Int. J. Dev. Biol. 68: 55 - 64 (2024)
TGF-β signaling molecules in Hydra: role of BMP and BMP inhibitors during pattern formation
Open Access | Review | Published: 21 May 2024
Abstract
Understanding the evolution of body plans has been one of the major areas of investigation in developmental and evolutionary biology. Cnidaria, the sister group to bilaterians, provides an opportunity to elucidate the origin and evolution of body axes. Hydra, a freshwater cnidarian, is a useful model to study signaling pathways governing pattern formation, which are conserved up to vertebrates including humans. The transforming growth factor β (TGF-β) signaling pathway is one of the fundamental pathways that regulate axis formation and organogenesis during embryonic development. In this article, we discuss the TGF-β pathway members identified in Hydra along with other cnidarians with an emphasis on bone morphogenetic proteins (BMPs) and their inhibitors. TGF-β members, especially those involved in BMP signaling pathway, are mainly involved in maintaining the Organizer region and patterning the body axis in Hydra. Identification of other members of this pathway in Hydra and fellow cnidarians would provide insights into the evolution of body axes and pattern formation in more complex metazoans.
Keywords
Hydra, evolution, pattern formation, BMP, BMP inhibitors, TGF-β
Introduction
Seven conserved pathways, Wnt, transforming growth factor-β (TGF-β), Receptor Tyrosine Kinases, Hedgehog, Janus kinase/signal transducer and activator of transcription (JAK/STAT), Notch and nuclear receptor pathways drive cell signaling during the embryonic development and are used repeatedly during later developmental processes in metazoans (Gerhart, 1999 and Barolo and Posakony, 2002). Many of these signaling pathways act by ligand binding to their respective receptors, activating transcription factors leading to regulation of gene expression (Trompouki et al., 2011). Alterations in spatio-temporal expression patterns of transcription factors and signaling components not only increase the extent of cellular response but also affect the complexity of cross talk between different pathways (Itasaki and Hoppler, 2010). Interestingly, several components of these seven signaling pathways have been identified in basal phyla such as Porifera (Liongue and Ward, 2013; Borisenko et al., 2016, 2019) and Cnidaria (Hobmayer et al., 2000; Miller et al., 2000; Reinhardt et al., 2004; Technau et al., 2005; Käsbauer et al., 2007; Putnam et al., 2007) and are seen to be conserved up to humans. This clearly shows that many of these signaling pathways had already evolved to a greater or lesser extent in the common ancestors of the bilaterians.
The TGF-β signaling pathway is one of the fundamental signal transduction machineries crucial for early embryogenesis, axis formation, organogenesis, and development. This pathway plays an important role in providing positional information during embryogenesis in both invertebrates and vertebrates (Hobmayer et al., 2001). The pathway is known to regulate diverse cellular processes including cell proliferation, differentiation, migration, apoptosis, and adult tissue homeostasis in vertebrates. Deregulation of the pathway leads to several pathological conditions including cancer (Massagué et al., 2000). The entire TGF-β superfamily can be divided into two groups: (1) bone morphogenetic proteins (BMPs), the most prominent TGF-β ligands, which induce signaling by the canonical small mothers against decapentaplegic (Smad)-dependent pathway mediated by two types of serine/threonine kinase receptors, BMP receptor (BMPR) -I and -II, through activation of Smad 1, 5 and 8 (Chen and Shen, 2004) or by the Smad-independent pathway, which involves TGF-β Activated Tyrosine Kinase 1 (TAK1) and Mitogen Activated Protein Kinase (MAPK) (Zhang and Li, 2005) and (2) the TGFs, activin, nodal and myostatin, which act through Smads 2 and 3 (Wharton and Derynck, 2009). Signals are mediated through a heterotetrameric receptor unit composed of two transmembrane type I and type II receptors. Ligand binding to the type II receptors causes activation of serine-threonine kinase domain of the type I receptors and results in receptor Smad (R-Smad) phosphorylation. Phosphorylated R-Smads form hetero-oligomeric pairing with common/collaborating Smads (Co-Smads), enter the nucleus and regulate gene expression. Two inhibitory Smads (I-Smads), Smads 6 and 7 also exist, which regulate TGF-β signaling by inhibiting the R-Smads and Co-Smads. It is evident from the sequence similarities that these Smads arose due to gene duplication; their evolutionary origin and functions, however, remain unclear. In this review article, we discuss key molecules involved in establishment of the axis in Hydra and other cnidarians since this can potentially provide insights into axial patterning during metazoan evolution.
Axis formation in cnidarians
Cnidarians, the sister group of bilateria, possess an oral-aboral axis with a radial symmetry as opposed to the distinct anterior-posterior, dorsal-ventral and left-right axes found in bilaterians, which are regulated by Wnt, TGF-β/BMP and Nodal signaling pathways, respectively (Watanabe et al., 2014a). In bilaterians, axial patterning is predominantly controlled by the Wnt pathway, which also helps in the establishment of the Organizer, specification of dorsal-ventral and anterior- posterior axes and imparts positional information during vertebrate and invertebrate embryonic development. Owing to their unique phylogenetic position and relatively simple body plan, molecular mechanisms governing formation of body axes can be better understood by studying cnidarians. Nematostella vectensis, Clytia hemisphaerica and Hydra are some of the best studied cnidarian models to understand axial patterning. In Nematostella, an anthozoan cnidarian, 13 Wnt genes are expressed along the oral-aboral axis in the developing embryo and larval forms, while only Wnt1 and Wnt3 are shown to possess the secondary axis inductive capacity (Kraus et al., 2016). In the hydrozoan Clytia hemisphaerica, role of two Frizzled receptors and the maternally deposited Wnt3 ligand in the establishment of oral/aboral axis has been reported (Momose and Houliston, 2007; Momose et al., 2008). Similar role for Wnt signaling in the establishment of oral axis in the colonial Hydrozoan Hydractinia (Duffy et al., 2010) and in the anthozoan cnidarian Acropora millepora (Hayward et al., 2015) has been reported. This suggests that the dependency on Wnt pathway members for establishing axial patterning predates the cnidarian-bilaterian split.
Similarly, components of TGF-β/BMP signaling pathway, which are involved in dorsoventral axis formation in vertebrates and correspond to establishing oral-aboral axis in cnidarians were investigated and resulted in the identification of several components of the TGF-β/BMP pathway and their inhibitors. It is interesting to note, however, that the regulation of directive axis formation in cnidarians is quite different from that observed in the bilaterians. In Nematostella, overlapping expression patterns of decapentaplegic (dpp) and its inhibitor Chordin are seen during the development of the embryo on one side of the blastopore, while in Drosophila and Xenopus, dpp and its antagonists, short gastrulation gene (sog) and its ortholog Chordin are expressed in opposing domains (Hayward et al., 2002; Matus et al., 2006; Rentzsch et al., 2006). A gradient of BMPs and asymmetric expression of its inhibitors, dpp and Chordin on one side of the oral end of the developing embryo also suggest presence of a secondary axis that acts perpendicular to the oral-aboral axis and their role in secondary axis patterning in Nematostella (Finnerty et al., 2004; Matus et al., 2006; Saina et al., 2009; Genikhovich and Technau, 2017). One of the BMP-interacting proteins, repulsive guidance molecule (RGM) is also shown to be co-expressed along with BMP2/4 and Chordin and has been shown to play a role in developing bilateral symmetry in Nematostella (Leclère and Rentzsch, 2014). A similar expression of dpp is also seen in the anthozoan coral, Acropora, suggesting a role in establishing the directive axis (Hayward et al., 2002). This suggests that the molecular toolkit of major signaling pathways for the formation of dorsal-ventral and left-right axes in bilaterians is already present in the cnidarians.
Axial patterning in Hydra
Hydra, a freshwater hydrozoan, is one of the most favorite models to study axis formation due to its simple body plan, oral-aboral axis and radial symmetry (Ghaskadbi, 2020a, Ghaskadbi, 2020b; Wang et al., 2020). It exhibits a simple, yet defined body plan with a single oral-aboral axis comprising of a hypostome that serves as mouth surrounded by a few tentacles at the oral end, and a basal disc at the aboral end using which it attaches to the substratum. The cnidarian oral opening is often compared to the posterior end of bilaterians, where wnt signaling is active and is required for posterior growth (Meinhardt, 2002; Martin and Kimelman, 2009; Petersen and Reddien, 2009). Bilaterians are broadly classified into protostomes and deuterostomes based on the site of gastrulation that forms endodermal tissue during blastopore formation and gives rise to either mouth or anus. In both protostomes and deuterostomes, the vegetal pole cells and animal pole cells form endoderm and oral/anterior structures, respectively. While the formation of anus from the site of gastrulation in deuterostome is not questioned, development of mouth from the site of gastrulation in protostomes is disputed (Martindale, 2013). For example, in priapulid, an ecdysozoan worm, the gastrulation pattern and the molecular markers expressed are similar to those seen in deuterostomes (Martín-Durán et al., 2012) suggesting a need to study gastrulation patterns in protostomes.
Establishment and maintenance of the polarity of anterior-posterior axis is often controlled by Wnt signaling along with TGF pathway members which are conserved across different animal phyla. In case of cnidarians, the oral structure is developed from the animal pole cells as seen in bilaterians (Martindale, 2013) and shows expression of Wnt pathway members, thus conferring cnidarian oral axis similarity to that of posterior end of bilaterians. In deuterostomes, including echinoderms and hemichordates, Wnt signaling aids in posteriorization of neural ectoderm, while it induces oral nervous system in another anthozoan cnidarian, Nematostella suggesting that the bilaterian posterior end is similar to the cnidarian oral side (Watanabe et al., 2014b). This is also supported by the fact that Hydra hypostome shows expression of wnt and Brachyury (Technau et al., 1999; Hobmayer et al., 2000), the posterior expressing genes in vertebrates suggesting that the marginal zone of vertebrates and the Hydra head Organizer are similar and the absence of head specific genes such as Nkx in the hypostome further supports the close similarity between oral-aboral axis of Hydra and posterior-anterior axis of vertebrates (Grens et al., 1996; Meinhardt, 2012; Holstein, 2022, 2024). Also, the expression of Wnt-3 in the posterior tip of Hydractinia larvae that develops into the oral pole confirms that the oral side of cnidarians is equivalent to the posterior pole of bilaterians (Duffy et al., 2010). In Hydra, HyWnt, an orthologue of wnt3A is expressed in the hypostome, which acts as the head organizer and plays a role in controlling axial polarity and patterning (Hobmayer et al., 2000; Broun and Bode, 2002). In addition to the Wnt/β-catenin pathway, recent findings suggest involvement of Hippo/YAP pathway in establishing new axis during budding by regulating YAP (Yorkie in Drosophila), that acts upstream of the Wnt pathway (Brooun et al., 2022). Presence of the first YAP was reported from Trichoplax adhaerens, a placozoan. All components of the core machinery of the modern Hippo/YAP pathway are seen in Nematostella (Hilman et al., 2011). During regeneration on the oral side, expression of Hippo/YAP pathway components is regulated followed by activation of Wnt-PCP pathway members. This suggests interaction between these two pathways during regeneration and in determining anterior-posterior polarity (Schaffer et al., 2016). Furthermore, Wnt has been shown to act directly on different components of TGF-β pathway during secondary axis patterning in Hydra. Work from our laboratory has shown the roles of two BMP antagonists Noggin and Gremlin in axis formation and tentacle patterning in Hydra (Chandramore et al., 2010; Krishnapati et al., 2020). Over the past few years, quite a few players from the TGF-β pathway have been identified in Hydra and have been shown to play key roles in axial patterning (Table 1). These studies suggest presence of coordinated signaling among Hippo-Wnt-TGF-β pathways in determining axial patterning in Hydra.
Table 1
Expression domain and possible functions of TGF-β signaling pathway members in Hydra
| Gene name | Expression domain in adult polyps | Possible functions | References |
|---|---|---|---|
| Hysmad1 | Body column except basal disc | Nematocyte and germ cell specification, oogenesis | Hobmayer et al., 2001 |
| HyBMP5-8a | Body column except basal disc | ? | Reinhardt et al., 2004 |
| HyBMP5-8b | Base of the tentacles, lower 1/3 of body column | Tentacle formation, patterning of lower body column | Reinhardt et al., 2004 |
| chordin | Head region | Organizer formation | Rentzsch et al., 2006, 2007 |
| nodal | Presumptive budding zone | Determination of biradial symmetry | Watanabe et al., 2014a |
| activin | Ubiquitous expression towards oral side | ? | Watanabe et al., 2014a |
| noggin | Hypostome, base of the tentacles,lower body column, basal disc | Organizer maintenance, tentacle formation | Chandramore et al., 2010; Krishnapati et al., 2020 |
| gremlin | Budding zone | Budding | Krishnapati et al., 2020 |
TGF-β signaling pathway in Hydra
In addition to the Wnt pathway, another highly conserved pathway in defining the three axes during development and patterning in both invertebrates and vertebrates is the TGF-β pathway. Several members of TGF-β signaling pathway are involved in dorsal-ventral axis patterning in bilaterians, such as, Drosophila (invertebrate) and Xenopus (vertebrate). Though initially thought to have evolved in bilaterians, discovery of several components of TGF-β pathway in the basal phyla, such as, cnidarians (Samuel et al., 2001 Hayward et al., 2002; Matus et al., 2006) and poriferans (Suga et al., 1999) revealed their conserved role in development and patterning different tissues and their early evolution.
The initial discovery of TGF-β pathway in Hydra came from the identification of Hysmad1, an orthologue of receptor Smads (Hobmayer et al., 2001). Structurally, the protein Hysmad1 shows similarity to both R-Smads and Co-Smads of higher metazoans and belongs to the Smad 1-5-9 subgroup. Thirteen out of the 18 conserved amino acid residues that help in the Smad2-Smad anchor for receptor activation (SARA) -interaction and a phosphorylation motif at the C-terminus are present in Hysmad1 indicating the presence of an active BMP/Smad signaling in Hydra (Hobmayer et al., 2001). This has been further confirmed by the identification of two BMP5-8 orthologues, HyBMP5-8a and HyBMP5-8b in Hydra (Reinhardt et al., 2004). Expression of HySmad1 transcripts in the interstitial oocyte precursor cells and nurse cells during oocyte differentiation indicates its role in oogenesis in Hydra, which confirms conservation of similar functions in higher metazoans such as in Drosophila (Xie and Spradling, 1998). While Hysmad1 appears to have a uniform expression along the body axis during budding and regeneration with increased levels during oogenesis, expression of the ligands HyBMP5-8a can be detected throughout the body column and HyBMP5-8b at the base of the tentacles and in the lower one third of the body column in adult Hydra (Reinhardt et al., 2004). Graded expression of HyBMP5-8b along the body axis in Hydra suggests its role in axial patterning that confers positional information during budding and regeneration.
Analysis of proteomic and transcriptomic data revealed altered levels of several components of Wnt and TGF pathway, which showed differentially upregulated Wnt ligands and their downstream target genes. Members of TGF signaling pathway, Activin, BMP2/4 and BMP5/8c are downregulated, while the BMP ligands, Noggin and Chordin are strongly upregulated during head regeneration (Petersen et al., 2015). Interestingly, members of Cerberus-Gremlin-DAN family are downregulated during head regeneration (Reddy et al., 2019). This suggests that both Wnt and TGF signaling pathways are used during patterning of the opposite ends of the axis in Hydra. Expression of wnt3A and HyBMP5-8b at the oral and aboral ends in Hydra, thus is analogous to the expression of Wnt and BMP pathway members during dorsoventral patterning in Xenopus (Cho et al., 1991; Hobmayer et al., 2000). It is also interesting to note that BMPs and other genes including Nodal, Brachyury, GATA, Forkhead and Snail, needed for mesodermal specification and patterning in vertebrates are identified in Hydra, which lacks mesoderm (Technau and Bode, 1999; Technau and Scholz, 2003; Nakamura et al., 2011; Watanabe et al., 2014a). Many of these vertebrate mesodermal marker genes are expressed in the endoderm of Hydra; similarly, expression of these several mesodermal marker genes is also seen in basal endoderm in Nematostella, and are often referred to as mesendodermal markers. This suggests that the bilaterian mesoderm could have descended from the endoderm of their diploblastic ancestors (Technau, 2020).
Nodal and Activin
An important feature of the vertebrate body plans is the presence of a left-right (L/R) axis that is distinctive and is conserved in almost all vertebrates. The molecular asymmetry that establishes symmetry in external structures is directly under the control of several morphogens (Boutet et al., 2017). For example, L/R asymmetry at the molecular level is mainly controlled by the expression of nodal-lefty-pitx cassette during embryogenesis (Boutet et al., 2017). Nodal, a member of the TGF-β superfamily originally discovered in the genetic studies in mouse embryos, mediates signaling through activin receptors followed by phosphorylation and nuclear localization of Smad2 transcription factor (Conlon et al., 1991; Schier et al., 2003). Though originally discovered for its role in mesoderm induction, role of nodal as endoderm inducer in vertebrates strengthened the idea of mesendoderm induction (Schier et al., 2003). Further, its instructive role in developing L/R axis is also evident by its expression on the left-hand side of lateral plate mesoderm which is well conserved in vertebrates (Levin et al., 1995). This was further confirmed by nodal misexpression on the right-hand side, which resulted in abnormalities in determining the organ systems along the axis (Levin et al., 1997). Another BMP inhibitor, cerberus, expressed on the right-hand side of the embryonic node also helps in maintaining L/R asymmetry by binding directly to the Nodal protein thereby repressing Nodal signaling on the right-hand side (Vandenberg et al., 2013; Li et al., 2017). Yet another gene of Cerberus/Dan family, caronte (car), expressed on the left-hand side of the lateral plate mesoderm, maintains L/R asymmetry by inducing nodal expression and antagonizes the BMP pathway (Yokouchi et al., 1999). This mechanism of nodal induction by car is also mimicked by noggin suggesting that BMP pathway plays a crucial role in repressing nodal expression (Capdevila et al., 2000). It is also to be noted that the inhibition of Nodal by BMPs in establishing the L/R axis is a conserved phenomenon in vertebrates (Capdevila et al., 2000).
Though the left-hand sided activity of Nodal signaling is conserved in vertebrates, right-hand side expression is seen in echinoderms and hemichordates, suggesting an axis inversion in these organisms (Duboc et al., 2005). In Hydra, nodal is expressed asymmetrically in the budding region of the main body axis and in the early buds when the secondary axis perpendicular to the main axis is generated and controls biradial asymmetry (Watanabe et al., 2014a). The downstream target gene, pitx is also expressed in the same region suggesting that both belong to the same molecular cassette in Hydra (Watanabe et al., 2014a). Hydra nodal has been shown to play a role in breaking the symmetry during bud formation and may act in co-ordination with Wnt pathway members during bud formation (Watanabe et al., 2014a). The role of β-catenin in inducing nodal and pitx was shown during bud evagination suggesting that β-catenin-nodal-pitx act as the core signaling cassette in controlling axial patterning in Hydra (Watanabe et al., 2014a). Overexpression of nodal upon the removal of a pharmacological inhibitor A-83-01, a specific inhibitor for Activin-receptor-like kinase 4/5/7 in Hydra perturbs the biradial symmetry and confirms its role in controlling the symmetry in Hydra (Watanabe et al., 2014a). BMP inhibitors such as members of the Cerberus/Dan family have also been shown to inhibit nodal signaling in vertebrates including humans (Hsu et al., 1998; Piccolo et al., 1999). In Hydra, gremlin, one of the BMP antagonists that belongs to DAN family, has been reported by us (Krishnapati et al., 2020). It is interesting to see that the expression of gremlin during initial stages of budding persists even as the buds develop (Krishnapati et al., 2020), while the expression of nodal, that is expressed at the presumptive budding zone, disappears during bud development (Watanabe et al., 2014a). It is known that Gremlin2 blocks interaction of Nodal to its type II receptor thereby inhibiting nodal signaling (Nolan et al., 2016). This suggests existence of a possible antagonism between gremlin and nodal even in Hydra.
Activin, originally identified from the ovarian fluid for its ability to release follicle stimulating hormone from the pituitary was later shown to have diverse roles in growth and development (Ying et al., 1997). Activin is a polypeptide belonging to the TGF-β family that binds to four transmembrane activin receptors (type I, IB, II, IIB) and follistatin, one of the inhibitors of the BMP pathway. Binding of activin to its type II receptor leads to phosphorylation of type I receptor followed by phosphorylation of Smad2 and Smad3 (Xia et al., 2009). Binding of follistatin to activin neutralizes its activity by preventing it from interacting with its own receptors (Thompson et al., 2005). Both activin and follistatin have been shown to play roles in mesoderm induction in amphibians. They act as potent inducers and result in the formation of a secondary axis upon ectopic expression in the Xenopus embryos (Dohrmann et al., 1993). This was further confirmed by injecting activin receptor mRNA, which led to defects in axis formation and mesoderm induction suggesting that activin-activin receptor system is highly regulated during embryogenesis (Hemmati-Brivanlou et al., 1992). In Hydra, activin-like genes (ActL1, 3, 4) are expressed in the endoderm in upper body column excluding the hypostome, while ActL2 shows expression in the overall body column in Hydra. Similarly, in Nematostella, expression of activin in the endoderm of tentacle buds was shown to have a role in morphogenesis (Matus et al., 2006). However, their role in axis asymmetry during bud formation similar to nodal was not identified (Watanabe et al., 2014a).
Chordin
Embryonic pattern formation is regulated by the interactions between BMPs and their antagonists, Chordin, Noggin and Follistatin. While BMPs determine ventral cell fate, the inhibitors, Chordin, Noggin and Follistatin act as dorsalizing factors and are expressed in the Organizer. Though all these genes encoding BMP antagonists are expressed simultaneously in the amphibian embryo during gastrulation, they show distinct spatio-temporal expression patterns in other organisms (Streit and Stern, 1999). Among these, chordin, the homolog to Drosophila sog, was first identified in a differential screen of zygotic dorsal-specific cDNAs and is secreted from the Organizer in amphibian embryos (Sasai et al., 1994). Chordin shows characteristic four cysteine-rich repeats, each containing 10 cysteines. The first two cysteine-rich domains bind to BMP2 and BMP4 and form a transient complex that prevents their interaction with cell surface receptors, which is disassociated by the BMP1/metalloproteinases of the Tolloid family (Piccolo et al., 1996). This mechanism of binding and dissociation of chordin-BMP complex results in varying concentrations of BMPs and is well conserved in establishing dorsal-ventral axis in invertebrates and vertebrates (Piccolo et al., 1996; Marqués et al., 1997).
The ability to induce a secondary axis on the ventral side of the host upon transplantation is the best example of the Organizer activity. In amphibian embryos, the dorsal lip of blastopore acts as the Spemann-Mangold Organizer and has the ability to generate cell polarity and specific cell fate in the neighboring tissues (Spemann et al., 1924; Gerhart et al., 2001). It is interesting to note several similarities between vertebrate and Hydra Organizers in terms of expression of many genes. For example, marker genes such as β-catenin, noggin and chordin expressed in the vertebrate Organizer are also seen in Hydra and overexpression of these Hydra genes result in secondary axis formation in vertebrate embryos (Hobmayer et al., 2000, Rentzsch et al., 2007; Chandramore et al., 2010). Similarly, Hensen’s node, the avian equivalent to Spemann-Mangold Organizer also secretes molecules such as Noggin, Chordin and Nodal (Chapman et al., 2002; Katsu et al., 2012). This suggests that the signals responsible for maintaining the Organizer activity are similar and are conserved across phyla in both invertebrates and vertebrates.
Despite the presence of similarities in gene expression patterns, the Hydra Organizer is not viewed as a direct equivalent to the vertebrate Organizer. In Hydra, the Organizer primarily contributes to establishing the oral-aboral axis, mainly controlled by Wnt signaling. In vertebrates, even though, Wnt from the Organizer aids in anterior-posterior axis establishment, its formation from the blastopore remains unaffected even in the absence of the Spemann-Organizer and suggests differences in axis formation between the Hydra Organizer and the Spemann-Organizer. This is comprehensively reviewed by Meinhardt (2006), wherein the differences and similarities between Hydra Organizer and vertebrate Organizer in axis formation and patterning the tissues are discussed.
In Hydra, the hypostome and basal disc act as Organizers with an ability to induce secondary axis upon transplantation (Browne 1909, Hicklin and Wolpert, 1973; Müller, 1996). However, the hypostome tissue alone, anterior to the tentacles and without the tentacle base is absorbed and does not give rise to formation of new Hydranths (Browne, 1909). Experiments conducted by Hicklin and associates showed for the first time that the lateral grafts from the foot region are also able to induce new axis suggesting Organizer activity of the basal disc (Hicklin and Wolpert 1973, Hicklin et al., 1973). However, the new axis always forms when the grafting is performed only at the distal end and not at the proximal end. This is possibly due to the inhibitory effects of head inducing gradients at the proximal end resulting in inhibiting the development of new hydranth from foot grafts. On the other hand, reported work from our laboratory has shown that both homoplastic and heteroplastic transplantations, using a piece of hypostome or a complete hypostome with tentacle base and without any conspicuous tentacles on upper, middle and basal regions resulted in successful grafting with more than 90 % accepted grafts (Kadu et al., 2012). Similar results are also seen by grafting a complete foot in the upper (with/without hypostome and tentacles), middle and basal regions during homoplastic and heteroplastic transplantations (Kadu et al., 2012). The ability to induce secondary axis either by the head or foot Organizers in Hydra is due to the presence of molecules that provide instructive signals for initiating fate determination and morphogenesis. Few such molecules like Wnt, Chordin have been identified and shown to have Organizer properties in Hydra. Role of Hydra chordin-like (hychdl) gene in Organizer formation was identified by its endodermal expression pattern during early stages of budding and regeneration, during which the new head Organizer is established. Similarly, role of Chordin in secondary axis formation perpendicular to the oral/aboral axis, comparable to budding in Hydra, was shown in Nematostella (Saina et al., 2009). Heterologous expression of hychdl mRNA in Zebrafish embryos resulted in inhibition of BMP signaling in a dose dependent manner (Rentzsch et al., 2007). Such inhibition of BMP signaling is possibly due to the presence of chordin-, follistatin- and insulin-like growth factor binding protein (IGFBP)- domains in Hychdl. Though follistatin has not been identified in Hydra so far, a single domain corresponding to three follistatin domains of human follistatin has been identified in Hydra Chordin. Follistatin is a known BMP inhibitor, while IGFBP inhibits TGF signaling indirectly due to its structural similarity to Twisted gastrulation (Tsg) that interacts with BMPs. This suggests that these two domains in Hychdl may also contribute to inhibition of BMP signaling in zebrafish embryos and that the BMP-chordin antagonism may have potential role in establishing axial polarity in Hydra.
Noggin
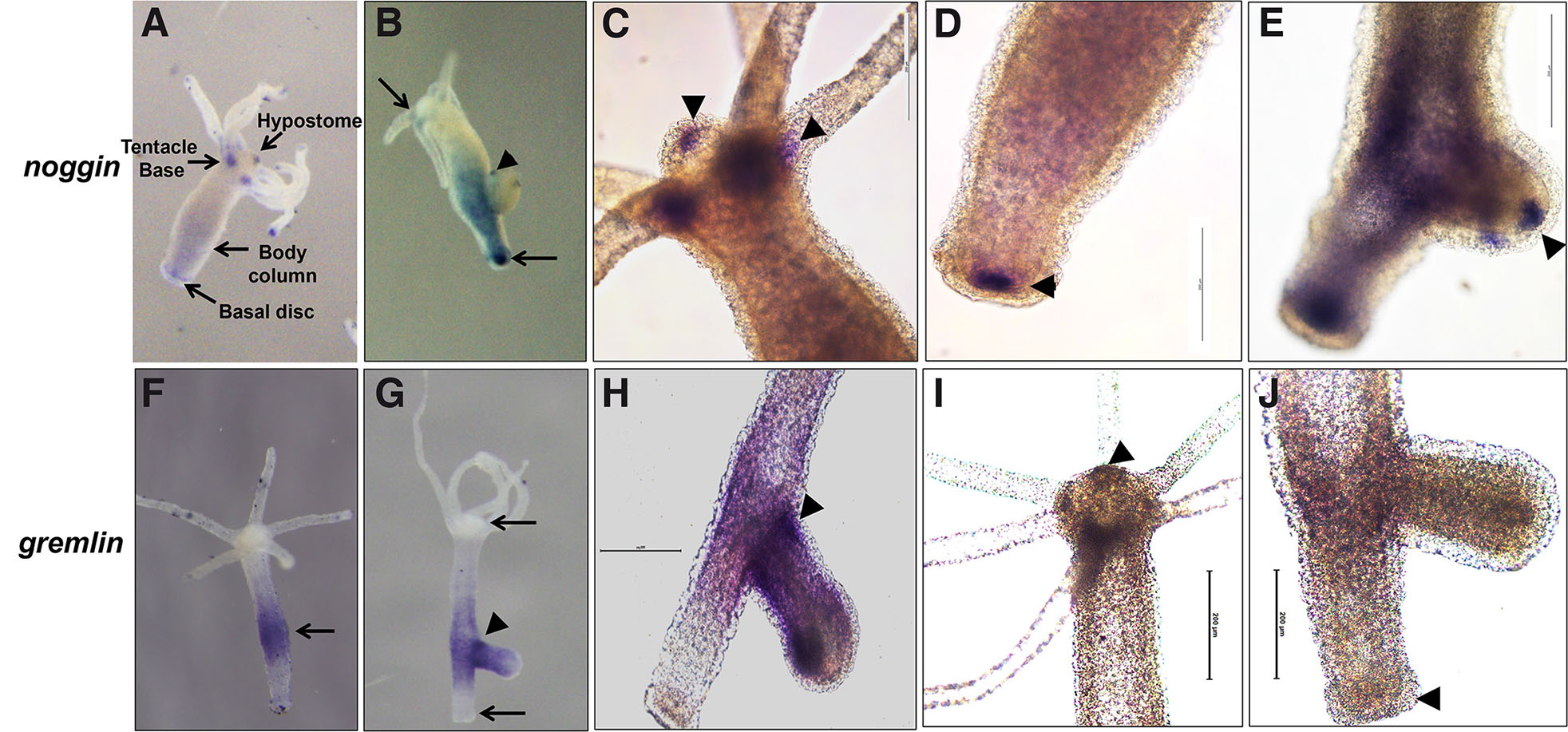
One of the important mechanisms of BMP pathway regulation is by binding of secretory molecules to BMP ligands, which prevent them from interacting with their cell surface receptors. So far, chordin, noggin and gremlin have been identified from Hydra and are shown to have role in axial and tentacle patterning (Rentzsch et al., 2007; Chandramore et al., 2010; Krishnapati et al., 2020). Identification of noggin in Hydra came from our own studies. We found that it is expressed in the hypostome, base of the tentacles and foot region and as distinct spots before the emergence of tentacles during budding (Chatterjee and Ghaskadbi 2001). Recent work from our laboratory further confirmed its endodermal expression in the hypostome, lower body column, base of the tentacles and basal disc (Krishnapati et al., 2020). To confirm the specific expression of noggin at the hypostome, basal disc and tentacle base, magnified images were taken (Fig. 1 C,D,E, current manuscript). Expression of noggin1 in the endoderm of tentacle base, tips and at the tentacle emergence zone in the developing buds has also been reported in Nematostella (Fig. 2C) suggesting that Noggin plays a role in patterning tentacles in cnidarians. Interestingly, BMP5-8b is also localized at the base of the tentacles in Hydra, which suggests possible interactions between Noggin and BMP during tentacle patterning. Expression of noggin in the head region comprising hypostome and base of the tentacles (Fig. 1C) and basal disc/foot (Fig. 1D) in Hydra is particularly interesting as both act as Organizers and induce secondary axis on transplantation (Browne, 1909; Hicklin and Wolpert, 1973; Hicklin et al., 1973; Müller, 1996; Kadu et al., 2012).
Fig. 1. Localization of noggin and gremlin in nonbudding and budding Hydra.
Expression of noggin is predominant in the hypostome, base of the tentacles, lower body column and in the basal region in non-budding polyp (A). During bud development, noggin is localized at the sites of tentacle emergence (arrowhead in B). High magnification images showing noggin expression in the hypostome and base of the tentacles (arrowhead in C), basal disc (arrowhead in D) and at tentacle emergence region (arrowhead in E). Gremlin is expressed mainly in the budding region and in the upper body column in the non-budding polyps (arrow in F) with increased expression during bud development (arrowhead in G) with no expression in the basal disc and hypostome (arrows in G). Magnified images showing gremlin expression in the budding Hydra (arrowhead in H). Absence of gremlin expression in the hypostome (arrowhead in I) and basal disc (arrowhead in J).
Structurally, Hydra Noggin shows characteristic Cysteine ring motif as seen in human Noggin that provides conformational rigidity to the protein (Groppe et al., 2002; Krishnapati et al., 2020). Although structural conservation does not fully imply functional conservation in higher metazoans, injection of Hydra noggin mRNA in two cell stage Xenopus embryos resulted in partial duplication of axis in 100 % embryos. When injected in UV-ventralized Xenopus embryos, Hydra Noggin partially rescued the embryos from the effects of UV irradiation. This dorsalizing effect of Hydra Noggin in Xenopus embryos was indeed due to inhibition of BMP signaling and its components as confirmed with the animal cap assay (Chandramore et al., 2010). These results demonstrate that Hydra Noggin functionally mimics a vertebrate Noggin and is conserved in higher metazoans.
Gremlin
Gremlin is yet another BMP inhibitor that shows dorsalizing effects like Noggin and Chordin. Gremlin, Cerberus and the tumor suppressor, DAN are structurally and functionally related proteins and belong to the same subfamily of BMP inhibitors (Ozaki et al., 1993; Bouwmeester et al., 1996). This is evident from the fact that all three binds to BMP2 and compete with Noggin suggesting structural/topological similarity, although no sequence similarity to Noggin and Chordin is observed (Hsu et al., 1998). It is interesting to note that gremlin is not expressed in the Organizer region during gastrulation in Xenopus embryos but shows axial patterning activities (Hsu et al., 1998). Gremlin transcripts form a concentration gradient from aboral to oral side along the oral-aboral axis in the anthozoan cnidarian, Nematostella vectensis. Expression of NvGrm is seen on the opposite side of Nvdpp expression in mid-late planula stages of Nematostella (Fig 2C; Rentzsch et al., 2006) and in the endoderm tissue surrounding the mouth in the late planula stages during polyp formation (Fig. 2D; Matus et al., 2006), while NvChd and NvBMP5-8 are expressed on the same side along the oral aboral axis (Fig 2D) suggesting the presence of complex interactions within the BMP signaling pathway in cnidarians.
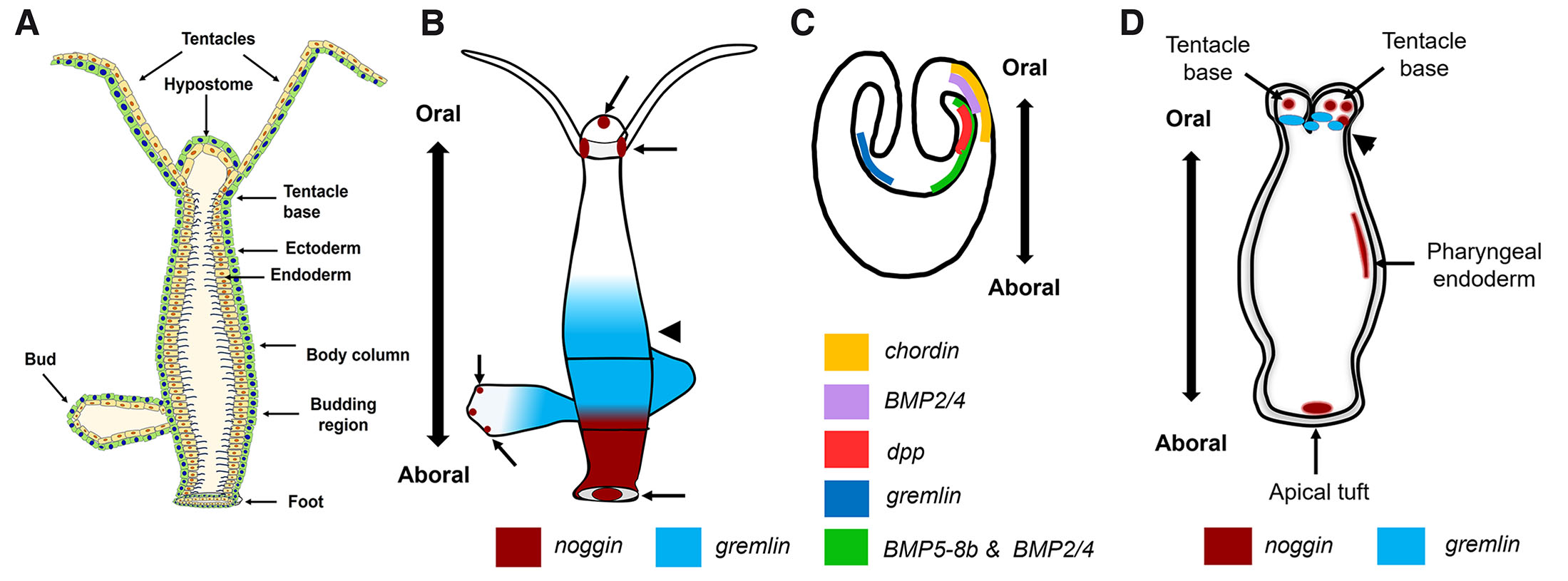
Fig. 2. Expression patterns of BMPs and BMP inhibitors in Hydra and Nematostella
Schematic representation of different morphological regions in adult Hydra (A). Representation of noggin and gremlin expression in Hydra (B) based on data from Krishnapati et al., 2020 and early (C) and late (D) planula stages of Nematostella vectensis (lateral views) based on data from Matus et al., 2006; Rentzsch et al., 2006; Saina et al., 2009. Endodermal expression of noggin is seen at the base of the tentacles, hypostome and in the basal disc in Hydra (arrows in A). Expression of chordin, gremlin and the ligands dpp, BMP2/4 and BMP5-8b on the oral side of early planula stage of Nematostella is shown (C). Expression of noggin is also seen at the base of the tentacles in the late planula stage of Nematostella, (arrows in D) and in the endoderm of apical tuft (D) that lie on the aboral side. Graded expression of gremlin is seen in the endoderm of budding region in Hydra (arrowhead in B) while gremlin is localized in the region surrounding mouth on the oral side of late planula stage of Nematostella (arrowhead in D).
Identification of gremlin in a fellow cnidarian prompted us to look for its presence in Hydra. We identified a putative gremlin-like 1 mRNA sequence of 483 bp from Hydra vulgaris. Using whole mount in situ hybridization (WISH), endodermal expression of gremlin in the budding region was reported with clean hypostome and foot regions (Krishnapati et al., 2020). The expression pattern of gremlin was further confirmed by taking magnified images showing no signal in the hypostome and foot regions (Fig. 1 I,J shown by arrowhead) and strong signal at the base of the bud (Fig. 1 G,H shown by arrowhead). An earlier study has reported increased neuronal cell density at the site of bud formation (Bode et al., 1973). A positive correlation between neuronal cell density and rate of budding in Hydra has also been demonstrated (Browne et al., 1978). Significant levels of gremlin transcripts in Xenopus neural crest cells and their role in neural crest induction and patterning has been reported (Hsu et al., 1998). Expression of gremlin during early stages of budding in Hydra, where the neuronal cell density is high, suggests its possible expression in the neuronal cell lineage and role in patterning the tissue during bud initiation and development. Interestingly, gremlin expression was undetectable in the hypostome (Fig. 1I) and basal region (Fig. 1J) that act as organizing centers in Hydra. This lack of expression in the Organizer regions also agrees with the expression pattern of gremlin in the Xenopus Organizer (Hsu et al., 1998) suggesting its primary role in tissue patterning during development.
One of the most interesting events that occur during the development of metazoan embryo is gastrulation, which results in the generation of three germ layers, ectoderm, endoderm and mesoderm. Basal metazoans belonging to phylum Placozoa, Porifera, Cnidaria and Ctenophora, however, show only two germ layers, ectoderm and endoderm and are hence diploblastic. The two germ layers carry out all the necessary functions responsible for morphogenesis, behavior and reproduction. Accumulating evidence shows the importance of TGF-β/BMP pathway in germ layer segregation during early embryonic development and patterning. In Hydra, the diploblastic nature is defined by two lineages of epitheliomuscular stem cells, the ectodermal and the endodermal epithelial stem cells. It is interesting to that the majority of BMP ligands and their inhibitors are expressed in the endoderm of Hydra and Nematostella (Matus et al., 2006; Krishnapati et al., 2020). Expression of HyBMP5-8b, and the inhibitors of BMPs, chordin, noggin and gremlin are observed in Hydra endoderm. Interestingly, single cell RNA sequencing using trajectory analysis of ectodermal and endodermal epithelial cells also revealed predominant expression of another BMP antagonist, DAN containing gene, t2758 in the endodermal epithelial cells in the foot and lower body column region (Sieberts et al., 2019). Similarly, BMP5-8, dpp, Smad1/5 and Smad4 are expressed in the endoderm of Nematostella. On the contrary, Smad1 is expressed in both the germ layers of Hydra. However, very little information is available pertaining to the presence of BMP receptors, their expression and roles in axis patterning in Hydra. The presence of active signaling of BMPRI was indirectly shown by ectopic expression of BMPRI, which induces NvHoxE and pSmad1/5 expression in the ectoderm (Genikhovich et al., 2015). Since the ligands are expressed in the endoderm and the Smads are seen in the ectoderm of Hydra, it would be interesting to look at the interlineage interactions to understand the communication between ligands, receptors and the Smad proteins (Hemmrich et al., 2012). Another important feature of Hydra is the presence of mesoglea between ectoderm and endoderm. Structurally, the acellular matrix is similar to vertebrate extracellular matrix and is rich in fibrillar collagen and laminin (Sarras et al., 1994; Zhang et al., 2002). Several cell adhesion proteins, ECM proteins and proteoglycans have been identified and shown to play crucial roles in morphogenesis, pattern formation, regeneration and cell-ECM interactions in Hydra (Sarras et al., 1993; Sarras 2012). Recent evidence suggests the role of Wnt/β-catenin signaling in the remodeling of mesoglea during axis formation in Hydra (Veschgini et al., 2023). Also, previous results have shown the importance of mesoglea during budding. Since members of the BMP pathway are shown to play key roles in secondary axis formation and patterning in Hydra, it would be interesting to look at the remodeling of mesoglea in relation to the TGFβ pathway.
To summarize, the presence of a large number of TGFβ signaling components in Hydra suggests that the pathway evolved early in evolution and was recruited for patterning of the body axis. Though Wnt/β-catenin signaling plays a major role in Organizer formation, concomitant expression of BMP inhibitors Chordin and Noggin in the hypostome and basal disc (which exhibit Organizer activity on transplantation) suggests that TGF-β/BMP pathways play crucial role in maintaining the Organizers in Hydra. Although, it was initially surprising to identify genes encoding proteins that participate in vertebrate dorsal-ventral and left-right axis in the radially symmetrical cnidarians, identification of a few components of both Wnt and TGF-β pathway members during embryonic development in poriferans (Adamska et al., 2007; Windsor Reid et al., 2018) also suggests that the molecular tool kit for axis formation evolved long before the cnidarian-bilaterian split. One expects that in the near future, more players in the TGF-β signaling pathway will be identified from Hydra and other cnidarians. This will give us a better idea of how cell signaling, crucial for multicellularity, evolved in simple metazoans. This, in turn, may provide us with vital clues regarding evolution of dorsal-ventral, anterior-posterior and left-right axes in more complex metazoans including humans.
Materials and Methods
Whole mount in situ hybridization
Whole-mount in situ hybridization (WISH) was performed using digoxigenin (DIG)-labeled RNA probes for gremlin and noggin as described (Krishnapati et al., 2020). Briefly, the complete coding sequences amplified were used for in vitro transcription reaction using Dig-RNA labeling kits (Roche) following manufacturer’s instructions. WISH was performed as previously described with few modifications. Polyps were relaxed in 2 % urethane for 2 min, proteinase K treatment was performed for 10 min and endogenous alkaline phosphatase was inhibited by heat-inactivation at 70 °C for 15 min in 2 X SSC. Prehybridization with tRNA for 2 h and hybridization with sense and anti-sense riboprobes for 48 h were followed at 60 °C. Post-hybridization washes were carried out with gradients of hybridization buffer and 2X SSC and stringency washes with 0.5X SSC, followed by incubation with anti-digoxigenin antibody. Following color development with Nitro Blue Tetrazolium/5-bromo-4-chloro 3-indolyl-phosphate p-toluidine salt (NBT/BCIP) and polyps were dehydrated in methanol grades. Whole polyps were imaged using Olympus SZX16 stereo microscope using DP71 camera and high magnification images were taken using Leica microscope DM5500 B using DFC450C camera.
Acknowledgements
We have been able to establish and maintain a hydra laboratory since the year 2000 due to the constant support from MACS-Agharkar Research institute, Pune. We thank all our lab members and collaborators who have contributed to our efforts to reintroduce the hydra model for teaching and research in India. Ms Rohini Londhe, who has ably maintained the hydra cultures over the years, deserves a special word of appreciation as she managed to maintain the hydra culture even during the difficult Covid-19 outbreak and lockdowns. KLS was supported by Young Scientist and NPDF grants from DST-SERB, Government of India, New Delhi while SG was supported by the Emeritus Scientist Scheme of Council for Scientific and Industrial Research (CSIR), New Delhi.
Abbreviations
BMP, Bone morphogenetic protein ; HyBMP, Hydra BMP ; HySmad, Hydra Smad ; Smad, Suppressor of mothers against decapentaplegic ; TGF-β, Transforming growth factor-β ; WISH, Whole mount in situ hybridization ;References
Adamska M., Matus D. Q., Adamski M., Green K., Rokhsar D. S., Martindale M. Q., Degnan B. M., (2007). The evolutionary origin of hedgehog proteins. Current biology : CB 17: R836-R837.
Barolo S., Posakony J. W. (2002). Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes & Development 16: 1167-1181.
Bode H., Berking S., David C. N., Gierer A., Schaller H., Trenkner E. (1973). Quantitative analysis of cell types during growth and morphogenesis in Hydra. Wilhelm Roux Archiv für Entwicklungsmechanik der Organismen 171: 269-285.
Borisenko I., Adamski M., Ereskovsky A., Adamska M. (2016). Surprisingly rich repertoire of Wnt genes in the demosponge Halisarca dujardini. BMC Evolutionary Biology 16: 123.
Borisenko I., Podgornaya O. I., Ereskovsky A. V. (2019). From traveler to homebody: Which signaling mechanisms sponge larvae use to become adult sponges?. In Intracellular Signalling Proteins. Elsevier.
Boutet A. (2017). The evolution of asymmetric photosensitive structures in metazoans and the Nodal connection. Mechanisms of Development 147: 49-60.
Bouwmeester T., Kim S.H., Sasai Y., Lu B., Robertis E. M. D. (1996). Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature 382: 595-601.
Brooun M., Salvenmoser W., Dana C., Sudol M., Steele R., Hobmayer B., McNeill H. (2022). The Hippo pathway regulates axis formation and morphogenesis in Hydra. Proceedings of the National Academy of Sciences 119: e2203257119.
Broun M., Bode H. R. (2002). Characterization of the head organizer in hydra. Development 129: 875-884.
Browne E. N. (1909). The production of new hydranths in Hydra by the insertion of small grafts. Journal of Experimental Zoology 7: 1-23.
Browne C. L., Davis L. E. (1978). The role of nerve cell density in the regulation of bud production in hydra. Wilhelm Roux's Archives of Developmental Biology 184: 95-108.
Capdevila J., Vogan K. J., Tabin C. J., Izpisúa Belmonte J. C. (2000). Mechanisms of Left–Right Determination in Vertebrates. Cell 101: 9-21.
Chandramore K., Ito Y., Takahashi S., Asashima M., Ghaskadbi S. (2010). Cloning of noggin gene from hydra and analysis of its functional conservation using Xenopus laevis embryos. Evolution & Development 12: 267-274.
Chapman S. C., Schubert F. R., Schoenwolf G. C., Lumsden A. (2002). Analysis of Spatial and Temporal Gene Expression Patterns in Blastula and Gastrula Stage Chick Embryos. Developmental Biology 245: 187-199.
Chatterjee S., Lahudkar S., Godbole N. N., Ghaskadbi S. (2001). Hydra constitutively expresses transcripts involved in vertebrate neural differentiation. Journal of Biosciences 26: 153-155.
Chen C., Shen M. M. (2004). Two Modes by which Lefty Proteins Inhibit Nodal Signaling. Current Biology 14: 618-624.
Cho K. W.Y., Blumberg B., Steinbeisser H., De Robertis E. M. (1991). Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67: 1111-1120.
Conlon F. L., Barth K. S., Robertson E. J. (1991). A novel retrovirally induced embryonic lethal mutation in the mouse: assessment of the developmental fate of embryonic stem cells homozygous for the 413.d proviral integration. Development 111: 969-981.
Dohrmann C. E., Hemmati-Brivanlou A., Thomsen G. H., Fields A., Woolf T. M., Melton D. A. (1993). Expression of Activin mRNA during Early Development in Xenopus laevis. Developmental Biology 157: 474-483.
Duboc V., Röttinger E., Lapraz F., Besnardeau L., Lepage T. (2005). Left-Right Asymmetry in the Sea Urchin Embryo Is Regulated by Nodal Signaling on the Right Side. Developmental Cell 9: 147-158.
Duffy D. J., Plickert G., Kuenzel T., Tilmann W., Frank U. (2010). Wnt signaling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development 137: 3057-3066.
Finnerty J. R., Pang K., Burton P., Paulson D., Martindale M. Q. (2004). Origins of Bilateral Symmetry: Hox and Dpp Expression in a Sea Anemone. Science 304: 1335-1337.
Gerhart J. (1999). 1998 warkany lecture: Signaling pathways in development. Teratology 60: 226-239.
Genikhovich G., Fried P., Prünster M. M., Schinko J. B., Gilles A. F., Fredman D., Meier K., Iber D., Technau U. (2015). Axis Patterning by BMPs: Cnidarian Network Reveals Evolutionary Constraints. Cell Reports 10: 1646-1654.
Genikhovich G., Technau U. (2017). On the evolution of bilaterality. Development 144: 3392-3404.
Gerhart J, (2001). Evolution of the organizer and the chordate body plan. The International journal of developmental biology 45: 133-153.
Ghaskadbi S. (2020a). Cell signaling molecules in hydra: insights into evolutionarily ancient functions of signaling pathways. The International Journal of Developmental Biology 64: 141-149.
Ghaskadbi S. (2020b). Hydra: A powerful biological model. Resonance 25: 1197-1213.
Grens A., Gee L., Fisher D. A., Bode H. R. (1996). CnNK-2,an NK-2 Homeobox Gene, Has a Role in Patterning the Basal End of the Axis in Hydra. Developmental Biology 180: 473-488.
Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A. N., Kwiatkowski W., Affolter M., Vale W. W., Belmonte J. C. I., Choe S. (2002). Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420: 636-642.
Hayward D. C., Grasso L. C., Saint R., Miller D. J., Ball E. E. (2015). The organizer in evolution–gastrulation and organizer gene expression highlight the importance of Brachyury during development of the coral, Acropora millepora. Developmental Biology 399: 337-347.
Hayward D. C., Samuel G., Pontynen P. C., Catmull J., Saint R., Miller D. J., Ball E. E. (2002). Localized expression of a dpp / BMP2 / 4 ortholog in a coral embryo. Proceedings of the National Academy of Sciences 99: 8106-8111.
Hemmrich G., Khalturin K., Boehm A.M., Puchert M., Anton-Erxleben F., Wittlieb J., Klostermeier U. C., Rosenstiel P., Oberg H.H., Domazet-Lošo T., Sugimoto T., Niwa H., Bosch T. C.G. (2012). Molecular Signatures of the Three Stem Cell Lineages in Hydra and the Emergence of Stem Cell Function at the Base of Multicellularity. Molecular Biology and Evolution 29: 3267-3280.
Hemmati-Brivanlou A., Wright D. A., Melton D. A. (1992). Embryonic expression and functional analysis of a Xenopus activin receptor. Developmental Dynamics 194: 1-11.
Hicklin J., Wolpert L. (1973). Positional information and pattern regulation in hydra: formation of the foot end. Development 30: 727-740.
Hicklin J., Hornbruch A., Wolpert L., Clarke M. (1973). Positional information and pattern regulation in hydra: the formation of boundary regions following axial grafts. Development 30: 701-725.
Hilman D., Gat U. (2011). The Evolutionary History of YAP and the Hippo/YAP Pathway. Molecular Biology and Evolution 28: 2403-2417.
Hobmayer B., Rentzsch F., Kuhn K., Happel C. M., von Laue C. C., Snyder P., Rothbächer U., Holstein T. W. (2000). WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407: 186-189.
Hobmayer B., Rentzsch F., Holstein T. W. (2001). Identification and expression of HySmad1, a member of the R-Smad family of TGFβ signal transducers, in the diploblastic metazoan Hydra. Development Genes and Evolution 211: 597-602.
Holstein T. W. (2022). The role of cnidarian developmental biology in unraveling axis formation and Wnt signaling. Developmental Biology 487: 74-98.
Holstein T. W. (2024). The significance of Ethel Browne's research on Hydra for the organizer concept. Cells & Development In Press: 203907.
Hsu D. R., Economides A. N., Wang X., Eimon P. M., Harland R. M. (1998). The Xenopus Dorsalizing Factor Gremlin Identifies a Novel Family of Secreted Proteins that Antagonize BMP Activities. Molecular Cell 1: 673-683.
Itasaki N., Hoppler S. (2010). Crosstalk between Wnt and bone morphogenic protein signaling: A turbulent relationship. Developmental Dynamics 239: 16-33.
Kadu V., Ghaskadbi S., Ghaskadbi S., (2012). Induction of secondary axis in hydra revisited: New insights into pattern formation. International journal of molecular and cellular medicine 1: 11-20.
Katsu K., Tokumori D., Tatsumi N., Suzuki A., Yokouchi Y. (2012). BMP inhibition by DAN in Hensen's node is a critical step for the establishment of left–right asymmetry in the chick embryo. Developmental Biology 363: 15-26.
Käsbauer T., Towb P., Alexandrova O., David C. N., Dall'Armi E., Staudigl A., Stiening B., Böttger A. (2007). The Notch signaling pathway in the cnidarian Hydra. Developmental Biology 303: 376-390.
Kraus Y., Aman A., Technau U., Genikhovich G. (2016). Pre-bilaterian origin of the blastoporal axial organizer. Nature Communications 7: 11694.
Krishnapati L. S., Khade S., Trimbake D., Patwardhan R., Nadimpalli S. K., Ghaskadbi S. (2020). Differential expression of BMP inhibitors gremlin and noggin in Hydra suggests distinct roles during budding and patterning of tentacles. Developmental Dynamics 249: 1470-1485.
Liongue C., Ward A. C. (2013). Evolution of the JAK-STAT pathway. JAK-STAT 2: e22756.
Leclère L., Rentzsch F. (2014). RGM Regulates BMP-Mediated Secondary Axis Formation in the Sea Anemone Nematostella vectensis. Cell Reports 9: 1921-1930.
Levin M., Johnson R. L., Sterna C. D., Kuehn M., Tabin C. (1995). A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82: 803-814.
Levin M. (1997). Left‐right asymmetry in vertebrate embryogenesis. BioEssays 19: 287-296.
Li G., Liu X., Xing C., Zhang H., Shimeld S. M., Wang Y. (2017). Cerberus–Nodal–Lefty–Pitx signaling cascade controls left – right asymmetry in amphioxus. Proceedings of the National Academy of Sciences 114: 3684-3689.
Marqués G., Musacchio M., Shimell M. J., Wünnenberg-Stapleton K., Cho K. W.Y., O'Connor M. B. (1997). Production of a DPP Activity Gradient in the Early Drosophila Embryo through the Opposing Actions of the SOG and TLD Proteins. Cell 91: 417-426.
Martin B. L., Kimelman D. (2009). Wnt Signaling and the Evolution of Embryonic Posterior Development. Current Biology 19: R215-R219.
Martín-Durán J. M., Janssen R., Wennberg S., Budd G. E., Hejnol A. (2012). Deuterostomic Development in the Protostome Priapulus caudatus. Current Biology 22: 2161-2166.
Martindale M. Q. (2013). Evolution of Development: The Details Are in the Entrails. Current Biology 23: R25-R28.
Massagué J., Blain S. W., Lo R. S. (2000). TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 103: 295-309.
Matus D. Q., Pang K., Marlow H., Dunn C. W., Thomsen G. H., Martindale M. Q. (2006). Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proceedings of the National Academy of Sciences 103: 11195-11200.
Meinhardt H. (2002). The radial‐symmetric hydra and the evolution of the bilateral body plan: an old body became a young brain. BioEssays 24: 185-191.
Meinhardt H. (2006). Primary body axes of vertebrates: Generation of a near‐Cartesian coordinate system and the role of Spemann‐type organizer. Developmental Dynamics 235: 2907-2919.
Meinhardt H. (2012). Modeling pattern formation in hydra: a route to understanding essential steps in development. The International Journal of Developmental Biology 56: 447-462.
Miller M. A., Steele R. E. (2000). Lemon Encodes an Unusual Receptor Protein-Tyrosine Kinase Expressed during Gametogenesis in Hydra. Developmental Biology 224: 286-298.
Momose T., Houliston E. (2007). Two Oppositely Localised Frizzled RNAs as Axis Determinants in a Cnidarian Embryo. PLoS Biology 5: e70.
Momose T., Derelle R., Houliston E. (2008). A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development 135: 2105-2113.
Müller W. A., (1996). Head formation at the basal end and mirror-image pattern duplication in Hydra vulgaris. The International journal of developmental biology 40: 1119-1131.
Nakamura Y., Tsiairis C. D., Özbek S., Holstein T. W. (2011). Autoregulatory and repressive inputs localize Hydra Wnt3 to the head organizer. Proceedings of the National Academy of Sciences 108: 9137-9142.
Nolan K., Kattamuri C., Rankin S. A., Read R. J., Zorn A. M., Thompson T. B. (2016). Structure of Gremlin-2 in Complex with GDF5 Gives Insight into DAN-Family-Mediated BMP Antagonism. Cell Reports 16: 2077-2086.
Ozaki T., Sakiyama S. (1993). Molecular cloning and characterization of a cDNA showing negative regulation in v-src-transformed 3Y1 rat fibroblasts.. Proceedings of the National Academy of Sciences 90: 2593-2597.
Petersen C. P., Reddien P. W. (2009). Wnt Signaling and the Polarity of the Primary Body Axis. Cell 139: 1056-1068.
Petersen H. O., Höger S. K., Looso M., Lengfeld T., Kuhn A., Warnken U., Nishimiya-Fujisawa C., Schnölzer M., Krüger M., Özbek S., Simakov O., Holstein T. W., (2015). A Comprehensive Transcriptomic and Proteomic Analysis of Hydra Head Regeneration. Molecular biology and evolution 32: 1928-1947.
Piccolo S., Sasai Y., Lu B., De Robertis E. M. (1996). Dorsoventral Patterning in Xenopus: Inhibition of Ventral Signals by Direct Binding of Chordin to BMP-4. Cell 86: 589-598.
Piccolo S., Agius E., Leyns L., Bhattacharyya S., Grunz H., Bouwmeester T., Robertis E. M. D. (1999). The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397: 707-710.
Putnam N. H., Srivastava M., Hellsten U., Dirks B., Chapman J., Salamov A., Terry A., Shapiro H., Lindquist E., Kapitonov V. V., Jurka J., Genikhovich G., Grigoriev I. V., Lucas S. M., Steele R. E., Finnerty J. R., Technau U., Martindale M. Q., Rokhsar D. S. (2007). Sea Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organization. Science 317: 86-94.
Reddy P. C., Gungi A., Ubhe S., Pradhan S. J., Kolte A., Galande S., (2019). Molecular signature of an ancient organizer regulated by Wnt/β-catenin signalling during primary body axis patterning in Hydra. Communications biology 2: 434.
Reinhardt B., Broun M., Blitz I. L., Bode H. R. (2004). HyBMP5-8b , a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Developmental Biology 267: 43-59.
Rentzsch F., Anton R., Saina M., Hammerschmidt M., Holstein T. W., Technau U. (2006). Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: Implications for the evolution of axial patterning. Developmental Biology 296: 375-387.
Rentzsch F., Guder C., Vocke D., Hobmayer B., Holstein T. W. (2007). An ancient chordin-like gene in organizer formation of Hydra. Proceedings of the National Academy of Sciences 104: 3249-3254.
Saina M., Genikhovich G., Renfer E., Technau U. (2009). BMPs and Chordin regulate patterning of the directive axis in a sea anemone. Proceedings of the National Academy of Sciences 106: 18592-18597.
Samuel G., Miller D., Saint R. (2001). Conservation of a DPP/BMP signaling pathway in the nonbilateral cnidarian Acropora millepora. Evolution & Development 3: 241-250.
Sarras M. P. Jr. (2012). Components, structure, biogenesis and function of the Hydra extracellular matrix in regeneration, pattern formation and cell differentiation. The International Journal of Developmental Biology 56: 567-576.
Sarras M. P. Jr., Zhang X., Huff J. K., Accavitti M. A., St. John P.L., Abrahamson D. R. (1993). Extracellular Matrix (Mesoglea) of Hydra vulgaris. Developmental Biology 157: 383-398.
Sarras M. P. Jr., Yan L., Grens A., Zhang X., Agbas A., Huff J. K., St. John P.L., Abrahamson D. R. (1994). Cloning and Biological Function of Laminin in Hydra vulgaris. Developmental Biology 164: 312-324.
Sasai Y. (1994). Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79: 779-790.
Schaffer A. A., Bazarsky M., Levy K., Chalifa-Caspi V., Gat U. (2016). A transcriptional time-course analysis of oral vs. aboral whole-body regeneration in the Sea anemone Nematostella vectensis. BMC Genomics 17: 718.
Schier A. F. (2003). Nodal Signaling in Vertebrate Development. Annual Review of Cell and Developmental Biology 19: 589-621.
Siebert S., Farrell J. A., Cazet J. F., Abeykoon Y., Primack A. S., Schnitzler C. E., Juliano C. E. (2019). Stem cell differentiation trajectories in Hydra resolved at single-cell resolution. Science 365: eaav9314.
Spemann H., Mangold H. (2001). Induction of embryonic primordia by implantation of organizers from a different species. 1923 [Originally published in Archiv für Mikroskopische Anatomie und Entwicklungsmechanik, 100 (1924): 599-563].. The International Journal of Developmental Biology 45: 13-38.
Streit A., Stern C. D. (1999). Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mechanisms of Development 82: 51-66.
Suga H., Ono K., Miyata T. (1999). Multiple TGF‐β receptor related genes in sponge and ancient gene duplications before the parazoan–eumetazoan split 1. FEBS Letters 453: 346-350.
Technau U., Bode H. R. (1999). HyBra1 , a Brachyury homologue, acts during head formation in Hydra. Development 126: 999-1010.
Technau U., Scholz C. B., (2003). Origin and evolution of endoderm and mesoderm. International Journal of Developmental Biology 47: 531-539.
Technau U., Rudd S., Maxwell P., Gordon P. M.K., Saina M., Grasso L. C., Hayward D. C., Sensen C. W., Saint R., Holstein T. W., Ball E. E., Miller D. J. (2005). Maintenance of ancestral complexity and non-metazoan genes in two basal cnidarians. Trends in Genetics 21: 633-639.
Technau U. (2020). Gastrulation and germ layer formation in the sea anemone Nematostella vectensis and other cnidarians. Mechanisms of Development 163: 103628.
Thompson T. B., Lerch T. F., Cook R. W., Woodruff T. K., Jardetzky T. S. (2005). The Structure of the Follistatin:Activin Complex Reveals Antagonism of Both Type I and Type II Receptor Binding. Developmental Cell 9: 535-543.
Trompouki E., Bowman T. V., Lawton L. N., Fan Z. P., Wu D.C., DiBiase A., Martin C. S., Cech J. N., Sessa A. K., Leblanc J. L., Li P., Durand E. M., Mosimann C., Heffner G. C., Daley G. Q., Paulson R. F., Young R. A., Zon L. I. (2011). Lineage Regulators Direct BMP and Wnt Pathways to Cell-Specific Programs during Differentiation and Regeneration. Cell 147: 577-589.
Vandenberg L. N., Levin M. (2013). A unified model for left–right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Developmental Biology 379: 1-15.
Veschgini M., Suzuki R., Kling S., Petersen H. O., Bergheim B. G., Abuillan W., Linke P., Kaufmann S., Burghammer M., Engel U., Stein F., Özbek S., Holstein T. W., Tanaka M. (2023). Wnt/β-catenin signaling induces axial elasticity patterns of Hydra extracellular matrix. iScience 26: 106416.
Wang R., Steele R. E., Collins E.M. S. (2020). Wnt signaling determines body axis polarity in regenerating Hydra tissue fragments. Developmental Biology 467: 88-94.
Watanabe H., Schmidt H. A., Kuhn A., Höger S. K., Kocagöz Y., Laumann-Lipp N., Özbek S., Holstein T. W. (2014a). Nodal signalling determines biradial asymmetry in Hydra. Nature 515: 112-115.
Watanabe H., Kuhn A., Fushiki M., Agata K., Özbek S., Fujisawa T., Holstein T. W. (2014b). Sequential actions of β-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nature Communications 5: 5536.
Wharton K., Derynck R. (2009). TGFβ family signaling: novel insights in development and disease. Development 136: 3691-3697.
Windsor Reid P. J., Matveev E., McClymont A., Posfai D., Hill A. L., Leys S. P., (2018). Wnt signaling and polarity in freshwater sponges. BMC evolutionary biology 18: 12.
Xia Y., Schneyer A. L. (2009). The biology of activin: recent advances in structure, regulation and function. Journal of Endocrinology 202: 1-12.
Xie T., Spradling A. C. (1998). decapentaplegic Is Essential for the Maintenance and Division of Germline Stem Cells in the Drosophila Ovary. Cell 94: 251-260.
Ying S.Y., Zhang Z., Furst B., Batres Y., Huang G., Li G. (1997). Activins and Activin Receptors in Cell Growth. Experimental Biology and Medicine 214: 114-122.
Yokouchi Y., Vogan K. J., Pearse R. V., Tabin C. J. (1999). Antagonistic Signaling by Caronte , a Novel Cerberus -Related Gene, Establishes Left–Right Asymmetric Gene Expression. Cell 98: 573-583.
Zhang X., Fei K., Agbas A., Yan L., Zhang J., O'Reilly B., Deutzmann R., Sarras M. P. Jr. (2002). Structure and function of an early divergent form of laminin in hydra: a structurally conserved ECM component that is essential for epithelial morphogenesis. Development Genes and Evolution 212: 159-172.
Zhang J., Li L. (2005). BMP signaling and stem cell regulation. Developmental Biology 284: 1-11.