Int. J. Dev. Biol. 69: 91 - 99 (2025)
Characterization of somatic testicular cells during human development: fetal, peripubertal, adolescent and adult human testis from healthy and infertility related disease
Open Access | Developmental Expression Pattern | Published: 13 June 2025
Abstract
The transcription factor GATA4 is found in Sertoli and Leydig cells, whereas SOX9 is exclusive to Sertoli cells, being both factors essential for the normal development of murine and human fetal testis. In turn, the steroidogenic acute regulatory protein (STAR) is specifically expressed in Leydig cells. Nevertheless, the function of STAR, GATA4 and SOX9 in peripubertal, adolescent and adult testes in Klinefelter syndrome and azoospermic patients remains poorly understood. To characterize the developmental expression of STAR, GATA4 and SOX9 in human testicular somatic cells, we performed immunofluorescence using fetal, peripubertal, adolescent and adult testes. Our findings demonstrate that STAR is absent in early fetal stages, but present in Leydig cells from 12 weeks of gestation, as well as in peripubertal, adolescent and adult Klinefelter patients, in the adult testis with idiopathic azoospermia and in men showing normal spermatogenesis. GATA4 was expressed in both Sertoli and Leydig cells during all the studied developmental stages and in peripubertal, adolescent and adult patients with and without spermatogenesis. SOX9 was mainly expressed in Sertoli cells in fetal, peripubertal, adolescent and adult Sertoli cell patients. In patients with Klinefelter syndrome as well as in men with or without spermatogenesis SOX9 was also found in Leydig cells. Our findings support the premise that STAR is a key steroidogenic protein for androgen development in the fetal testis, that GATA4 regulates Sertoli and Leydig cells during testis development and that SOX9 regulates the development of Sertoli cells and is present in the Leydig cells of patients with azoospermia.
Keywords
male germ cells, testicular somatic cells, Klinefelter syndrome, azoospermia
Introduction
Spermatogenesis is a highly complex process involving a large diversity of cell types, hormones, paracrine, genetic and epigenetic factors. It takes place in the seminiferous tubules and begins with the proliferation of spermatogonia, concluding with the release of mature spermatozoa into the lumen of the testicular tubule (Neto et al., 2016; Fabregues and Matorras, 2025). In human spermatogenesis, apart from the germinal cells, there are two cells which play an important role: Sertoli cells and Leydig cells (Fabregues and Matorras, 2025). Sertoli cells are a type of sustentacular cells which are a structural component of seminiferous tubules. They are stimulated by the FSH (follicle stimulating hormone), and their main function is to nourish developing sperm cells during spermatogenesis (Fabregues and Matorras, 2025). Leydig cells are the predominant cells in the intertubular space (Holstein et al., 2003). They are stimulated by the LH (luteinizing hormone) and their main function is androgen production (Fabregues and Matorras, 2025).
The steroidogenic acute regulatory protein (STAR)-related lipid transfer (START) domain is formed by 210 aminoacid-residues and binds lipids, including sterols (Ponting and Aravind, 1999; Manna et al., 2016). In mammals, the START domain is found in 15 different proteins, grouped into six different subfamilies in terms of sequence and ligand similarities. STAR (also known as STARD1) is a member of the first subfamily (STARD1/STARD3 subfamily) (Meier and Clark, 2012). This protein binds cholesterol and helps its transfer from the outer to the inner mitochondrial membrane. As the conversion of cholesterol to pregnenolone in the inner membrane of the mitochondria is the first enzymatic reaction of steroidogenesis, the delivery of cholesterol by STAR can be considered the first rate limiting step of steroidogenesis (Stocco and Clark, 1996; Tugaeva and Sluchanko, 2019; Jiang and Jorgensen, 2024).
STAR transcript can be detected in several urogenital human organs, such as adrenal gland, kidney, ovary and testis (Meier and Clark, 2012). In mice, STAR is detected in the cytoplasm of fetal Leydig cells of 13.5 days post coitum (dpc) and its expression increases during development. The absence of the expression of STAR at 11.5 dpc suggests that STAR androgen production begins in differentiated Leydig cells, in case of mice at 12.5-13.0 dpc (Warita et al., 2013). In humans, STAR is known to be expressed in adult Leydig cells, but also in Leydig cells of fetal testes ranging from 14.5-19 weeks of gestation (WG) (Pollack et al., 1997; Wang et al., 2022). However, there is no data about the expression of STAR in first trimester fetal testes and it is unknown if the same expression pattern happens in human and mice nor its expression in the absence of spermatogenesis.
GATA transcription factors are structurally-related zinc finger proteins that recognize a consensus DNA sequence, known as a GATA motif, which is an essential cis-acting element in the promoters and enhancers of a variety of genes (Orkin, 1992). Members of the GATA family, namely GATA4, GATA5 and GATA6, are expressed in gut epithelium, heart, yolk sac endoderm, gonads and a limited number of other tissues in mouse embryonic development (Arceci et al., 1993; Soudais et al., 1995; Morrisey et al., 1996, 1997; Narita et al., 1996; Fujiwara, 2017). GATA4 transcript and protein can be detected in the mouse gonadal ridges of both sexes as early as 11.5 dpc and in remains expressed in Sertoli and Leydig cells throughout development and adulthood (Viger et al., 1998; Ketola et al., 1999; Viger et al., 2022). Treatment of Leydig or Sertoli tumor cell lines with gonadotropins increases the steady state level of GATA4 transcripts (Heikinheimo et al., 1997; Ketola et al., 1999), suggesting hormonal regulation of this transcription factor. Gonadotropin or androgen action is not, however, a prerequisite for the basal expression of Gata4 in the testis, as the presence of GATA4 was demonstrated in Sertoli and Leydig cells in genetically hypogonadal mice and in rat Sertoli cells after chemical abolition of Leydig cells (Narita et al., 1996; Kyrönlahti et al., 2011; Schrade et al., 2015, 2016).
Sox9 is a Sry-box–containing gene that encodes a transcriptional activator. During mouse gonadogenesis, SOX9 is detected in the male gonad from 11.5-12.5 dpc (Kobayashi et al., 2005). From this stage onwards, SOX9 expression is restricted to the Sertoli cell lineage and persists in the adulthood (Sekido et al., 2004; Ming et al., 2022). In humans, SOX9 is present in chromosome 17. The mutation of SOX9 in humans causes a number of sex and skeletal developmental abnormalities known as Campomelic dysplasia (Foster et al., 1994). SOX9 is important during sex determination in mammals to induce (somatic) testis formation from a bipotential gonad (Bi et al., 2001; Vining et al., 2021). Furthermore, most patients with 46XY karyotype and heterozygous for SOX9 show variable male-to-female sex reversal (Bi et al., 2001; Croft et al., 2018). Mice with a Sox9 heterozygous mutation die at birth with a syndrome similar to that of human Campomelic dysplasia. In contrast to humans, XY Sox9 +/- mice form normal appearing testes (Bi et al., 2001). Mice containing a homozygous mutation in Sox9 die soon after 11.5 dpc because of cardiovascular defects (Bi et al., 2001). In vitro culture of mouse 11.5 dpc genital ridges of XY Sox9 -/- results in gonads lacking testicular cords and Sertoli cell marker expression, but with the expression of ovarian-specific markers (Chaboissier et al., 2004; Yao et al., 2004). Therefore, SOX9 is essential for diverting an intrinsically ovarian program of organogenesis towards testis formation. There are only few studies about the characterization of testicular somatic cells in infertility related diseases, such as Klinefelter syndrome and azoospermia (Van Saen et al., 2015; Savchuk et al., 2019; Tirumalasetty et al., 2024; Martin-Inaraja et al., 2025). In this study, we characterized the somatic cell developmental in Sertoli and Leydig evaluating the expression of testicular somatic cell proteins (STAR, GATA4 and SOX9). We analyzed in fetal, peripubertal, adolescent and adult testis with some specific conditions as Klinefelter syndrome and idiopathic secretory azoospermia as well as in patients with azoospermia but with normal spermatogenesis (vasectomy and obstructive azoospermia).
Results
Expression of STAR, GATA4 and SOX9 in fetal human male gonads.
Three different proteins were identified in important somatic cell types on the human testis development: SOX9 for Sertoli cells, GATA4 for Sertoli and Leydig cells and STAR for Leydig cells. In the case of STAR expression, clear differences were noticed at different stages of fetal development. At 9-10 WG, STAR was absent, however, some expression was observed at the end of the first trimester, at 12 WG (Fig. 1, Table 1). The expression of STAR increased during development, being very significant at 14 WG and afterwards (Fig. 2, Table 1). As expected, STAR expression only appeared between the seminiferous tubules, in the interstitial space, where Leydig cells are located.
Fig. 1. Characterization of somatic testicular cells in human fetal gonads from first trimester.
Immunofluorescence on histological sections of testis from 9 to 12 weeks of gestation (WG) for VASA (red), GATA4 (green), STAR (grey) and DAPI (blue) (A) and VASA (red), SOX9 (green), STAR (grey) and DAPI (blue) (B). Germ cells (white asterisk), Sertoli cells (red asterisk), Leydig cells (yellow asterisk). Scale bars are 100 μm.
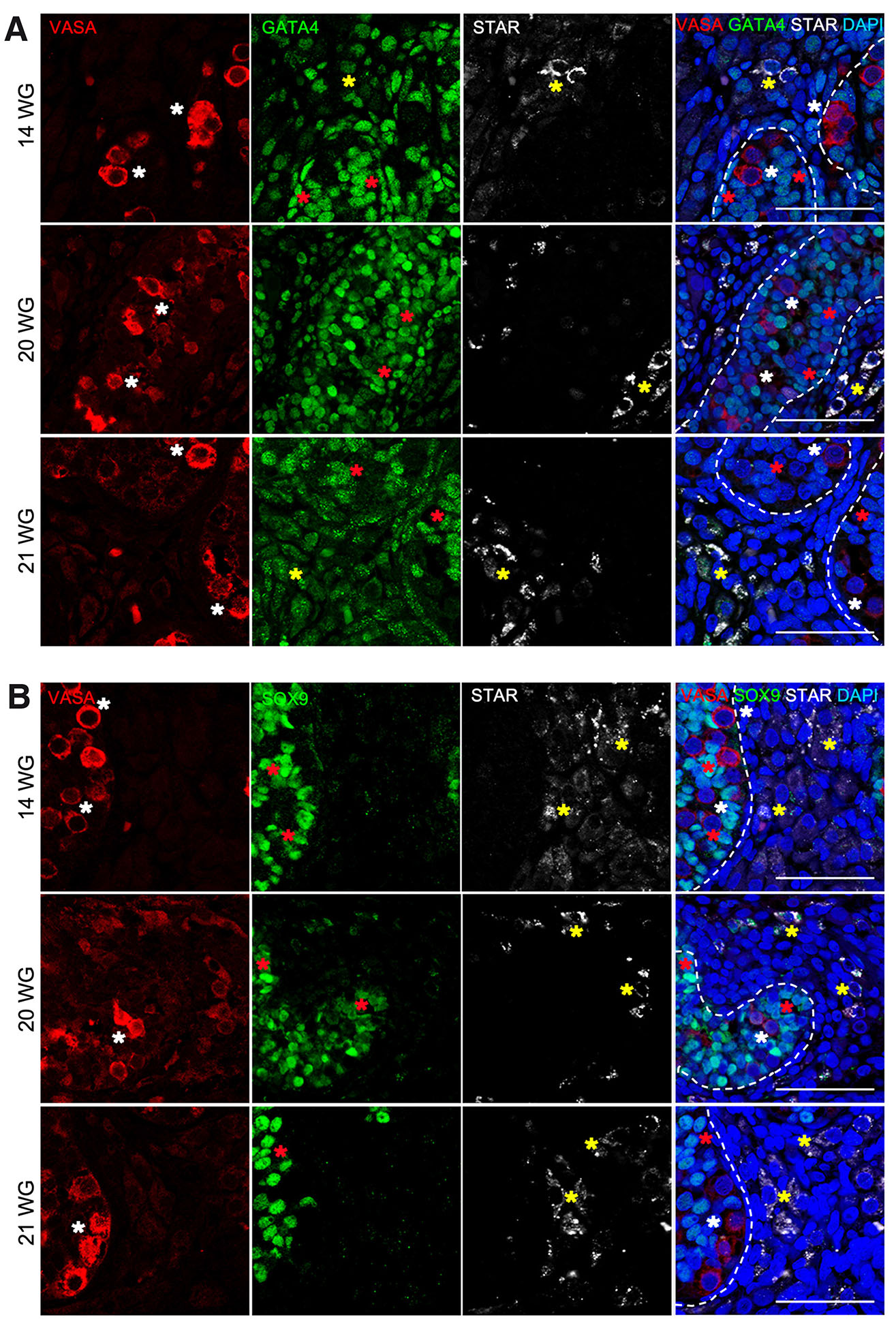
Fig. 2. Characterization of somatic testicular cells in human fetal gonads from second trimester.
Immunofluorescence on histological sections of testis from 14 to 21 weeks of gestation (WG) for VASA (red), GATA4 (green), STAR (grey) and DAPI (blue) (A) and VASA (red), SOX9 (green), STAR (grey) and DAPI (blue) (B). Germ cells (white asterisk), Sertoli cells (red asterisk), Leydig cells (yellow asterisk). Scale bars are 100 μm.
Table 1
Summary of somatic and germ cell markers expression during human testis development
| Fetus | Klinefelter syndrome | Normal spermatogenesis | Idiopathic SA | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <12 WG | 12WG | >12 WG | Peripubertal patients | Adolescent patients | Adult patients with vasectomy or OA | Adult patients | |||||||||||||||
| SC | LC | GC | SC | LC | GC | SC | LC | GC | SC | LC | GC | SC | LC | GC | SC | LC | GC | SC | LC | GC | |
| STAR (Leydig cells) |
n/a | - | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a |
| GATA4 (Sertoli and Leydig cells) |
+ | - | n/a | + | + | n/a | + | + | n/a | + | + | n/a | + | + | n/a | + | + | n/a | + | + | n/a |
| SOX9 (Sertoli cells) |
+ | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | n/a | n/a | + | - (except patient 4) | n/a | + | n/a | n/a |
| VASA (Germ cells) |
n/a | n/a | + (except 9WG) | n/a | n/a | + | n/a | n/a | + | n/a | n/a | - | n/a | n/a | - | n/a | n/a | + (except patient 4) | n/a | n/a | + |
SC, Sertoli cells; LC, Leydig cells; GC, Germ cells; OA, Obstructive Azoospermia; SA, Secretory Azoospermia; +, expression of marker; -, no expression; n/a, not applicable
During fetal development, GATA4 was clearly present in the Sertoli cells (9 WG to 21 WG), but also in interstitial cells where Leydig cells are located (12 WG to 21 WG). Its expression was nuclear, but with spots (Fig. 1, Fig. 2, Table 1). SOX9 expression was restricted to the Sertoli cells, with a defined nuclear expression from 9 WG to 21 WG (Fig. 1, Fig. 2, Table 1). The expression of GATA4 and SOX9 remained constant during different fetal stages from 9 WG to 21 WG (Fig. 1, Fig. 2, Table 1).
Expression of STAR, GATA4 and SOX9 in testes of Klinefelter syndrome patients
At the moment of testicular tissue sampling, the Klinefelter syndrome patients were 13, 15 and 19 years old. STAR expression was detected in all the samples, located in the interstitial space between tubules in a cytoplasmic spotty pattern. No differences were noticed between the three patients of different ages (Fig. 3, Table 1). GATA4 and SOX9 expression was evident in all the Klinefelter syndrome patients. Similar to fetal samples, GATA4 was expressed in Sertoli cells inside the seminiferous tubules, but also in Leydig cells between the tubules (Fig. 3, Table 1). Its staining was nuclear but less spotty in the Klinefelter syndrome patients than in the fetal testis. In addition, SOX9 showed a pronounced expression inside the tubules with a nuclear staining (Fig. 3, Table 1), similar to that observed in fetal testis and also outside of tubules in one patient (Fig. 3, #15 years patient). We noticed that germ cells (VASA-positive) were not present in the testicular tissue sampling analyzed (Fig. 3, Table 1).
Fig. 3. Characterization of somatic testicular cells in human prepubertal and pubertal testis of Klinefelter syndrome patients.
Immunofluorescence on histological sections of testis from prepubertal and pubertal Klinefelter syndrome patients of 13,15 and 19 years-old for VASA (red), GATA4 (green), STAR (grey) and DAPI (blue) (A) and VASA (red), SOX9 (green), STAR (grey) and DAPI (blue) (B). Germ cells (white asterisk), Sertoli cells (red asterisk), Leydig cells (yellow asterisk). Scale bars are 100 μm.
Expression of STAR, GATA4 and SOX9 in the testis of adult patients with azoospermia
Two groups of patients were constituted regarding the presence of spermatogenesis at the moment of testicular biopsy for testicular sperm extraction (TESE). The first group was composed of adult patients diagnosed with azoospermia in whom there was evidence of spermatogenesis (corresponding to 2 cases of post-vasectomy azoospermia and 1 case of obstructive azoospermia) (Fig. 4, Table 1). The second group was composed of adult patients diagnosed with azoospermia, but showing absence of spermatozoa after sample analysis (all of them corresponding to idiopathic secretory azoospermia) (Fig. 5, Table 1).
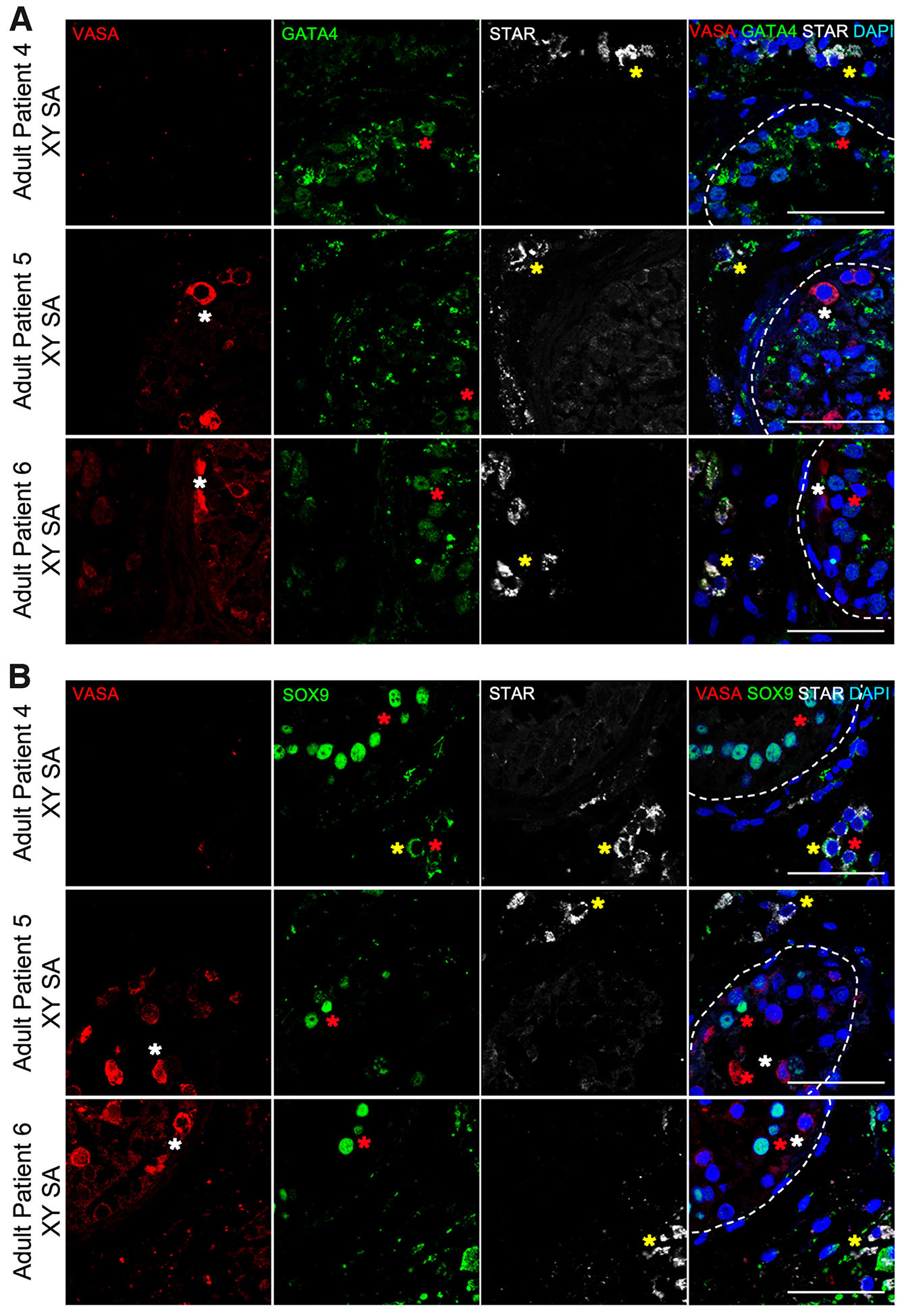
Fig. 4. Characterization of somatic testicular cells in human adult testis of azoospermic patients positive for spermatozoa.
Immunofluorescence on histological sections of testis from adult azoospermic patients for VASA (red), GATA4 (green), STAR (grey) and DAPI (blue) (A) and VASA (red), SOX9 (green), STAR (grey) and DAPI (blue) (B). Germ cells (white asterisk), Sertoli cells (red asterisk), Leydig cells (yellow asterisk). Scale bars are 100 μm.
Fig. 5. Characterization of somatic testicular cells in human adult testis of azoospermic patients negative for spermatozoa.
Immunofluorescence on histological sections of testis from adult azoospermic patients for VASA (red), GATA4 (green), STAR (grey) and DAPI (blue) (A) and VASA (red), SOX9 (green), STAR (grey) and DAPI (blue) (B). Germ cells (white asterisk), Sertoli cells (red asterisk), Leydig cells (yellow asterisk). Scale bars are 100 μm.
The expression of STAR was detected in all patients and it was similar between both groups of adult patients and comparable to that of Klinefelter syndrome patients. STAR was expressed in the interstitial Leydig cells and in a cytoplasmic spotty pattern (Fig. 4, Fig. 5, Table 1).
GATA4 expression was observed in Sertoli cells inside seminiferous tubules, but also in the interstitial space in Leydig cells. There was no difference between adult patients groups, the positive and negative for spermatozoa (Fig. 4, Fig. 5, Table 1).
Finally, SOX9 expression in all adult samples followed the same pattern as in Klinefelter syndrome patients and fetal testis, giving a clear nuclear staining only in the somatic cells inside the seminiferous tubules (Fig. 4, Fig. 5, Table 1). Nevertheless, in adult negative samples for spermatozoa (patient #4), some cells in the interstitial space had a clearly cytoplasmic staining for SOX9 not seen before (Fig. 5, Table 1).
Expression of VASA during testis development and adulthood in patients with infertility
In male testis from 5-10 WG, most germ cells are OCT4 (or POU5F1) positive and expressed no or low levels of VASA (Fig. 1, Table 1), whereas VASA is expressed in fetal germ cells from 10 to 21WG (Fig. 1, Fig. 2, Table 1). In Klinefelter syndrome testis, we did not find any VASA positive germ cells (Fig. 3, Table 1). In adult testis, we found VASA positive germ cells in almost all samples (Fig. 4, Fig. 5, Table 1), except in one azoospermic patient (patient #4) without spermatogenesis (Fig. 5, Table 1).
Discussion
This study provides the expression pattern of steroidogenic protein STAR and the transcription factors GATA4 and SOX9 in human testis during fetal development, peripubertal, adolescent Klinefelter syndrome patients and two groups of adult azoospermic patients (with and without spermatogenesis) (Table 1). The two main types of azoospermia are: secretory azoospermia, where spermatogenesis is compromised, and obstructive azoospermia, where spermatogenesis is normal but the spermatozoa cannot go outside, such in cases after vasectomy. Both have been addressed in our study.
STAR appears to play an essential role in the acute response of steroidogenic cells facilitating the intramitochondrial translocation of sterol to the cholesterol side-chain cleavage enzyme. STAR transcript is abundant in second trimester testis, where it has been observed in Leydig cells (Pollack et al., 1997; Van Saen et al., 2015; Savchuk et al., 2019; Jiang and Jorgensen, 2024). In agreement with both studies, we have observed STAR from 12-21 WG in fetal Leydig cells as a result of fetal testicular androgen formation. In addition, we report the increase of STAR protein from fetal stages up to adulthood supporting the physiological function and normal development of Leydig cells. After the fetal testosterone peak at 12 WG, Leydig cells slowly regress to mature again after birth to produce testosterone during puberty up to adulthood (Prince, 2001). In consistency with our data in adult testis, Leydig cell hyperplasia can occur in Klinefelter Syndrome patients and azoospermic patients consistent with increased STAR expression and over-expression of genes involved in steroidogenesis (Wikström et al., 2004; D’Aurora et al., 2015, 2017; Hauptman et al., 2021).
In agreement with data described previously (Ketola et al., 2000; Guo et al., 2021), we report that GATA4 protein is present in Sertoli and Leydig cells and absent from the germ cells during fetal development and adulthood. This is also in agreement with several animal studies which detected GATA4 expression only in somatic cells, mainly in Sertoli cells, during testis development (Viger et al., 1998; Ketola et al., 1999; Kyrönlahti et al., 2011; Viger et al., 2022). Given that Sertoli cells proliferate only during the fetal and prepubertal periods, the intense GATA4 expression in fetal Sertoli cells may suggest a role in the proliferation of these testicular supportive cells. In fetal Sertoli cells, the strongest GATA4 expression level coincides with high serum follicle-stimulating hormone (FSH) levels at the beginning of the second trimester (Kaplan et al., 1976), suggesting that in vivo, GATA4 may be under gonadotropic control during the fetal period. In general, Leydig cells showed consistently lower GATA4 expression when compared to Sertoli cells. A connection between GATA4 and steroidogenesis is supported by the fact that in Leydig cells GATA4 expression is most intense in the second trimester and during puberty, when testosterone production is at its peak (Tapanainen et al., 1981). Furthermore, GATA4 was low in Sertoli and Leydig cells in patients with azoospermia, suggesting that androgen action could influence GATA4 expression. In Klinefelter syndrome testes, in general, there are few somatic cells (Martin-Inaraja et al., 2025) and we found no difference on the GATA4 expression pattern in Sertoli or Leydig cells in comparison with fetal and adult testis.
In light of our findings, we propose that a normal testicular response to gonadotropins as well as androgen action are needed for normal GATA4 expression in the human testis, particularly in Leydig cells. However, gonadotropin and/or androgen actions are not prerequisites for the basal expression of GATA4 in Sertoli and Leydig cells. A gonadotropin stimulus may be more important for GATA4 expression during fetal development than postnatally, given that Klinefelter Syndrome and adult azoospermic patients (idiopathic secretory azoospermia, vasectomy and obstructive azoospermia) had no effect on GATA4 expression. However, it is likely that not only gonadotropins and androgens but also other factors may be involved in the regulation of GATA4 in Sertoli and Leydig cells. Recently, GATA4 expression has been shown to be intense in testicular somatic cell tumors, such as Sertoli and Leydig cell tumors (Siltanen et al., 1999; Laitinen et al., 2000). Hence, it was proposed that GATA4 may influence cell proliferation during tumorigenesis in human somatic cell-derived gonadal tumors (Morrisey et al., 1996; Narita et al., 1996).
During testis development, SOX9 expression in Sertoli cells was maintained. SOX9 is a Sertoli-cell marker crucial during male sexual development (Hemendinger et al., 2002; Guo et al., 2021). Its function is well studied during sexual differentiation (De Santa Barbara et al., 1998; Svingen and Koopman, 2013; Lindeman et al., 2021), however its continuous expression in the testis during development suggests an additional role in spermatogenesis. Our results showed that SOX9 protein is present specifically in Sertoli cells during first and second trimester in agreement with previous studies in mouse and human (Kobayashi et al., 2005; Van Saen et al., 2015). Van Saen and colleagues (2015) have claimed SOX9 expression in fetal interstitial cells in human testis (Van Saen et al., 2015), however we were unable to confirm that claim in fetal testis. Nevertheless, analysis of SOX9 expression after birth revealed, in addition to expression in Sertoli cells, that some patients (1 in 3 Klinefelter Syndrome patients and 1 in 3 azoospermic patients) expressed SOX9 protein in interstitial Leydig cells. This may suggest a role for SOX9 in Leydig cells in infertility related diseases, such as Klinefelter syndrome and azoospermia (Daigle et al., 2015). In general, Sertoli cells showed strong SOX9 expression suggesting a link between SOX9 protein and androgen production. In agreement, an up-regulation of SOX9 in Sertoli cells has been observed in Sertoli cell-only syndrome (SCOS) azoospermic patients (Lan et al., 2013).
Finally, VASA (or DDX4) is a cytoplasmic protein expressed in post-migratory germ cell precursors from 10WG until adulthood with normal spermatogenesis (Altman et al., 2014; Eguizabal et al., 2016). As we expected we found germ cells with expression for VASA during human testis development with the exception of Klinefelter syndrome patients and patient without spermatogenesis.
Conclusion
This study supports the premise that STAR is a key steroidogenic protein for androgen development in the fetal testis. In addition, GATA4 regulates Sertoli and Leydig cells during testis development and SOX9 regulates the development of Sertoli cells and is present in the Leydig cells of patients with azoospermia. In conclusion, the expression of these three key genes, STAR, GATA4 and SOX9 was detected during the development from fetal to adult human testicular somatic cells and its expression was found to be increased in Klinefelter syndrome and in azoospermic patients. These data could point out to potential causes of germ cell loss and pathological features such as fibrosis and hyalinization in patients with infertility related diseases.
Material and Methods
Ethical permits
Human peripubertal and adolescent samples from patients diagnosed with Klinefelter syndrome and human adult samples diagnosed with azoospermia were obtained from Cruces University Hospital (Spain), according to an investigation project approved by the Basque Committee of Ethics and Clinical Research (PI2014205). The collection and use of human fetal material from elective abortions (without medical indication) was approved by the Medical Ethical Committee of the Leiden University Medical Center (P08.087). Oral and written information was given also to these patients and all of them signed an informed consent.
Human fetal, peripubertal, adolescent and adult sample collection
Calculation of the gestational age (in weeks and days) was based on measurements of crown-rump length obtained by ultrasonography. In total six human fetal testis from 9, 10, 12, 14, 20 and 21 WG were included in this study and were isolated in 0.9% NaCl solution (232225, Fresenius Kabi).
The peripubertal and adolescent population consisted in three patients aged 13, 15 and 19 years old, all of them with Klinefelter syndrome. All of them were under some treatment with testosterone and were subjected to a testicular biopsy in search of testicular spermatozoa for a future Assisted Reproduction Technique (ART) as well as of spermatogonia. During this testicular biopsy, an aliquot was taken for the purpose of the present study. In none of them spermatozoa or spermatogonia were recovered.
Regarding the adult population, all of them had peripheral levels of gonadotropins and testosterone within the normal ranges, a normal karyotype and a normal testicular size. Their ages were from 32 to 48 years old. Two of them had a previous vasectomy because of previous children and four had an idiopathic azoospermia. All of them were subjected to a TESE with the aim of obtaining testicular sperm to be used in ART. None of them was under hormonal treatment. Testicular biopsies revealed spermatozoa in three of them (1/4 azoospermia; 2/2 vasectomies).
Immunofluorescence staining and imaging
The testes were fixed in 4 % paraformaldehyde for 2 h, dehydrated with 70%, 96% and 3 cycles of 100% of ethanol for 1h each, cleared in 2 cycles of xylene for 1h each and embedded in paraffin wax in 3 cycles of 1h and 20min each. Serial section (5 µm) were de-paraffined, rehydrated and incubate in an antigen-retrieval solution (0.01M citric buffer, pH 6). The immunofluorescence staining was performed using the following antibodies and their dilutions: STAR (sc-166821, SantaCruz, 1:50), GATA4 (sc-9053, SantaCruz, 1:250 and ab84593, Abcam, 1:250), SOX9 (AB5535, Merck Millipore, 1:500), VASA (AF2030, R&D, 1:2000). Alexa Flour 488, Alexa Flour 555 and Alexa Flour 647 from Invitrogen were used as secondary antibodies (all in 1:500). Slides were counterstained with DAPI (dilution 1:5000; Life Technologies) and mounted in ProLong Gold Antifade Mountant (Life Technologies). Images were taken using a FluoView FV500 confocal microscope (Olympus).
Acknowledgements
We would like to thank the Basque Biobank for Research (Biobanco Vasco) for the human peripubertal and adolescent samples of patients diagnosed with Klinefelter syndrome and the adult samples from humans diagnosed with azoospermia; the Centre for Contraception, Abortion and Sexuality (CASA) in Leiden and Den Haag for all the efforts to collect and provide the human fetal material. We also thank A. Orrantia for technical assistance. Finally, we apologize to our colleagues whose work was not cited due to space limitations.
Abbreviations
ART, Assisted Reproduction Techniques ; dpc, days post coitum ; FSH, follicle-stimulating hormone ; OA, obstructive azoospermia ; SA, secretory azoospermia ; SCOS, Sertoli cell-only syndrome ; STAR, steroidogenic acute regulatory protein ; TESE, testicular sperm extraction ; WG, weeks of gestation ;Declarations
Author contributions
MM and LH: collection and/or assembly of data, data analysis and interpretation, and manuscript writing and final approval of manuscript. CR and SS: final approval of manuscript and financial support. RM, AE, M and BP: sample collection, clinical characterization and final approval of manuscript. SMCSL and CE: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Funding
This work was supported by Fundación MERCK Salud (Grant FSALUD17/004), Fundación Salud de la Mujer-DEXEUS (Grant FDEXEUS15/001), Health Department of the Basque Government (Grant 2019111068, 2019/4703, 2020111058, 2020333032, 2021333012, 2021333057, 2022333044, 2022111067, 2023333035, 2023333036, 2024333042), Economic Development and Infrastructures Department of the Basque Government (KK-2020/00068), Fundación Inocente Inocente (FII22/001 and FII18/003), EITB Maratoia (BIO24/CA/026), Project “PI18/01299” and “PI21/01187”, funded by Instituto de Salud Carlos III and co-funded by European Union (ERDF) “A way to make Europe”, “ICI21/00095” funded by Instituto de Salud Carlos III and co-funded by European Union (NextGenerationEU), RICORS: (RD21/0017/0024 and RD24/0014/0025) Red Española de Terapias Avanzadas TERAV ISCIII funded by “Instituto de Salud Carlos III (ISCIII)” and co-funded by European Union (NextGenerationEU) “Plan de Recuperación Transformación y Resiliencia”, COST Action CA20119 (ANDRONET) supported by COST (European Cooperation in Science and Technology) (www.cost.eu) and ORCHID-NET (https://www.orchid-net.com/). MM was supported by the Jesus Gangoiti Barrera Foundation and the Health Department of the Basque Country Government. LH was supported by the Jesus Gangoiti Barrera Foundation and the Asociación Española contra el Cáncer (AECC). SMCSL was funded by the European Research Council Consolidator Grant OVOGROWTH (ERC-CoG-2016-725722) and by the Dutch Research Council (VICI-2018-345 91819642).
References
Altman E., Yango P., Moustafa R., Smith J. F., Klatsky P. C., Tran N. D. (2014). Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. REPRODUCTION 148: 417-427.
Arceci R. J., King A. A. J., Simon M. C., Orkin S. H., Wilson D. B. (1993). Mouse GATA-4: a Retinoic Acid-Inducible GATA-Binding Transcription Factor Expressed in Endodermally Derived Tissues and Heart. Molecular and Cellular Biology 13: 2235-2246.
Bi W., Huang W., Whitworth D. J., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proceedings of the National Academy of Sciences 98: 6698-6703.
Chaboissier M.C., Kobayashi A., Vidal V. I. P., Lützkendorf S., van de Kant H. J. G., Wegner M., de Rooij D. G., Behringer R. R., Schedl A. (2004). Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131: 1891-1901.
Croft B., Ohnesorg T., Hewitt J., Bowles J., Quinn A., Tan J., Corbin V., Pelosi E., van den Bergen J., Sreenivasan R., Knarston I., Robevska G., Vu D. C., Hutson J., Harley V., Ayers K., Koopman P., Sinclair A. (2018). Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nature Communications 9: 5319.
D’Aurora M., Ferlin A., Di Nicola M., Garolla A., De Toni L., Franchi S., Palka G., Foresta C., Stuppia L., Gatta V. (2015). Deregulation of sertoli and leydig cells function in patients with klinefelter syndrome as evidenced by testis transcriptome analysis. BMC Genomics 16: 156.
D’Aurora M., Ferlin A., Garolla A., Franchi S., D’Onofrio L., Trubiani O., Palka G., Foresta C., Stuppia L., Gatta V. (2017). Testis Transcriptome Modulation in Klinefelter Patients with Hypospermatogenesis. Scientific Reports 7: 45729.
Daigle M., Roumaud P., Martin L. J. (2015). Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Molecular and Cellular Biochemistry 407: 209-221.
De Santa Barbara P., Bonneaud N., Boizet B., Desclozeaux M., Moniot B., Sudbeck P., Scherer G., Poulat F., Berta P. (1998). Direct Interaction of SRY-Related Protein SOX9 and Steroidogenic Factor 1 Regulates Transcription of the Human Anti-Müllerian Hormone Gene. Molecular and Cellular Biology 18: 6653-6665.
Eguizabal C., Herrera L., De Oñate L., Montserrat N., Hajkova P., Izpisua Belmonte J. C. (2016). Characterization of the Epigenetic Changes During Human Gonadal Primordial Germ Cells Reprogramming. Stem Cells 34: 2418-2428.
Fabregues F., Matorras R., (2025). The role of endogenous LH in the male [Papel de la LH endógena en el varón]. In LH function, structure and evidence [LH, funcionamiento, estructura y evidencia]. Meysis, Madrid.
Foster J. W., Dominguez-Steglich M. A., Guioli S., Kwok C., Weller P. A., Stevanović M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N., Brook J. D., Schafer A. J. (1994). Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372: 525-530.
Fujiwara T. (2017). GATA Transcription Factors: Basic Principles and Related Human Disorders. The Tohoku Journal of Experimental Medicine 242: 83-91.
Guo J., Sosa E., Chitiashvili T., Nie X., Rojas E. J., Oliver E., DonorConnect Plath K., Hotaling J. M., Stukenborg J. B., Clark A. T., Cairns B. R., (2021). Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell 28: 764-778.e4.
Hauptman D., Perić M. H., Marić T., Bojanac A. K., Sinčić N., Zimak Z., Kaštelan Ž, Ježek D. (2021). Leydig Cells in Patients with Non-Obstructive Azoospermia: Do They Really Proliferate?. Life 11: 1266.
Heikinheimo M., Ermolaeva M., Bielinska M., Rahman N. A., Narita N., Huhtaniemi I. T., Tapanainen J. S., Wilson D. B. (1997). Expression and Hormonal Regulation of Transcription Factors GATA-4 and GATA-6 in the Mouse Ovary 1. Endocrinology 138: 3505-3514.
Hemendinger R. A., Gores P., Blacksten L., Harley V., Halberstadt C. (2002). Identification of a Specific Sertoli Cell Marker, Sox9, for Use in Transplantation. Cell Transplantation 11: 499-505.
Holstein A.F., Schulze W., Davidoff M. (2003). Understanding spermatogenesis is a prerequisite for treatment. Reproductive Biology and Endocrinology 1: 107.
Jiang K., Jorgensen J. S. (2024). Fetal Leydig cells: What we know and what we don't. Molecular Reproduction and Development 91: e23739.
Kaplan S. L., Grumbach M. M., Aubert M. L., (1976). The ontogenesis of pituitary hormones and hypothalamic factors in the human fetus: maturation of central nervous system regulation of anterior pituitary function. Recent Progress in Hormone Research 32: 161-243.
Ketola I., Rahman N., Toppari J., Bielinska M., Porter-Tinge S. B., Tapanainen J. S., Huhtaniemi I. T., Wilson D. B., Heikinheimo M. (1999). Expression and Regulation of Transcription Factors GATA-4 and GATA-6 in Developing Mouse Testis 1. Endocrinology 140: 1470-1480.
Ketola I., Pentikäinen V., Vaskivuo T., Ilvesmäki V., Herva R., Dunkel L., Tapanainen J. S., Toppari J., Heikinheimo M. (2000). Expression of Transcription Factor GATA-4 during Human Testicular Development and Disease 1. The Journal of Clinical Endocrinology & Metabolism 85: 3925-3931.
Kobayashi A., Chang H., Chaboissier M. C., Schedl A., Behringer R. R. (2005). Sox9 in Testis Determination. Annals of the New York Academy of Sciences 1061: 9-17.
Kyrönlahti A., Euler R., Bielinska M., Schoeller E. L., Moley K. H., Toppari J., Heikinheimo M., Wilson D. B. (2011). GATA4 regulates Sertoli cell function and fertility in adult male mice. Molecular and Cellular Endocrinology 333: 85-95.
Laitinen M. P. E., Anttonen M., Ketola I., Wilson D. B., Ritvos O., Butzow R., Heikinheimo M. (2000). Transcription Factors GATA-4 and GATA-6 and a GATA Family Cofactor, FOG-2, Are Expressed in Human Ovary and Sex Cord-Derived Ovarian Tumors*. The Journal of Clinical Endocrinology & Metabolism 85: 3476-3483.
Lan K.C., Chen Y.T., Chang C., Chang Y.C., Lin H.J., Huang K.E., Kang H.Y. (2013). Up-Regulation of SOX9 in Sertoli Cells from Testiculopathic Patients Accounts for Increasing Anti-Mullerian Hormone Expression via Impaired Androgen Receptor Signaling. PLoS ONE 8: e76303.
Lindeman R. E., Murphy M. W., Agrimson K. S., Gewiss R. L., Bardwell V. J., Gearhart M. D., Zarkower D. (2021). The conserved sex regulator DMRT1 recruits SOX9 in sexual cell fate reprogramming. Nucleic Acids Research 49: 6144-6164.
Manna P. R., Stetson C. L., Slominski A. T., Pruitt K. (2016). Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 51: 7-21.
Martin-Inaraja M., Romayor I., de Cámara A. S., Herrera L., Palacios A., Villaverde M., Santos S., Vesga M. , Barreña B., Aransay A. M., Anguita J., Solorzano E., Prieto M. B., Matorras R., Eguizabal C. (2025). Generation, establishment and characterization of three pluripotent stem cell lines (CVTTHi002-A, CVTTHi003-A and CVTTHi004-A) from primary testicular somatic cells isolated from two prepuberal and one peripuberal Klinefelter Syndrome (47 XXY) patients. Stem Cell Research 83: 103657.
Meier R. K., Clark B. J. (2012). Angiotensin II-Dependent Transcriptional Activation of Human Steroidogenic Acute Regulatory Protein Gene by a 25-kDa cAMP-Responsive Element Modulator Protein Isoform and Yin Yang 1. Endocrinology 153: 1256-1268.
Ming Z., Vining B., Bagheri-Fam S., Harley V. (2022). SOX9 in organogenesis: shared and unique transcriptional functions. Cellular and Molecular Life Sciences 79: 522.
Morrisey E. E., Ip H. S., Lu M. M., Parmacek M. S. (1996). GATA-6: A Zinc Finger Transcription Factor That Is Expressed in Multiple Cell Lineages Derived from Lateral Mesoderm. Developmental Biology 177: 309-322.
Morrisey E. E., Ip H. S., Tang Z., Lu M. M., Parmacek M. S. (1997). GATA-5: A Transcriptional Activator Expressed in a Novel Temporally and Spatially-Restricted Pattern during Embryonic Development. Developmental Biology 183: 21-36.
Narita N., Heikinheimo M., Bielinska M., White R. A., Wilson D. B. (1996). The Gene for Transcription Factor GATA-6 Resides on Mouse Chromosome 18 and Is Expressed in Myocardium and Vascular Smooth Muscle. Genomics 36: 345-348.
Neto F. T. L., Bach P. V., Najari B. B., Li P. S., Goldstein M. (2016). Spermatogenesis in humans and its affecting factors. Seminars in Cell & Developmental Biology 59: 10-26.
Orkin S.H. (1992). GATA-binding transcription factors in hematopoietic cells. Blood 80: 575-581.
Pollack S. E., Furth E. E., Kallen C. B., Arakane F., Kiriakidou M., Kozarsky K. F., Strauss J. F. (1997). Localization of the Steroidogenic Acute Regulatory Protein in Human Tissues 1. The Journal of Clinical Endocrinology & Metabolism 82: 4243-4251.
Ponting C. P., Aravind L. (1999). START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends in Biochemical Sciences 24: 130-132.
Prince F.P. (2001). The triphasic nature of Leydig cell development in humans, and comments on nomenclature. Journal of Endocrinology 168: 213-216.
Savchuk I., Morvan M.L., Antignac J.P., Kurek M., Le Bizec B., Söder O., Svechnikov K. (2019). Ontogenesis of human fetal testicular steroidogenesis at early gestational age. Steroids 141: 96-103.
Schrade A., Kyrönlahti A., Akinrinade O., Pihlajoki M., Häkkinen M., Fischer S., Alastalo T.P., Velagapudi V., Toppari J., Wilson D. B., Heikinheimo M. (2015). GATA4 Is a Key Regulator of Steroidogenesis and Glycolysis in Mouse Leydig Cells. Endocrinology 156: 1860-1872.
Schrade A., Kyrönlahti A., Akinrinade O., Pihlajoki M., Fischer S., Rodriguez V. M., Otte K., Velagapudi V., Toppari J., Wilson D. B., Heikinheimo M. (2016). GATA4 Regulates Blood-Testis Barrier Function and Lactate Metabolism in Mouse Sertoli Cells. Endocrinology 157: 2416-2431.
Sekido R., Bar I., Narváez V., Penny G., Lovell-Badge R. (2004). SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Developmental Biology 274: 271-279.
Siltanen S., Anttonen M., Heikkilä P., Narita N., Laitinen M., Ritvos O., Wilson D. B., Heikinheimo M. (1999). Transcription Factor GATA-4 Is Expressed in Pediatric Yolk Sac Tumors. The American Journal of Pathology 155: 1823-1829.
Soudais C., Bielinska M., Heikinheimo M., MacArthur C. A., Narita N., Saffitz J. E., Simon M. C., Leiden J. M., Wilson D. B. (1995). Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development 121: 3877-3888.
Stocco D. M., Clark B. J. (1996). Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochemical Pharmacology 51: 197-205.
Svingen T., Koopman P. (2013). Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes & Development 27: 2409-2426.
Tapanainen J., Kellokumpu-Lehtinen P., Pelliniemi L., Huhtaniemi I. (1981). Age-Related Changes in Endogenous Steroids of Human Fetal Testis during Early and Midpregnancy*. The Journal of Clinical Endocrinology & Metabolism 52: 98-102.
Tirumalasetty M. B., Bhattacharya I., Mohiuddin M. S., Baki V. B., Choubey M. (2024). Understanding testicular single cell transcriptional atlas: from developmental complications to male infertility. Frontiers in Endocrinology 15: 1394812.
Tugaeva K. V., Sluchanko N. N. (2019). Steroidogenic Acute Regulatory Protein: Structure, Functioning, and Regulation. Biochemistry (Moscow) 84: 233-253.
Van Saen D., Pino Sánchez J., Ferster A., van der Werff ten Bosch J., Tournaye H., Goossens E. (2015). Is the protein expression window during testicular development affected in patients at risk for stem cell loss?. Human Reproduction 30: 2859-2870.
Viger R. S., Mertineit C., Trasler J. M., Nemer M. (1998). Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125: 2665-2675.
Viger R. S., de Mattos K., Tremblay J. J. (2022). Insights Into the Roles of GATA Factors in Mammalian Testis Development and the Control of Fetal Testis Gene Expression. Frontiers in Endocrinology 13: 902198.
Vining B., Ming Z., Bagheri-Fam S., Harley V. (2021). Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination. Genes 12: 486.
Wang D., Hildorf S., Ntemou E., Mamsen L. S., Dong L., Pors S. E., Fedder J., Clasen-Linde E., Cortes D., Thorup J., Andersen C. Y. (2022). Organotypic Culture of Testicular Tissue from Infant Boys with Cryptorchidism. International Journal of Molecular Sciences 23: 7975.
Warita K., Mitsuhashi T., Fukui S., Ohta K., Suzuki S., Miki T., Takeuchi Y., Yokoyama T., Kitagawa H., Sugawara T., Hoshi N. (2013). Immunohistochemical analysis of steroidogenic acute regulatory protein (StAR) and StAR-binding protein (SBP) expressions in the testes of mice during fetal development. Reproductive Biology 13: 92-95.
Wikström A. M., Raivio T., Hadziselimovic F., Wikström S., Tuuri T., Dunkel L. (2004). Klinefelter Syndrome in Adolescence: Onset of Puberty Is Associated with Accelerated Germ Cell Depletion. The Journal of Clinical Endocrinology & Metabolism 89: 2263-2270.
Yao H. H.C., Matzuk M. M., Jorgez C. J., Menke D. B., Page D. C., Swain A., Capel B. (2004). Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Developmental Dynamics 230: 210-215.