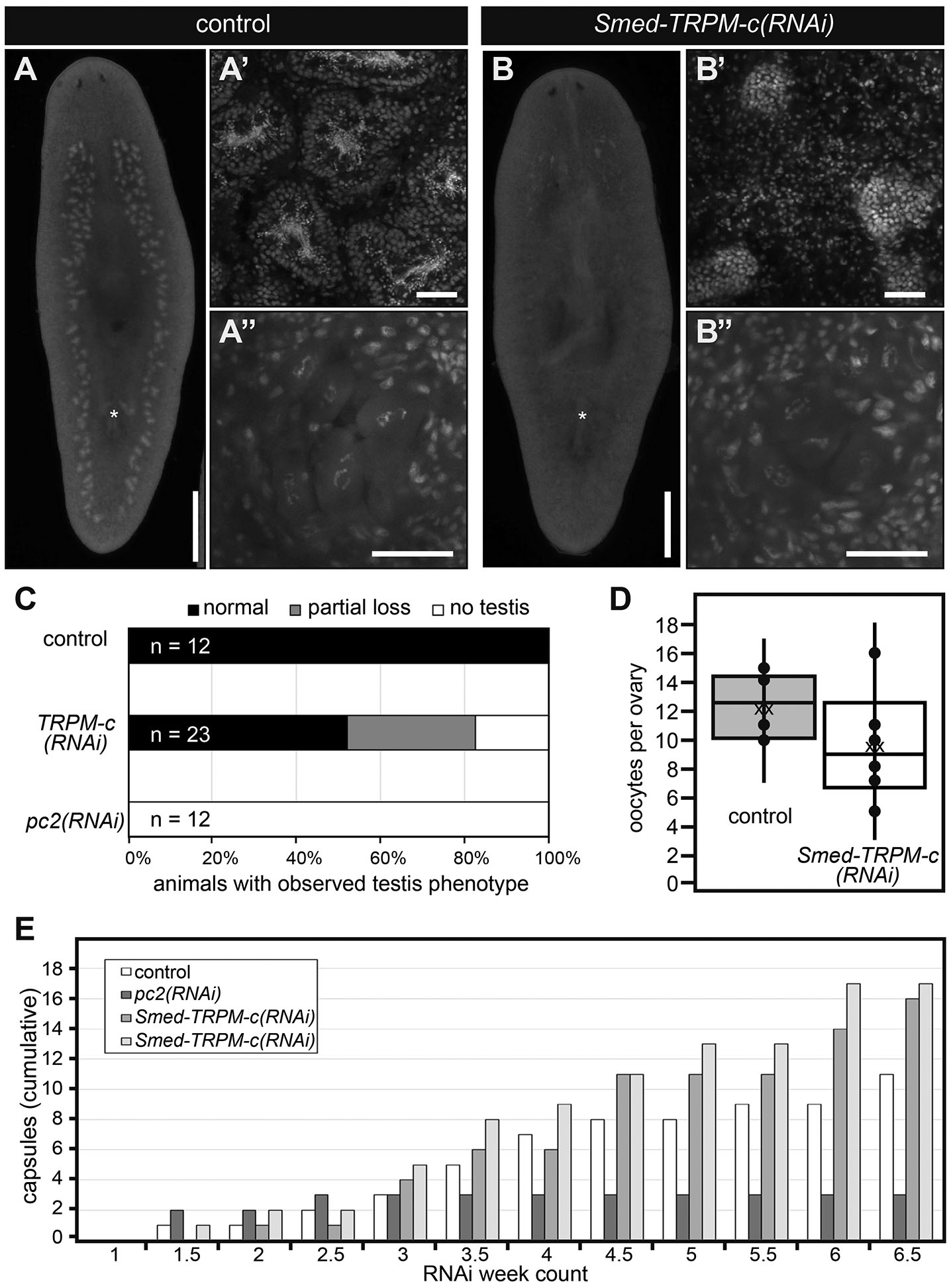
RNAi experiments performed using dsRNA corresponding to full-length Smed-TRPM-c ORF were performed to verify the phenotype observed upon knockdown of this gene using partial sequence. Groups of juvenile S. mediterranea were fed dsRNA mixed with liver twice per week, and a positive control group for RNAi efficacy (Smed-prohormone convertase 2; pc2(RNAi)) along with a negative control group (Luciferase(RNAi)) were included for comparison. After six weeks of RNAi, samples of comparable size were stained with DAPI and analyzed by fluorescence stereo and confocal microscopy. DAPI staining revealed normal testis morphology and distribution in all of the Luciferase(RNAi) samples (n = 12; Fig. 4 A-A’,C). As was expected from previous studies (Collins et al., 2010), animals from the pc2(RNAi) group lacked testes and showed progressively less motility (Fig. 4C; not shown), indicating that the RNAi regimen was effective. Interestingly, about half of the animals in the Smed-TRPM-c(RNAi) group (n = 23) displayed fewer and underdeveloped testes in comparison to control samples (Fig. 4 B-B’,C). In contrast, analysis of ovaries did not reveal noticeable changes in morphology between Luciferase(RNAi) and Smed-TRPM-c(RNAi) (Fig. 4 A”,B”), and quantification of the number of oocytes present per ovary did not reveal statistically significant a difference in Smed-TRPM-c(RNAi) and control planarians (Fig. 4D; mean 12.2 oocytes/ovary in Luciferase(RNAi) vs. 9.6 oocytes/ovary Smed-TRPM-c(RNAi); unpaired two-tailed student’s t-test, p > 0.05). These results indicate that Smed-TRPM-c is primarily required to support normal sperm development in planarians.
Fig. 4. Smed-TRPM-c supports sperm development.
(A,B) Analysis of DAPI-stained samples by whole-mount fluorescence stereomicroscopy reveals testis lobe distribution and presence of copulatory apparatus (asterisks) in Luciferase(RNAi) (control; A) and Smed-TRPM-c(RNAi) (B) planarians. Analyses of same samples by single-plane confocal microscopy reveal normal sperm development in Luciferase(RNAi) planarians (A’) and sperm development disruption in Smed-TRPM-c(RNAi) (B’), whereas development of oocytes seems comparable (A” and B”). Scale bars, 1 mm (A,B) and 50 μm (A′-B′ and A”-B”). (C,D) Quantification of gonad development phenotypes (as in A,B) revealed normal testes distribution (black bar; C) in all control samples, and partial (gray bar) or complete loss (white bar) of productive testes in almost half of all Smed-TRPM-c(RNAi) planarians (n = 23). Complete loss of testes was observed in all pc2(RNAi) planarians (n = 12). (D) Box and whisker plot show a slight decrease in the number of oocytes per ovary in Smed-TRPM-c(RNAi) samples (white) when compared to Luciferase(RNAi) (gray). This difference was not statistically significant (t-test, p > 0.05). Dots represent individual data points, “xx” marks the mean. (E) Analysis of cumulative egg capsule production as a readout of functional somatic reproductive structures reveals continuous production of capsules in Luciferase(RNAi) (control, white bars) and two groups of Smed-TRPM-c(RNAi) planarians (lighter shades of gray), whereas pc2(RNAi) planarians (dark gray) ceased producing capsules 2.5 weeks into RNAi treatments.
To further check for pleiotropic defects related to maintenance of sexual maturation, production of egg capsules was analyzed in sexually mature Smed-TRPM-c(RNAi) planarians. Planarians, like most flatworms, produce ectolecithal eggs that lack the nutritional components necessary for early development (Gremigni, 1983). Instead, the embryo is encapsulated with yolk cells produced by yolk glands (vitellaria) that develop at the end of sexual maturation using modifications to oogenic genetic programs (Rouhana et al., 2017; Issigonis et al., 2022). Importantly, production and deposition of egg capsules occur independently of presence or absence of functional gametes (Steiner et al., 2016). To test the requirement of Smed-TRPM-c in vitellaria maintenance and function, sexually mature planarians were subjected to six weeks of RNAi treatments and the number of egg capsules deposited was recorded after each dsRNA feeding as an indicator of vitellaria presence and function. The number of capsules deposited by Luciferase(RNAi) control planarians accumulated continuously over the course of RNAi treatment (Fig. 4E) as previously observed (Rouhana et al., 2017; Steiner et al., 2016). In contrast, pc2(RNAi) planarians produced capsules only during the first two weeks of treatment (Fig. 4E), which was expected given that pc2 function is required for maintenance of sexual maturation (Collins et al., 2010). Smed-TRPM-c(RNAi) planarians produced egg capsules continuously over the course of the experiment reaching even higher quantities than the Luciferase(RNAi) group (Fig. 4E), indicating that Smed-TRPM-c is not required for yolk cell development, maintenance, or function. Hatchling production was not quantified due to difficulties that would emerge from the considerable subset of Smed-TRPM-c(RNAi) animals that do produce sperm (Figs. 2H and 4C), as well as the already low and variable hatching rate observed for capsules of laboratory cultures of S. mediterranea (22% to 48%; Steiner et al., 2016). Nevertheless, the fact that Smed-TRPM-c(RNAi) planarians produced capsules as often as control animals indicates that in addition to yolk glands, structures that participate in capsule formation, such as the gonopore and genital atrium, were functional in Smed-TRPM-c(RNAi) planarians.
In addition to testing for capsule formation and deposition, changes in behavior were investigated in Smed-TRPM-c(RNAi) to assess the possibility of neuronal functions or non-specific knockdown of TRP paralogs with functions in S. mediterranea sensory behaviors (Arenas et al., 2017; Ross et al., 2018; Ross et al., 2024). Chemotaxis response was indirectly tested by tracking the fraction of planarians that ate during RNAi feedings, for which no differences were observed (data not shown). To test for changes in behavioral response to temperature, an arena composed of two cold (17°C) and two hot (30°C) quadrants was manufactured using a design similar to that described by Arenas et al., (2017). Luciferase(RNAi) spent most of their time in the cold quadrants and avoided hot temperature quadrants (Supplementary Fig. S4), as expected from findings by Arenas et al., (2017). No significant difference was observed in the time spent in cold and hot quadrants between Smed-TRPM-c(RNAi) and control groups (Supplementary Fig. S4). This result supports the notion that Smed-TRPM-c is directly required for sperm development, and that spermatogenesis defects in Smed-TRPM-c(RNAi) animals are not indirect outcomes from compromised neuronal functions.
Smed-TRPM-c supports development and/or maintenance of nanos(+) presumptive germline stem cells in asexual planarians
The phenotype observed from RNAi analyses in sexual planarians indicated that Smed-TRPM-c supports early stages of spermatogenesis. To further investigate this hypothesis, expression of Smed-TRPM-c was disrupted in asexual planarians. An asexual strain of S. mediterranea that reproduces exclusively through fission and regeneration contains a chromosomal translocation assumed to impede sexual maturation (Newmark and Sanchez Alvarado, 2002; Newmark et al., 2008). However, planarians in this asexual strain still contain presumptive germline stem cells located in the dorsolateral position equivalent to where testes develop in sexual planarians (Wang et al., 2007). Groups of asexual S. mediterranea were subjected to six-weeks of Luciferase or Smed-TRPM-c dsRNA feedings. Upon completion of dsRNA feedings, the presence of presumptive germline stem cell clusters was visualized using nanos(+) marker expression by WMISH. At the end of the 6-week RNAi treatment clusters of nanos(+) cells were quantified in both Luciferase(RNAi) control planarians (Fig. 5 A-A’) and Smed-TRPM-c(RNAi) planarians (Fig. 5 B-B’). Calculating the abundance of nanos(+) clusters per millimeter of animal length revealed a decrease in the number of nanos(+) clusters in Smed-TRPM-c knockdowns (Fig. 5C; see Supplementary Fig. S5 for individual nanos(+) cluster counts plotted against animal length). Statistical analysis using two-tailed Student’s t-test indicated that the difference in number of clusters per millimeter of animal length between Smed-TRPM-c(RNAi) and control planarians is significant (p < 0.01). This reduction in relative number of nanos(+) clusters corroborates with the loss of testis lobes observed in Smed-TRPM-c(RNAi) sexual planarians (Figs. 2 B,C,H and 4 B,C). Given these results, we propose that Smed-TRPM-c contributes to spermatogenesis through regulation of establishment, maintenance, and/or expansion of germline stem cells.
Fig. 5. Smed-TRPM-c is required for maintenance of presumptive germline stem cells in asexual planarians.
(A,B) Distribution of nanos(+) germline stem cell clusters in control Luciferase(RNAi) (A) and Smed-TRPM-c(RNAi) (B) asexual planarians are displayed in bright field microscopy images of samples subjected to whole-mount in situ hybridization. Insets in (A’ and B’) show 5-fold magnified view. Scale bar, 1 mm. (C) Box and whisker plot displaying quantitative analysis of nanos(+) cluster number in relation to planarian body length observed in Luciferase(RNAi) and Smed-TRPM-c(RNAi) planarians. Dots represent individual data points and “x” marks the mean. Statistical significance (Student’s t-test, p < 0.01) is indicated by two asterisks.
Smed-TRPM-c has domains present in mammalian TRPMs and is predicted to form a homotetrameric transmembrane channel
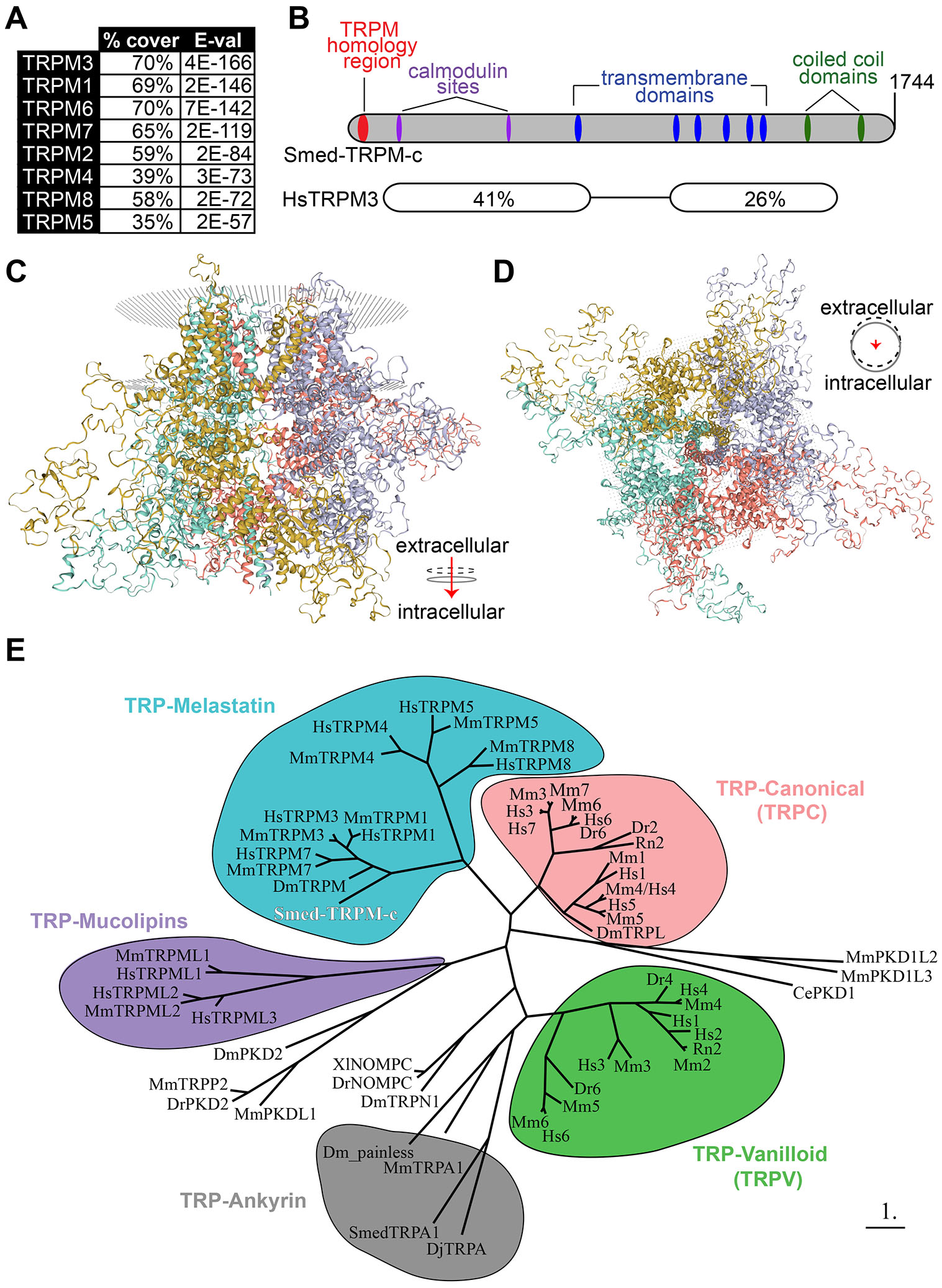
BLASTP analysis of the protein sequence coded in the longest identified open reading frame (5,235 nts; GenBank: PQ273721.1) identified TRPM3 as the closest human homolog to this planarian gene (Fig. 6A; E-value = 4E-166), which corroborates with the analysis of reference cDNA contigs for Smed-TRPM-c deposited on PlanMine (Supplementary Table S1). Smed-TRPM-c contains long regions of conserved sequence that include several predicted structural features present in mammalian TRPMs (Fig. 6B). These structural features include the N-terminal TRPM Homology Region (MHR) that is characteristic of members of the TRPM family (Grimm et al., 2003; Wagner et al., 2008; Wang et al., 2018; Winkler et al., 2017; Supplementary Fig. S6), six transmembrane domains (Supplementary Fig. S7), two calmodulin binding sites found in human TRPM3 (Holakovska et al., 2012) and two C-terminal coiled-coil domains (Fig. 6B). The MHR of Smed-TRPM-c and human TRPMs are 26-37% identical (Supplementary Fig. S6). Additionally, the serine residue at the 1107 position of mouse TRPM7, which is important in modulation of channel activity in response to phosphatidylinositol 4,5-bisphosphate levels (Zhelay et al., 2018) is conserved in Smed-TRPM-c (Supplementary Fig. S7). Analysis of quaternary structure based on protein homology modeling (SWISS-MODEL; Waterhouse et al., 2018; Bertoni et al., 2017) predicted a homotetrameric transmembrane channel as the top formation for Smed-TRPM-c (Fig. 6 C,D). Each unit in the complex is predicted to contribute to the central pore of the channel (Fig. 6D). To infer the phylogenetic relationship between Smed-TRPM-c and TRPs characterized in other experimental models, a phylogeny tree based on maximum-likelihood (Dereeper et al., 2008) was generated (Fig. 6E). In this analysis, Smed-TRPM-c groups with a subset of TRPMs that includes mammalian TRPM1, 3, and 7, as well as TRPM from Drosophila, which are expressed in spermatogenic cells in mammals (Li et al., 2010) and mediate calcium influx during egg activation in Drosophila (Hu et al., 2019), respectively. Altogether, these analyses indicate that Smed-TRPM-c shares sequence and structural features that are typical of mammalian TRPMs and close sequence conservation with those expressed in germ cells of other animals.
Fig. 6. Predicted protein structure and phylogenetic analysis of Smed-TRPM-c.
(A) The top eight human matches in BLASTP analysis against Smed-TRPM-c are TRPM proteins. (B) Architecture of Smed-TRPM-c illustrated using a bar diagram that includes predictions of transmembrane (blue) and coiled coil (green) domains identified by SMART (Simple Modular Architecture Research Tool; Letunic et al., 2021), as well as a TRP-Melastatin (TRPM) specific homology region (Grimm et al., 2003; red), and two calmodulin binding sites identified by the Calmodulin Target Database (http://calcium.uhnres.utoronto.ca/ctdb/ctdb/home.html; magenta). Regions of sequence conservation identified by pairwise BLASTP between Smed-TRPM-c and human TRPM3 (isoform 1; NP_066003.3), including percent identity and corresponding E-values. (C,D) The predicted quaternary structure of Smed-TRPM-c as a transmembrane homotetramer according to SWISS-MODEL structural modeling (Waterhouse et al., 2018; Bertoni et al., 2017) based on homology to mouse TRPM7 (Duan et al., 2018). The complex of four subunits is shown in its frontal view (C) and intra to extra cellular view (D). Each of four subunits are shown with different color and the phospholipid bilayer is represented by gray dots. (E) Phylogenetic tree using maximum-likelihood principle depicts closer association of Smed-TRPM-c (red bold font) with TRPM from Drosophila melanogaster (Dm) and mammalian TRPM3/7/1 orthologs, than with other characterized TRPs. Groups of TRPs belonging to the melastatin (blue), canonical (pink), vanilloid (green), ankyrin (gray), and mucolipins (purple) families are grouped in bubbles. Abbreviated names showing only first letter of genus and species names (e.g., “Hs” for Homo sapiens) were utilized for TRPC and TRPV genes. Scale bar represents 1 substitution per amino acid position.
Intracellular enzymatic domains are present in C-termini of a few mammalian TRPMs (i.e., TRPM2, TRPM6, and TRPM7), which is not characteristic of other TRP channels (reviewed by Scharenberg, 2005; Cabezas-Bratesco et al., 2015). Examination using bioinformatic domain recognition software and BLAST analyses did not identify a kinase domain homologous to those present in mammalian TRPM6 and TRPM7 amongst any of the planarian TRPMs. The closest sequence to match the kinase domain of human TRPM6 and TRPM7 in S. mediterranea is that of a eukaryotic elongation factor 2 kinase ortholog (data not shown). In contrast, a match for the NUDIX hydrolase domain present in the C-terminus of human TRPM2 was detected in C-termini of Smed-TRPM-a1 and Smed-TRPM-f, as well as in DjTRPMa from the planarian D. japonica (Supplementary Fig. S8). These findings support the notion that TRPM channel fusions with NUDIX hydrolase enzymatic domains predate the cnidarian-bilaterian split, as hypothesized from detection of this domain in TRPM2 from the cnidarian Nematostella vectensis (Kühn et al., 2016). Interestingly, all three of the planarian proteins that possess homology to the hydrolase domain (Smed-TRPM-a1, DjTRPMa, and Smed-TRPM-f) are preferentially expressed in neurons (Fig. 1 and Supplementary Table S1; Inoue et al., 2014).
Discussion
Our research revealed 21 potential TRPM family members in the planarian S. mediterranea. Expression of two of these was detected in testes, while some of the others were expressed in the gut, head tip, and subsets of neurons (Fig. 1). Smed-TRPM-c expression is enriched in testes (Figs. 1, 3, and Supplementary Fig. S1), which parallels expression of TRPM3 in mouse testis (Jang et al., 2012) and in rat spermatogenic cells (Li et al., 2010). Expression in the ovaries was only observed in a subset of animals possibly due to incomplete development of the ovaries, which occurs towards the end of sexual maturation. Smed-TRPM-c is required to maintain normal distribution and development of active testes in the dorsolateral anatomy of sexual planarians (Figs. 2, 4) and presumptive germ cell clusters in asexual planarians (Fig. 5; Supplementary Fig. S5). We hypothesize that Smed-TRPM-c contributes to spermatogenesis via cell-autonomous mechanisms based on detection of expression of this gene in the outer layer of germ cells in testis lobes, which is where spermatogonial stem cells and spermatogonia reside (Fig. 3 D-D’, Supplementary Fig. S1). We also hypothesize that Smed-TRPM-c works to regulate sperm development in the earliest steps of spermatogenesis (i.e., establishment, proliferation, and/or maintenance of germline stem cells), based on the observation that presumptive spermatogonial stem cell clusters are less abundant upon reduction of Smed-TRPM-c expression in asexual S. mediterranea (Fig. 5; Supplementary Fig. S5). These results place Smed-TRPM-c as a sensor of either cellular or environmental cues that drive sperm development in S. mediterranea.
Members of the TRPM channel family are known to be activated by cold (TRPM8; McKemy et al., 2002) and hot temperatures (TRPM3; Vriens et al., 2011), as well as by chemical ligands such as the steroid hormone pregnenolone sulphate and spermine (reviewed by Huang et al., 2020). The stimuli that activate Smed-TRPM-c remain unknown. However, our preferred hypothesis is that Smed-TRPM-c is responsive to temperature and regulates cell-autonomous mechanisms in the male germline. This is based on three indirect observations: 1) A member of the TRPM family that displays preferential expression during spermatogenesis in mammals protects germ cells from damages induced by cold-shock (TRPM8; Borowiec et al., 2016); 2) Smed-TRPM-c shares the highest sequence conservation with TRPM3 amongst mammalian orthologs, and mammalian TRPM3 is responsive to heat; 3) sexual reproduction and development in planarians are heavily regulated by temperature (see below); and 4) we didn’t detect any sperm development defects upon knockdown of orthologs of genes involved in pregnenolone sulphate metabolism (data not shown).
Planarians inhabit freshwater ecosystems all over the world, with Antarctica and islands where colonization has not occurred being possible exceptions (Vila-Farré and Rink, 2018). Most species in the genus Schmidtea are primarily distributed in northern Europe, while S. mediterranea has been mainly reported to inhabit southern European regions such as Tunisia, Italy, and Catalonia (Lázaro et al., 2011). Exclusively fissiparous populations (i.e., asexually reproducing through fission and regeneration) of S. mediterranea and other planarian species are not uncommon. However, sexual reproduction by hermaphroditism is the predominant and ancestral mechanism for reproduction in planarians. Development, size, and activity of planarian reproductive structures in the wild follow seasonal patterns, and some planarian species even switch between sexual and fissiparous reproductive strategies (Dahm, 1958; Ball and Reynoldson, 1981; Nodono and Matsumoto, 2022). Temperature has long-been an abiotic factor of interest in modulation of planarian reproductive strategies. For example, S. mediterranea testes, ovaries, and the copulatory apparatus shrink and eventually disappear in water temperatures above 20°C in (Harrath et al., 2004). However, the environmental and molecular factors that regulate this sexual maturation and reproduction in the wild remain to be uncovered.
Some evidence suggests that temperature works to tune sexual maturity in planarians through independent tissue-specific pathways, rather than through a master regulator. For example, Schmidtea (Dugesia) lugubris exhibits regressed testes at temperatures below 5°C, but these grow and become fertile when transferred to 10°C (Reynoldson et al., 1965). In contrast, S. lugubris ovaries are populated with oocytes even at temperatures below 5°C, indicating that testes and ovaries do not follow the same temperature restrictions. In another example, Polycelis tenuis produces capsules below 5°C, indicating that vitellaria are present and functional, but these capsules are sterile. Given that gametes are not required for capsule production in planarians (Steiner et al., 2016), the observation that P. tenuis produces capsules at low temperatures indicates that vitellaria are fully developed and functional, while their sterility suggests that sperm, ova, or both are absent or dysfunctional. In contrast, the planarian species Dendrocoelum lacteum produces fertile capsules at temperatures as low as 1°C to 5°C, indicating that functional oocytes, sperm, and vitellaria are present and functional in D. lacteum at these low temperatures. The stimulus (or stimuli) that activates Smed-TRPM-c remains to be found. However, Smed-TRPM-c does not seem to function as a “master switch” that modulates reproductive maturation across tissues. Instead, Smed-TRPM-c seems to serve primarily in the testis and promote specification, proliferation, and/or maintenance of spermatogonial germline stem cells. Tissue-specific functions of sensory receptors like Smed-TRPM-c provide a mechanism by which planarians can evolve reproductive system strategies in ways that maximize fitness in the different freshwater ecosystems that they inhabit.
Materials and Methods
Planarian cultures
A laboratory line of sexual S. mediterranea (Zayas et al., 2005) was used for all experiments except for those indicated as specifically using asexual animals, in which case specimens from the CIW4 clonal laboratory strain were used (Newmark and Sánchez Alvarado, 2000). Asexual animals were maintained in 1X Montjuïc salts at 21°C. Sexual animals were maintained in 0.75X Montjuïc salts at 18°C. Planarians were maintained in the dark except during weekly or biweekly feedings with beef calf liver, which were done on bench tops at room temperature. Laboratory colonies of S. mediterranea sexual strain were maintained and amplified mainly through transverse amputation and regeneration. Therefore, the majority of sexual animals used in this study originated from regenerated fragments. Animals of comparable size were chosen at the start of every experiment, checked for absence of gonopore on the ventral posterior (when juvenile stage was desired), and starved for at least one week prior to experimentation or fixation.
Identification of TRPM homologs and generation of riboprobes for whole-mount in situ hybridization
TRPM homologs in S. mediterranea were identified from transcriptomes of sexual (dd_Smes_v1 prefix) and asexual (dd_Smed_v6 prefix) strains deposited in PlanMine (Rozanski et al., 2019) using human TRPM3 (NCBI GenBank ID NP_066003.3) as input in TBLASTN searches. Redundant records were identified by pairwise BLASTN comparisons and removed. GeneArt Strings DNA fragments (ThermoFisher, Waltham, MA) were synthesized for each S. mediterranea TRPM homolog to contain ~500 bps of reference contig sequence flanked by SP6 (sense) and T3 (antisense) promoter sequences (Supplementary File 1). These custom-ordered DNA fragments were amplified by PCR using primers corresponding to SP6 and T3 promoter sequences that included an additional T7 promoter sequence at their 5’-ends (5′-GAATTTAATACGACTCACTATAGGGCGATTTAGGTGACACTATAGAAGAGAAC-3′ and 5′-GAATTTAATACGACTCACTATAGGGCGAATTAACCCTCACTAAAGGGAAC-3′, respectively) as described in Counts et al., (2017). PCR fragments were purified using DNA Clean & Concentrator-5 kits as per the manufacturer (Zymo Research, Irvine, CA), eluted in 20 µL or RNase-free water, and used as templates for synthesis of riboprobes labeled with Digoxigenin-11-UTP (Roche, distributed by Millipore Sigma, Burlington, MA) using T3 RNA polymerase (Promega, Madison, WI).
Cloning of Smed-TRPM-c cDNA
Full-length mRNA sequence for Smed-TRPM-c was predicted from matching contigs (dd_Smed_v6_11377_0_1, dd_Smes_v1_41098_1_4, uc_Smed_v2_27847, others) identified in in PlanMine (Rozanski et al., 2019) and primers for 5’ and 3’ RACE [5’-AGGAAGCAATGATTTACCGGGAGAAAG-3’ and 5’-CAAGTGGTCAGTAGAGCGCATTGACTAC-3’] were designed based on this prediction. Full-length Smed-TRPM-c ORF was amplified using Long PCR Master Mix (Promega, Madison, WI) from sexual S. mediterranea cDNA synthesized using oligo(dT) and N6 primers. The oligo primers used for amplification of Smed-TRPM-c ORF were 5’-GTCACAGTGGCATTGTAGCCAATCCCTC-3’ and 5’- ATTGGCGAGTAAATCGCTTTGCATTGC-3’. The forward primer is position immediately upstream from the first 19 nucleotides of the ORF (5’-ATGAAAAAATCTAAAAAAA-3’) according to records on PlanMine. Primers were not designed to include this region due to rich A/T content. Amplicons were ligated into the vector pGEM-T (Promega, Madison, WI) and verified by Sanger sequencing.
Bioinformatic analysis of protein structure
The transmembrane and coiled coil domains of the predicted Smed-TRPM-c full-length ORF were identified using the Normal SMART program (Letunic et al., 2021). The starting motif of the TRPM Homology Region (MHR) was derived from Grimm et al., (2003). The putative calmodulin binding sites of Smed-TRPM-c were identified with the Calmodulin Target Database (http://calcium.uhnres.utoronto.ca/ctdb/ctdb/home.html). N-terminus TRPM homology region analysis was performed by alignment of TRPM Homology Region of different TRPM channels (Grimm et al., 2003) using ClustalW 2.1 (Thompson et al., 1994). The multiple alignment was then input into BoxShade (https://github.com/mdbaron42/pyBoxshade/releases) using the default settings. Predictive models of three-dimensional Smed-TRPM-c structure were obtained using SWISS-MODEL structural modeling (https://swissmodel.expasy.org/; Waterhouse et al., 2018; Bertoni et al., 2017) based on homology to mouse TRPM7 (Duan et al., 2018) and using default settings.
Smed-TRPM-c phylogenetic analysis
TRP protein sequences were obtained from analyses published in Inoue et al., (2014) and Arenas et al., (2017). The predicted amino acid sequence of Smed-TRPM-c was aligned with those of TRP proteins from these studies using ClustalW and used as input to generate a phylogenetic tree using PhyML and TreeDyn in phylogeny.fr (Dereeper et al., 2008). Default settings were used across programs.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as described by Pearson et al., (2009) with modifications in fixation steps following King and Newmark (2013). Large sexually mature animals (~1.0 cm or larger) were used, therefore the N-acetylcysteine (NAC) treatment, as well as the initial fixation step and the Proteinase K treatment were prolonged. Planarians were placed in a solution of 10% NAC in PBS for 11 minutes with gentle agitation, then fixed in 4% formaldehyde in PBS containing 0.3% Triton X-100 (PBSTx) and rocked for 1 hour at 4°C. The animals were then gradually dehydrated in methanol and kept at -20°C overnight. The following day, the samples were gradually rehydrated in PBSTx and bleached in formamide bleaching solution (King and Newmark, 2013) for 2 hours under a bright light. After bleaching, the samples were rinsed in PBSTx and placed in a Proteinase K solution containing 10μg/ml of Proteinase K (ThermoFisher, Waltham, MA) in PBSTx with 0.1% (w/v) SDS for 12 minutes with gentle agitation. The samples were post-fixed for 10 minutes with 4% formaldehyde in PBSTx. The steps for hybridization and post-hybridization washes were performed as indicated by Pearson et al., (2009). For colorimetric in situ hybridization, samples were incubated in blocking solution containing 5% horse serum in TNTx (consisting of 0.1M Tris pH 7.5, 0.15M NaCl, and 0.3% Triton X-100) for at least 2 hours. Samples subjected to colorimetric signal detection were incubated in anti-Digoxigenin-AP antibody solution (1:4000 dilution; Roche Diagnostics, Mannheim, Germany) overnight while rocking at 4°C. The post-antibody incubation washes with TNTx and signal development were performed as in Pearson et al., (2009) for colorimetric signal detection. After development, the samples were mounted in 80% glycerol/20% PBS and imaged using a Zeiss V.16 SteREO microscope equipped with a Canon EOS Rebel T3 camera. For fluorescent in situ hybridization, samples were incubated in blocking solution containing 5% horse serum and 1% Western Blocking Reagent (Roche Diagnostics, Mannheim, Germany) in TNTx for at least 2 hours. Samples used in FISH analyses were incubated in anti-DIG-POD antibody solution (1:2000 dilution; Roche Diagnostics, Mannheim, Germany) rocking overnight at 4°C. The FISH samples were developed using FAM tyramide solution as described by King and Newmark (2013), washed, and mounted in 80% glycerol/20% PBS and imaged using a Nikon C2+ confocal microscope with NIS Elements C software. Initial observations of distribution of expression of TRPM homologs were performed using riboprobes generated from GeneArt DNA fragments (Supplementary File S1), and Smed-TRPM-c expression was validated in colorimetric and FISH analyses using riboprobes generated from full-length ORF clones. Modifications of WMISH for double-FISH were performed as per King and Newmark (2013).
Disruption of Smed-TRPM-c expression by RNA-interference (RNAi)
Templates for in vitro transcription of dsRNA generated by PCR from GeneArt fragments (Supplementary File S1) using the aforementioned primers (see “generation of riboprobes for whole-mount in situ hybridization, above) or from cDNA cloned into pGEM-T (Promega, Madison, WI) using the following primers: 5’-GAATTTAATACGACTCACTATAGGGCGCCAAGCTATTTAGGTGACACTATAGAATACTC-3’ and 5’- GAATTAATTAACCCTCACTAAAGGGAGAATTTAATACGACTCACTATAGGGCGAATTGG-3’). PCR products were purified using DNA Clean & Concentrator-5 columns (Zymo Research, Irvine, CA), eluted in 20μl of RNase-free water and used as templates for in vitro transcription. DsRNA was synthesized using T7 RNA polymerase as described by Rouhana et al., (2013).
Depending on the experiment, groups of either 6 juvenile sexual planarians (lacking gonopores), mature sexual planarians actively laying capsules, or 10 asexual planarians 0.5-0.7 mm length, were fed to satiation with a liver solution containing approximately 100 ng/μL of gene-specific dsRNA twice per week for at least 6 weeks. As a negative control, one group of planarians was fed firefly Luciferase dsRNA which is not expressed in the planarian genome and has no effect on their sexual development nor behavior. To assess RNAi efficacy in real-time, some experiments included one group of planarians fed with Smed-pc2 dsRNA which is required for testes maintenance and normal planarian behavior (Reddien et al., 2005; Collins et al., 2010).
Analysis of testis distribution and sperm development
A week following completion of the last RNAi feeding, planarian testis distribution and anatomy were assessed by FISH and/or DAPI staining. The samples were fixed and bleached as described above for in situ hybridization and processed for FISH, or (without methanol dehydration and rehydration steps) for analysis by DAPI only. After bleaching, DAPI-only samples were washed in PBSTx twice and then incubated in DAPI (1:1000 dilution of 1mg/ml DAPI stock solution in PBSTx; ACROS Organics, Morris, NJ) overnight while rocking at 4°C. After incubating overnight, the samples were washed 4 times with PBSTx and then mounted on slides with 4:1 glycerol:PBS and imaged under UV light with a Zeiss V.16 SteREO microscope equipped with a Canon EOS Rebel T3 camera (for low magnification) or a Nikon C2+ confocal microscope using a 10X or 20X objective and running NIS Elements C software (for high magnification).
Analysis of ovarian anatomy, oocyte development, and egg capsule deposition
To assess possible disruptions to oogenesis, the number of oocytes per ovary of Smed-TRPM-c knockdown planarians were compared to Luciferase knockdown planarians. Juvenile sexual planarians fed gene-specific dsRNA for 6 weeks were stained with DAPI a week following the conclusion of the RNAi feeding regimen. The number of oocytes were counted using Z-stacks on a Nikon C2+ confocal microscope with NIS Elements C software. The ovaries of five Luciferase(RNAi) and five Smed-TRPM-c(RNAi) planarians were examined. Each count of oocytes was plotted as an individual point in a box and whisker plot. Statistical analysis was performed using unpaired two-tailed Student’s t-test. To assess egg capsule deposition, groups of 6 sexually mature planarians (1.0 – 1.5 cm in length) were used per group. The animals were fed dsRNA as described above. The cumulative number of egg capsules deposited was recorded after each dsRNA feeding.
Analysis of presumptive germline stem cell distribution in asexual planarians
To assess distribution of presumptive germline stem cells in asexual planarians, groups of asexual S. mediterranea were subjected to dsRNA feedings for six weeks, fixed a week following the last feeding, and processed for colorimetric in situ hybridization using a nanos riboprobe (Wang et al., 2007). After in situ hybridization, each animal was imaged with a Zeiss V.16 SteREO microscope equipped with a Canon EOS Rebel T3 camera and the length of each animal was determined by drawing a 3-point arc from the center of the head to the pharynx and to the center of the tail of each planarian image using Adobe Illustrator. The images were each assigned a random number and subjected to triple-blind analysis. The number of nanos(+) clusters were counted by three individuals, averaged, and plotted against the length of the animal. Statistical analysis was completed using two-tailed unequal variance Student’s t-test on the ratios between the number of nanos clusters and the length of the animal.
Thermotaxis assay
Thermotactic behavior of asexual planarians was tested with an aluminum temperature plate based on the design used by Arenas et al., (2017) (Supplementary Fig. S4). The aluminum temperature plate was controlled by a simple variable 12-volt power supply. The power supply was regulated by two temperature controllers (Elitech, Milpitas, CA). The temperature controllers fed four Peltier plates in diagonal quadrants. Each pair of Peltier plates were either powered by positive or negative 12-volt direct current. Positive current produced warmer above-ambient temperatures while the negative current produced below-ambient temperatures.
To assemble the apparatus, thermal silver paste was used to adhere the anodized aluminum plate to the Peltier plates. The aluminum plate was treated with a hydrophobic waterproofing spray while leaving an uncoated circle of diameter 8.6 cm in the center. Two temperature sensors were adhered to the perimeter of two adjacent quadrants of the untreated circle. The heat sink was adhered to the bottom of the Peltier plates using the same thermal paste. A small computer fan was attached to the bottom of the heat sink to assist with heat dispersion.
The untreated circle was filled with Montjuïc salts to a height of approximately 3 mm, forming a bubble of water for the samples to move through. Groups of 10 asexual planarians were subjected to six weeks of RNAi treatment and then starved for one week prior to experimentation. Four Luciferase(RNAi) planarians and five Smed-TRPM-c(RNAi) planarians were fed pureed beef liver mixed with colored chalk shavings (Hagoromo Co., Ltd., Kasugai, Japan) as per Hattori et al., (2018) prior to being placed on the temperature plate to increase the visibility of the planarians during the assay. The nine planarians were placed in the center of the circle together and their movements were recorded for 10 minutes. Heat map images were taken with a thermal camera (Hti-Xintai, Dongguan, Guangdong, China) at five-minute intervals. The percentage of time spent in cold quadrants for each individual planarian was calculated from the video recording and plotted on Excel. Statistical analysis was performed using two-tailed unequal variance Student’s t-test.
Supplementary Material
Acknowledgements
The authors would like to thank Donovan Christman M.S. for his assistance in the triple-blind analysis of nanos(+) clusters in sexual planarians, Jacob Rissler for help with RNAi experiments, John Curry for assembling the thermotaxis arena, as well as Ashot Kozak and Jananie Rockwood for attempts to test planarian TRPM activity in vitro. This work was supported by a grant from The Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R15HD082754) to LR.
References
Arenas O. M., Zaharieva E. E., Para A., Vásquez-Doorman C., Petersen C. P., Gallio M. (2017). Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nature Neuroscience 20: 1686-1693.
Ball I. R., Reynoldson T. B., (1981). British Planarians. Cambridge University Press, Cambridge.
Bertoni M., Kiefer F., Biasini M., Bordoli L., Schwede T. (2017). Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Scientific Reports 7: 10480.
Borowiec A.S., Sion B., Chalmel F., Rolland A. D., Lemonnier L., De Clerck T., Bokhobza A., Derouiche S., Dewailly E., Slomianny C., Mauduit C., Benahmed M., Roudbaraki M., Jégou B., Prevarskaya N., Bidaux G. (2016). Cold/menthol TRPM8 receptors initiate the cold‐shock response and protect germ cells from cold‐shock–induced oxidation. The FASEB Journal 30: 3155-3170.
Brandl H., Moon H.K., Vila-Farré M., Liu S.Y., Henry I., Rink J. C. (2016). PlanMine – a mineable resource of planarian biology and biodiversity. Nucleic Acids Research 44: D764-D773.
Cabezas-Bratesco D., Brauchi S., Gonzalez-Teuber V., Steinberg X., Valencia I., Colenso C. (2015). The Different Roles of The Channel-Kinases TRPM6 and TRPM7. Current Medicinal Chemistry 22: 2943-2953.
Christman D. A., Curry H. N., Rouhana L. (2021). Heterotrimeric Kinesin II is required for flagellar assembly and elongation of nuclear morphology during spermiogenesis in Schmidtea mediterranea. Developmental Biology 477: 191-204.
Chmura H. E., Williams C. T. (2022). A cross-taxonomic perspective on the integration of temperature cues in vertebrate seasonal neuroendocrine pathways. Hormones and Behavior 144: 105215.
Collins J. J., Hou X., Romanova E. V., Lambrus B. G., Miller C. M., Saberi A., Sweedler J. V., Newmark P. A. (2010). Genome-Wide Analyses Reveal a Role for Peptide Hormones in Planarian Germline Development. PLoS Biology 8: e1000509.
Counts J. T., Hester T. M., Rouhana L. (2017). Genetic expansion of chaperonin‐containing TCP‐1 (CCT/TRiC) complex subunits yields testis‐specific isoforms required for spermatogenesis in planarian flatworms. Molecular Reproduction and Development 84: 1271-1284.
Dahm A. G., (1958). Taxonomy and ecology of five species groups in the family Planariidae (Turbellaria Tricladida Paludicola). Nya Litografen, Malmö.
De Blas G. A., Darszon A., Ocampo A. Y., Serrano C. J., Castellano L. E., Hernández-González E. O., Chirinos M., Larrea F., Beltrán C., Treviño C. L. (2009). TRPM8, a Versatile Channel in Human Sperm. PLoS ONE 4: e6095.
Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., Gascuel O. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36: W465-W469.
Diver M. M., Lin King J. V., Julius D., Cheng Y. (2022). Sensory TRP Channels in Three Dimensions. Annual Review of Biochemistry 91: 629-649.
Duan J., Li Z., Li J., Hulse R. E., Santa-Cruz A., Valinsky W. C., Abiria S. A., Krapivinsky G., Zhang J., Clapham D. E. (2018). Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proceedings of the National Academy of Sciences 115: E8201-E8210.
Fincher C. T., Wurtzel O., de Hoog T., Kravarik K. M., Reddien P. W. (2018). Cell type transcriptome atlas for the planarian Schmidtea mediterranea. Science 360: eaaq1736.
Gremigni V., (1983). Platyhelminthes-Turbellaria. In Reproductive Biology of Invertebrates. Oogenesis, oviposition, and oosorption. Wiley, New York, NY.
Grimm C., Kraft R., Sauerbruch S., Schultz G., Harteneck C. (2003). Molecular and Functional Characterization of the Melastatin-related Cation Channel TRPM3. Journal of Biological Chemistry 278: 21493-21501.
Harrath A. H., Charni M., Sluys R., Zghal F., Tekaya S. (2004). Ecology and distribution of the freshwater planarian Schmidtea mediterranea in Tunisia. Italian Journal of Zoology 71: 233-236.
Hattori M., Miyamoto M., Hosoda K., Umesono Y. (2018). Usefulness of multiple chalk‐based food colorings for inducing better gene silencing by feeding RNA interference in planarians. Development, Growth & Differentiation 60: 76-81.
Holakovska B., Grycova L., Jirku M., Sulc M., Bumba L., Teisinger J. (2012). Calmodulin and S100A1 Protein Interact with N Terminus of TRPM3 Channel. Journal of Biological Chemistry 287: 16645-16655.
Hu Q., Wolfner M. F. (2019). The Drosophila Trpm channel mediates calcium influx during egg activation. Proceedings of the National Academy of Sciences 116: 18994-19000.
Huang Y., Fliegert R., Guse A. H., Lü W., Du J. (2020). A structural overview of the ion channels of the TRPM family. Cell Calcium 85: 102111.
Hyman L. H. (1951). North American Triclad Turbellaria. XII. Synopsis of the Known Species of Fresh-Water Planarians of North America. Transactions of the American Microscopical Society 70: 154.
Inoue T., Yamashita T., Agata K. (2014). Thermosensory Signaling by TRPM Is Processed by Brain Serotonergic Neurons to Produce Planarian Thermotaxis. The Journal of Neuroscience 34: 15701-15714.
Issigonis M., Browder K. L., Chen R., Collins J. J., Newmark P. A., (2023). A niche-derived non-ribosomal peptide triggers planarian sexual development. bioRxiv Preprint: 2023.12.06.570471.
Issigonis M., Newmark P. A. (2019). From worm to germ: Germ cell development and regeneration in planarians. In The Immortal Germline. Elsevier.
Issigonis M., Redkar A. B., Rozario T., Khan U. W., Mejia-Sanchez R., Lapan S. W., Reddien P. W., Newmark P. A. (2022). A Krüppel-like factor is required for development and regeneration of germline and yolk cells from somatic stem cells in planarians. PLOS Biology 20: e3001472.
Jang Y., Lee Y., Kim S. M., Yang Y. D., Jung J., Oh U. (2012). Quantitative analysis of TRP channel genes in mouse organs. Archives of Pharmacal Research 35: 1823-1830.
Khan U. W., Newmark P. A. (2022). Somatic regulation of female germ cell regeneration and development in planarians. Cell Reports 38: 110525.
Kashio M., Tominaga M. (2022). TRP channels in thermosensation. Current Opinion in Neurobiology 75: 102591.
Kenk R., (1935). Studies on Virginian triclads. Journal of the Elisha Mitchell Scientific Society 51: 79-125.
King R. S., Newmark P. A. (2013). In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Developmental Biology 13: 8.
Kobayashi K., Koyanagi R., Matsumoto M., Cabrera J. P., Hoshi M. (1999). Switching from Asexual to Sexual Reproduction in the Planarian Dugesia ryukyuensis: Bioassay System and Basic Description of Sexualizing Process. Zoological Science 16: 291-298.
Kobayashi K., Maezawa T., Tanaka H., Onuki H., Horiguchi Y., Hirota H., Ishida T., Horiike K., Agata Y., Aoki M., Hoshi M., Matsumoto M., (2017). The identification of ᴅ-tryptophan as a bioactive substance for postembryonic ovarian development in the planarian Dugesia ryukyuensis. Scientific Reports 7: 45175.
Kobayashi K., Matsumoto M., Hoshi M., (2004). [Switching mechanism from asexual to sexual reproduction in planarians]. Tanpakushitsu Kakusan Koso 49: 102-107.
Kühn F. J. P., Kühn C., Winking M., Hoffmann D. C., Lückhoff A. (2016). ADP-Ribose Activates the TRPM2 Channel from the Sea Anemone Nematostella vectensis Independently of the NUDT9H Domain. PLoS ONE 11: e0158060.
Lázaro E. M., Harrath A. H., Stocchino G. A., Pala M., Baguñà J., Riutort M. (2011). Schmidtea mediterraneaphylogeography: an old species surviving on a few Mediterranean islands?. BMC Evolutionary Biology 11: 274.
Lee N., Chen J., Sun L., Wu S., Gray K. R., Rich A., Huang M., Lin J.H., Feder J. N., Janovitz E. B., Levesque P. C., Blanar M. A. (2003). Expression and Characterization of Human Transient Receptor Potential Melastatin 3 (hTRPM3). Journal of Biological Chemistry 278: 20890-20897.
Lesko S. L., Rouhana L. (2020). Dynein assembly factor with WD repeat domains 1 (DAW1) is required for the function of motile cilia in the planarian Schmidtea mediterranea. Development, Growth & Differentiation 62: 423-437.
Letunic I., Khedkar S., Bork P. (2021). SMART: recent updates, new developments and status in 2020. Nucleic Acids Research 49: D458-D460.
Li S., Wang X., Ye H., Gao W., Pu X., Yang Z. (2010). Distribution profiles of transient receptor potential melastatin- and vanilloid-related channels in rat spermatogenic cells and sperm. Molecular Biology Reports 37: 1287-1293.
Madeira F., Madhusoodanan N., Lee J., Eusebi A., Niewielska A., Tivey A. R. N., Lopez R., Butcher S. (2024). The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Research 52: W521-W525.
Magley R. A., Rouhana L. (2019). Tau tubulin kinase is required for spermatogenesis and development of motile cilia in planarian flatworms. Molecular Biology of the Cell 30: 2155-2170.
Martínez-López P., Treviño C. L., de la Vega‐Beltrán J. L., De Blas G., Monroy E., Beltrán C., Orta G., Gibbs G. M., O'Bryan M. K., Darszon A. (2011). TRPM8 in mouse sperm detects temperature changes and may influence the acrosome reaction. Journal of Cellular Physiology 226: 1620-1631.
McKemy D. D., Neuhausser W. M., Julius D. (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52-58.
Nakagawa H., Sekii K., Maezawa T., Kitamura M., Miyashita S., Abukawa M., Matsumoto M., Kobayashi K. (2018). A comprehensive comparison of sex-inducing activity in asexual worms of the planarian Dugesia ryukyuensis: the crucial sex-inducing substance appears to be present in yolk glands in Tricladida. Zoological Letters 4: 14.
Newmark P. A., Sánchez Alvarado A. (2000). Bromodeoxyuridine Specifically Labels the Regenerative Stem Cells of Planarians. Developmental Biology 220: 142-153.
Newmark P. A., Alvarado A. S. (2002). Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics 3: 210-219.
Newmark P.A., Wang Y., Chong T. (2008). Germ Cell Specification and Regeneration in Planarians. Cold Spring Harbor Symposia on Quantitative Biology 73: 573-581.
Nodono H., Matsumoto M. (2022). Annual rhythmicity in the switching of reproductive mode in planarians. Zoology (Jena) 155: 126053.
Pearson B. J., Eisenhoffer G. T., Gurley K. A., Rink J. C., Miller D. E., Sánchez Alvarado A. (2009). Formaldehyde‐based whole‐mount in situ hybridization method for planarians. Developmental Dynamics 238: 443-450.
Plass M., Solana J., Wolf F. A., Ayoub S., Misios A., Glažar P., Obermayer B., Theis F. J., Kocks C., Rajewsky N. (2018). Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 360: eaaq1723.
Reddien P. W., Bermange A. L., Murfitt K. J., Jennings J. R., Sánchez Alvarado A. (2005). Identification of Genes Needed for Regeneration, Stem Cell Function, and Tissue Homeostasis by Systematic Gene Perturbation in Planaria. Developmental Cell 8: 635-649.
Reynoldson T. B., Young J. O., Taylor M. C., (1965). The effect of temperature on the life-cycle of four species of lake-dwelling triclads. Journal of Animal Ecology 34: 23-43.
Ross K. G., Molinaro A. M., Romero C., Dockter B., Cable K. L., Gonzalez K., Zhang S., Collins E.M. S., Pearson B. J., Zayas R. M. (2018). SoxB1 Activity Regulates Sensory Neuron Regeneration, Maintenance, and Function in Planarians. Developmental Cell 47: 331-347.e5.
Ross K. G., Alvarez Zepeda S., Auwal M. A., Garces A. K., Roman S., Zayas R. M. (2024). The Role of Polycystic Kidney Disease-Like Homologs in Planarian Nervous System Regeneration and Function. Integrative Organismal Biology 6: obae035.
Rouhana L., Tasaki J., Saberi A., Newmark P. A. (2017). Genetic dissection of the planarian reproductive system through characterization of Schmidtea mediterranea CPEB homologs. Developmental Biology 426: 43-55.
Rouhana L., Weiss J. A., Forsthoefel D. J., Lee H., King R. S., Inoue T., Shibata N., Agata K., Newmark P. A. (2013). RNA interference by feeding in vitro–synthesized double‐stranded RNA to planarians: Methodology and dynamics. Developmental Dynamics 242: 718-730.
Rozanski A., Moon H.K., Brandl H., Martín-Durán J. M., Grohme M. A., Hüttner K., Bartscherer K., Henry I., Rink J. C. (2019). PlanMine 3.0—improvements to a mineable resource of flatworm biology and biodiversity. Nucleic Acids Research 47: D812-D820.
Saberi A., Jamal A., Beets I., Schoofs L., Newmark P. A. (2016). GPCRs Direct Germline Development and Somatic Gonad Function in Planarians. PLOS Biology 14: e1002457.
Scharenberg A. M. (2005). TRPM2 and TRPM7: channel/enzyme fusions to generate novel intracellular sensors. Pflügers Archiv - European Journal of Physiology 451: 220-227.
Shen B. W., Perraud A.L., Scharenberg A., Stoddard B. L. (2003). The Crystal Structure and Mutational Analysis of Human NUDT9. Journal of Molecular Biology 332: 385-398.
Steiner J. K., Tasaki J., Rouhana L. (2016). Germline Defects Caused by Smed-boule RNA-Interference Reveal That Egg Capsule Deposition Occurs Independently of Fertilization, Ovulation, Mating, or the Presence of Gametes in Planarian Flatworms. PLOS Genetics 12: e1006030.
Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673-4680.
Vila-Farré M., C. Rink J. (2018). The Ecology of Freshwater Planarians. In Planarian Regeneration. (Ed. Rink Jochen C.) Springer New York, New York, NY.
Vila-Farré M., Rozanski A., Ivanković M., Cleland J., Brand J. N., Thalen F., Grohme M. A., von Kannen S., Grosbusch A. L., Vu H. T.K., Prieto C. E., Carbayo F., Egger B., Bleidorn C., Rasko J. E. J., Rink J. C. (2023). Evolutionary dynamics of whole-body regeneration across planarian flatworms. Nature Ecology & Evolution 7: 2108-2124.
Vriens J., Owsianik G., Hofmann T., Philipp S. E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., Oberwinkler J., Vennekens R., Gudermann T., Nilius B., Voets T. (2011). TRPM3 Is a Nociceptor Channel Involved in the Detection of Noxious Heat. Neuron 70: 482-494.
Wagner T. F.J., Loch S., Lambert S., Straub I., Mannebach S., Mathar I., Düfer M., Lis A., Flockerzi V., Philipp S. E., Oberwinkler J. (2008). Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nature Cell Biology 10: 1421-1430.
Wang L., Fu T.M., Zhou Y., Xia S., Greka A., Wu H. (2018). Structures and gating mechanism of human TRPM2. Science 362: eaav4809.
Wang Y., Stary J. M., Wilhelm J. E., Newmark P. A. (2010). A functional genomic screen in planarians identifies novel regulators of germ cell development. Genes & Development 24: 2081-2092.
Wang Y., Zayas R. M., Guo T., Newmark P. A. (2007). nanos function is essential for development and regeneration of planarian germ cells. Proceedings of the National Academy of Sciences 104: 5901-5906.
Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F. T., de Beer T. A. P., Rempfer C., Bordoli L., Lepore R., Schwede T. (2018). SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Research 46: W296-W303.
Winkler P. A., Huang Y., Sun W., Du J., Lü W. (2017). Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552: 200-204.
Zayas R. M., Hernández A., Habermann B., Wang Y., Stary J. M., Newmark P. A. (2005). The planarian Schmidtea mediterranea as a model for epigenetic germ cell specification: Analysis of ESTs from the hermaphroditic strain. Proceedings of the National Academy of Sciences 102: 18491-18496.
Zhelay T., Wieczerzak K. B., Beesetty P., Alter G. M., Matsushita M., Kozak J. A. (2018). Depletion of plasma membrane–associated phosphoinositides mimics inhibition of TRPM7 channels by cytosolic Mg2+, spermine, and pH. Journal of Biological Chemistry 293: 18151-18167.
Zeng A., Li H., Guo L., Gao X., McKinney S., Wang Y., Yu Z., Park J., Semerad C., Ross E., Cheng L.C., Davies E., Lei K., Wang W., Perera A., Hall K., Peak A., Box A., Sánchez Alvarado A. (2018). Prospectively Isolated Tetraspanin+ Neoblasts Are Adult Pluripotent Stem Cells Underlying Planaria Regeneration. Cell 173: 1593-1608.e20.
Zheng J., (2013). Molecular mechanism of TRP channels. Comprehensive Physiology 3: 221-242.