Int. J. Dev. Biol. 68: 65 - 78 (2024)
Molecular signaling directing neural plate border formation
Open Access | Review | Published: 9 July 2024
Abstract
During embryonic development, the vertebrate embryonic epiblast is divided into two parts including neural and superficial ectoderm. The neural plate border (NPB) is a narrow transitional area which locates between these parts and contains multipotent progenitor cells. Despite its small size, the cellular heterogeneity in this region produces specific differentiated cells. Signaling pathways, transcription factors, and the expression/repression of certain genes are directly involved in these differentiation processes. Different factors such as the Wnt signaling cascade, fibroblast growth factor (FGF), bone morphogenetic protein (BMP) signaling, and Notch, which are involved in various stages of the growth, proliferation, and differentiation of embryonic cells, are also involved in the determination and differentiation of neural plate border stem cells. Therefore, it is essential to consider the interactions and temporospatial coordination related to cells, tissues, and adjacent structures. This review examines our present knowledge of the formation of the neural plate border and emphasizes the requirement for interaction between different signaling pathways, including the BMP and Wnt cascades, the expression of its special target genes and their regulations, and the precise tissue crosstalk which defines the neural crest fate in the ectoderm at the early human embryonic stages.
Keywords
neural plate, neural plate border, neural crest cell, signaling pathway
Introduction
The human embryonic period, which occurs during the first 8 weeks after fertilization, is categorized using a morphological system. This system consists of 23 distinct Carnegie stages, with each stage representing a span of 2 to 3 days. The purpose of this staging system is to facilitate an understanding of the timing and sequence of embryonic development. The fetal period begins at the 9th week after fertilization and continues until birth. Fetal age is primarily determined through measurements as there is no equivalent morphological staging system available (Flierman et al., 2023). Gastrulation and formation of the three main germ layers are the major events during the 3rd week of embryo development (Sauka-Spengler and Bronner-Fraser, 2008a, Sauka-Spengler and Bronner-Fraser, 2008b). One key structure that develops during this period is the neural tube, which eventually forms the brain and spinal cord. In initial neurulation process, the neural tube is formed from the neural plate (Ravi et al., 2021). At the beginning of the third week, the central part of the ectoderm that is located on the developing notochord thickens and forms the neural plate or neuroectoderm. While the rest of the ectoderms forms surface ectoderm. The formation of neural crest cells determines the border between these two areas. This border has a very high sensitivity in terms of evolution because it organizes and forms various structures such as the formation of placodal derivatives, the development of skull bones, the formation of nerve tissues, and other structures (Grocott et al., 2012). The complete development and closure of the neural tube takes place between days 17 and 30 after gestation, equivalent Carnegie stages 8 to 12 in the embryonic period. The caudal eminence, an extension of the primitive streak, gives rise to various structures including the notochord, somites, vertebrae, and hindgut. From the caudal eminence, the neural cord emerges and forms the caudal part of the spinal cord. This process is known as secondary neurulation (Catala, 2020). What is involved in this process is the presence of huge gene regulatory networks (GRNs) that drive cells toward differentiation (Williams et al., 2022). However, the only available evidence to determine this border is the time of neurulation and their separation during neural tube formation (Williams et al., 2022, Yardley and García-Castro, 2012). In many studies, it has been reported that any defect in the formation, migration, and lack of regulation of cell division of these border cells leads to birth defects in the fetus (Siismets and Hatch, 2020, Gandhi et al., 2020). On the other hand, these border cells are affected by various factors in each region along the neural plate, and this causes their differentiation into specialized cells, and the absence of these factors in a specific region causes the formation of specialized cells in that region (Schille and Schambony, 2017).
Here, this study aims to investigate the factors affecting the border of the neural plate and determine the role of the factors affecting its formation. Several studies related to the examination of the neural plate border (NPB) have been published recently, and therefore, in addition to focusing on presenting their important and useful content, our attention has been on finding answers to the questions in the minds of researchers about the NPB.
An overview of genes involved in determining the boundary between neural and surface neural ectoderm
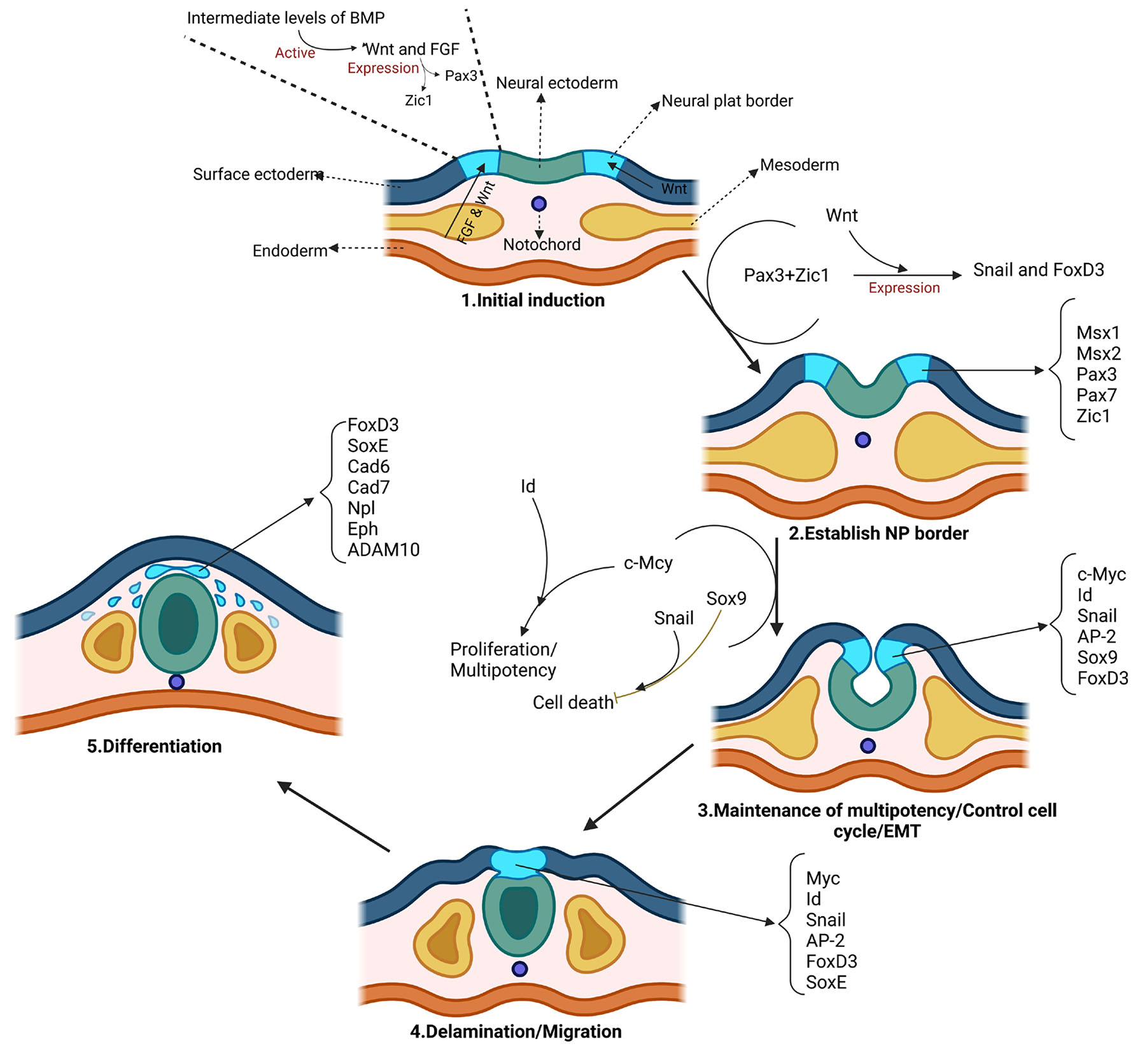
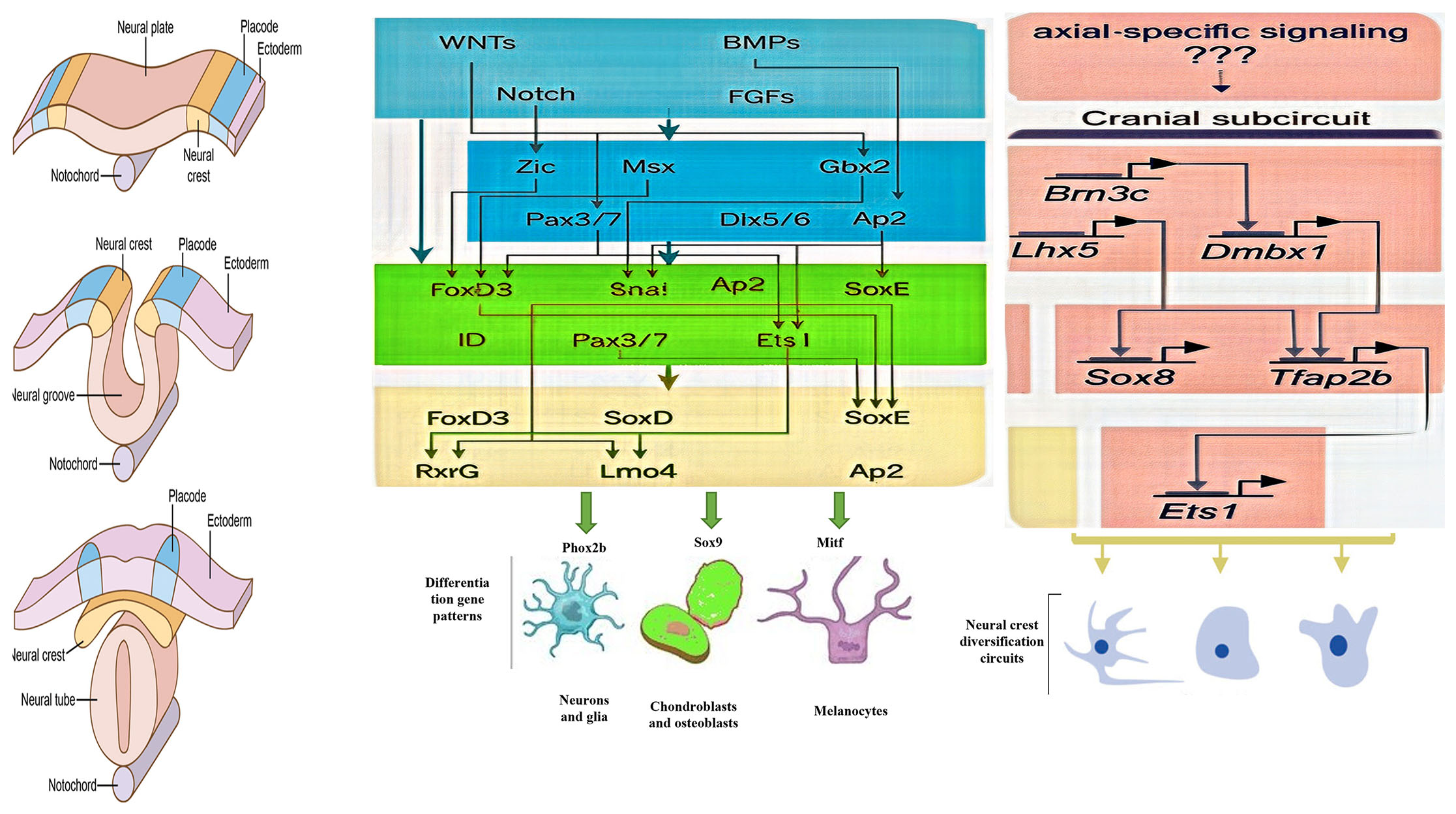
Delving into the molecular mechanisms that drive neurulation process is vital for deciphering the intricacies of embryonic development and organogenesis in vertebrates (Mirdass et al., 2023). According to the evidence and data available from previous studies, the development of neural crest cells from the beginning to the formation of specialized cells can be divided into 5 stages. These stages include initial induction, establishment of neural plate (NP) border, maintenance of multipotency; control of cell cycle and epithelial to mesenchymal transition (EMT); delamination; migration, and differentiation (Fig. 1). Although these stages are serial, the noteworthy point is that each stage is under the control of special regulators (Prasad et al., 2019).
These steps are involved in the fate determination of neural crest cells, along with several signaling factors. In the initial stage, with the secretion of factors such as Delta, Wnt, FGF, and BMP in the third week, it leads to the determination of neural crest cells (Tang et al., 2023, Roure et al., 2023). The effects of these factors in the next stage led to the expression of Msx, Pax3/7, Zic1, and Dlx3/5 factors at the border between neural ectoderm and superficial ectoderm. These signaling molecules in this border region led to the formation and specialization of neural crest cells. Neural plate boundary markers lead to the expression of signaling molecules and neural crest-determining genes such as Snail/Slug, AP-2, FoxD3, Twist, Id, cMyc, and Sox9/10. In the developmental process, a sequence of events occurs that enables neural crest cells to sustain their population through cell division and react to their surroundings by producing different cell receptor molecules, junctions, and metalloproteases (Sauka-Spengler and Bronner-Fraser, 2008a). Finally, the neural crest cells also migrate and differentiate into different cell types under the influence of this set of signaling factors. SoxE genes are one of gene specifier that play a significant role both in the early stages of neural crest formation and in the stages of differentiation into cartilage, neurons, and glia (Kelsh, 2006). It is not yet known precisely how the molecules that affect the growth, specialization, and movement of neural crest cells interact with each other. What is important to note is that there is no specific gene responsible for neural crest formation. Instead, it is a combination of these signaling molecules and other factors at specific times and places that directs this complex process (Méndez-Maldonado et al., 2020).
Fig. 1. Regulatory steps in the formation of the border between neural ectoderm and surface ectoderm.
Induction is initiated by signaling molecules such as fibroblast growth factor (FGF) from the underlying mesoderm, as well as Wnts from the mesoderm and the adjacent non-neural ectoderm at the border between the neural ectoderm and the surface ectoderm. Each of these molecules, if the bone morphogenetic protein (BMP) level is moderate, separately, or in interaction with each other, causes the expression of Pax3 and Zic1 molecules, which are responsible for determining the border between neural ectoderm and surface ectoderm. Pax3 and Zic1, dependent on Wnt, synergistically activate Snai and FoxD3 which are neural crest (NC) specifiers. These molecular interactions are the result of studies on Xenopus laevis, so these events may not be true for other organisms. The c-Myc-Id cassette is one of the molecules involved in the cell cycle that may play an important role in determining and maintaining the multipotent state. Sox9 can maintain trunk NC precursors by affecting anti-apoptotic factors such as Snai. The specific expression of early NC markers in the progenitor NC population causes their separation from the dorsal neuroepithelium. Moreover, these factors are involved in the regulation of many processes such as cell proliferation, stratification, and the initiation of epithelial-to-mesenchymal transition (EMT). FoxD3 and Sox10 play a role in the migration and delaminating of neural crest cells and also regulation of other factors such as Cad7, MMPs, ADAM10, Npl, and Eph. Abbreviations: NP, neural plate; Cad7, cadherin‑7; MMPs, matrix metalloproteases; ADAM10, a disintegrin and metalloprotease-10; Npl, neuropilins; Eph, ephrin type-A receptor 1.
The most important signaling factors in the determination of neural crest cells between neural and surface ectoderm
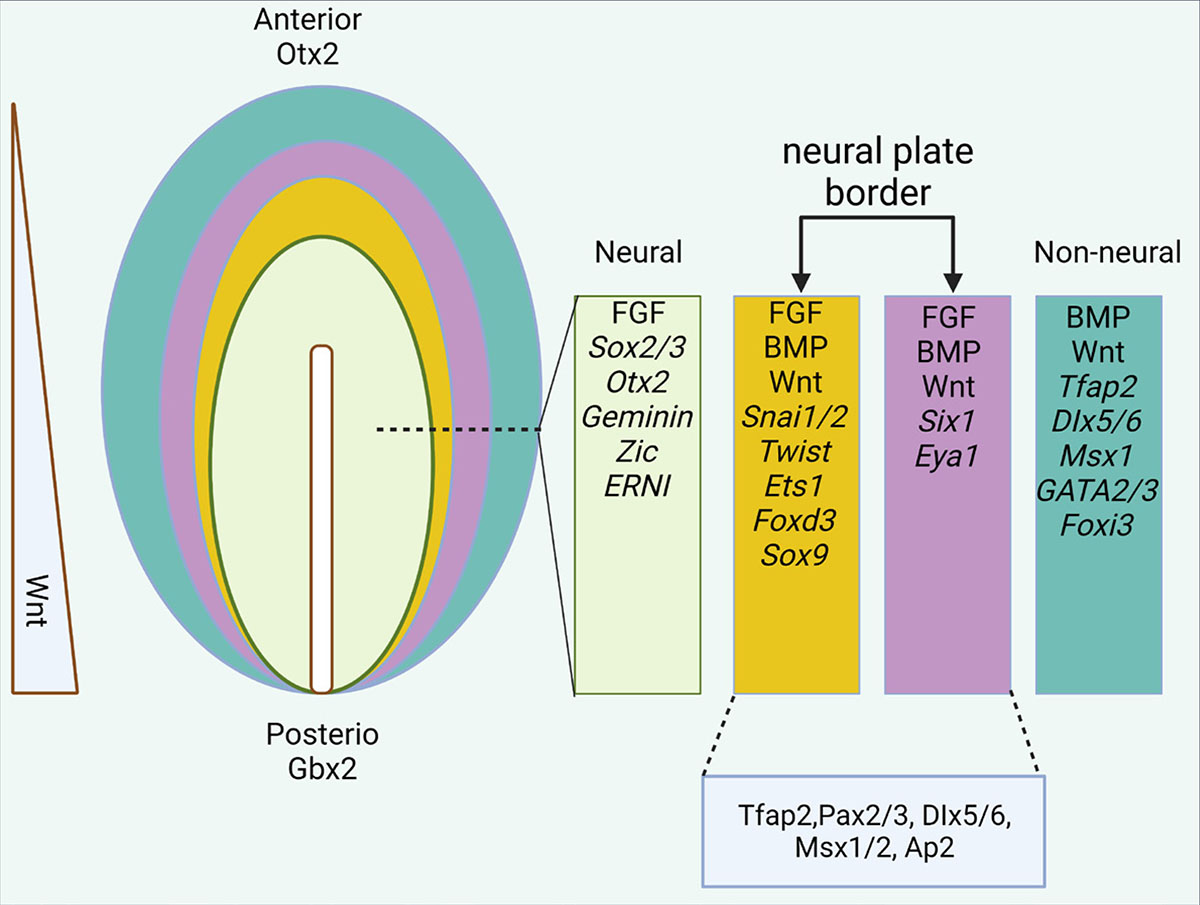
During embryonic development in many vertebrates, the main time for the formation of neural and surface ectoderm cells is before the gastrulation initiation. The dorsal ectoderm plays a crucial role in inducing the formation of the neural plate through the expression of genes such as Otx2, Sox3, ERNI, and Geminin. These genes are considered the most important signaling factors in determining neural crest cells between neural and surface ectoderm. (Bally-Cuif et al., 1995, Rex et al., 1997). In addition to the previously mentioned genes, other molecules such as FGFs, Wnt, and BMP are crucial in inducing the formation of the neural plate. These molecules play a significant role in the expression of pre-neural genes. (Stern and Downs, 2012, Rogers et al., 2011, Albazerchi and Stern, 2007). In regions of the epiblast where Wnt and BMP signals are active, they induce the expression of non-neuronal markers, including genes from Ap2, Dlx, Foxi, Gata2/3, and Msx transcription factor families (Fig. 2) (Pieper et al., 2012, Li and Cornell, 2007, Hoffman et al., 2007, Hans et al., 2007, Phillips et al., 2006). In Xenopus, it is proposed that the dorsolateral marginal zone (DLMZ) of the gastrula, which is located beneath the prospective neural crest, serves as the source of neural crest-inducing signals. The DLMZ expresses various Wnt and FGF ligands, as well as the BMP antagonist Chordin, all of which are known to play a role in neural crest induction. Additionally, the DLMZ expresses several other regulators of Wnt and BMP signaling, including Noggin, Cerberus, Frzb1, Dkk1, Sfrp2, and Crescent (Alasaadi et al., 2024). A recent study has also highlighted the importance of Snai2 in mesoderm formation and its involvement in regulating the signals originating from the DLMZ, thus making Snai2 a crucial factor in early neural crest development. As development progresses to the neurula stage, the DLMZ gives rise to the paraxial or intermediate mesoderm, which underlies the proper formation of the neural crest. Recombination experiments involving the DLMZ and animal caps, as well as grafts of the paraxial mesoderm into ventral epidermis, have shown the expression of neural crest markers (Li et al., 2019).
It is hypothesized that the interaction between the neural and surface ectoderm is necessary for the formation of the neural border (Groves and LaBonne, 2014). Experimental studies have shown that the grafting of neural plate cells on the surface ectoderm causes the formation of neural crest and placode cells (Selleck and Bronner-Fraser, 1995). In addition, Wnt, BMP, and FGF signals are necessary for the formation of the NPB (Yardley and García-Castro, 2012, Endo et al., 2002). In vitro observations have shown that mesodermal cells play an important role in the induction of neural ectoderm (Brugmann et al., 2004). Reports have shown that the hypoblast alone is not responsible for the formation of the neural border, but the inductive role of the underlying mesoderm cannot be ignored (Albazerchi and Stern, 2007, Richard et al., 2016). In the third week of embryonic development, the neural and surface ectoderm domains are specified (Meulemans and Bronner-Fraser, 2002, Sauka-Spengler and Bronner-Fraser, 2008b). As a result of inducing responses caused by the presence of factors such as Wnts and BMPs, it causes the expression of Tfap2, Pax3/7, Dlx3/5, and Msx1/2 factors at the NPB (Fig. 2) (Moody and LaMantia, 2015).
NPB progenitor cells are created in response to FGF, BMP, Wnt, and retinoic acid (RA) and finally differentiate into the neural crest and placodal ectodermal cells (Grocott et al., 2012, Sauka-Spengler and Bronner-Fraser, 2008a). Reports have shown that NPB expresses moderate levels of BMP signaling that along with levels of BMP agonists, cause their differentiation into specialized cells (de Crozé et al., 2011, Garnett et al., 2012). For example, in animal studies, it was found that high levels of the BMP antagonist, Noggin, cause the cells to differentiate towards the placode, while medium and high levels of Noggin cause the cells to differentiate towards the neural crest and neural plate, respectively (Hong and Saint-Jeannet, 2007, Park and Saint-Jeannet, 2008). Research on neural crest development has been conducted in various species, with Xenopus and chick studies providing valuable insights into the earliest inductive signaling events. Recent findings from these model organisms propose a two-step process for neural crest induction, involving FGF and Wnt signaling during gastrulation followed by Wnt and BMP signaling during neurulation to maintain the neural crest population. Despite the conservation of signaling pathways across species, differences exist in the source, timing, and regulation of neural crest development (Prasad et al., 2019, Rodrigues-Da-Silva et al., 2022). Understanding the diverse mechanisms underlying neural crest development is crucial for the advancement of clinical diagnostic and therapeutic strategies. because disruptions in neural crest development can lead to a range of severe human health conditions known as neurocristopathies, including malignant tumors like melanomas and neuroblastomas, rare syndromes such as Hirschsprung and Waardenburg syndromes, and structural abnormalities like cleft lip/palate and aganglionic megacolon. The expression patterns of neural crest markers at the beginning varies among different species. In Xenopus, most neural crest genes (Snai2, FoxD3, and Sox8/9) are first expressed shortly after the appearance of the NPB and before gastrulation is complete.
However, in the chick, Snai2 is first observed at a later stage (stage 6) and not strongly expressed until several hours after the NPB has formed (stage 8 also named as 4-somite stage) (Ponzoni et al., 2022, Ben Amar et al., 2022). Distinctive neural crest cells expressing a full complement of neural crest markers are not apparent until just before migration at stage 9/10. Despite these differences, the avian neural crest seems to be specified before gastrulation, while Xenopus neural folds do not maintain expression of neural crest markers without additional signals. In zebrafish, FoxD3 is expressed first and has a unique role early in gastrulation, while other neural crest markers label the neural crest towards the end of gastrulation. In mice, neural crest markers such as Sox9/10 and FoxD3 label the neural folds soon after the expression of NPB markers and before cranial neural crest migration. Other experiments, on the other hand, proposed a different mechanism for the induction of neural crest cells involving the interaction between neural and non-neural ectoderm (Rocha et al., 2020). Stuhlmiller conducted experiments using two different species of amphibians and demonstrated that when neural and epidermal tissues were placed in close proximity, they were able to generate neural crest cells (Stuhlmiller and García-Castro, 2012a). This finding was later supported by Pieper, who used pigmented and non-pigmented axolotl embryos in donor/host combinations and showed that both neural and epidermal tissues could give rise to neural crest cells (Pieper et al., 2012). Similar experiments conducted in Xenopus and chick, where neural tissue was grafted into lateral epidermis, also confirmed that both tissues were capable of producing neural crest cells in these species. Additionally, a recent study in Xenopus suggested that the neural non-neural ectoderm (NNE) loses its ability to generate neural crest cells towards the end of gastrulation, while the neural plate (NP) retains its competence until neurulation. These findings collectively supported a model in which neural crest induction occurs through interactions between the NP, NNE, and underlying mesoderm (Gouignard et al., 2021). Six1 and Eya1 play an important role in fate determining of the region referred to as the pre-placodal region (PPR). On the other hand, the expression level of Six1 has a direct effect on determining the cell fate towards the neural crest or placode. PPR contains undifferentiated placodal progenitors. Initially, certain genes like Foxi3 and Gata3 are broadly expressed in the non-neural ectoderm but eventually refine to the PPR (Sullivan et al., 2019). On the other hand, genes belonging to the Six and Eya family are newly expressed in the anterior NPB region, which extends from the first pair of somites to the most anterior regions of the neural plate. Subsequently, genes specific to sub- populations of placodes emerge in this region in response to local inducing signals. For instance, Pax2/8 genes appear in the otic and epibranchial placode region, Pax3 in the ophthalmic trigeminal ganglion, and Pax6 in the future lens and olfactory placodes. Otx2 and Gbx2 are expressed in the anterior and posterior epiblast, respectively, and their expression patterns are maintained through mutual repression during the induction and patterning of the neural plate. Ultimately, this process helps establish the boundary between the midbrain and hindbrain. As development progresses, additional changes occur in this region (Thawani and Groves, 2020, Seal and Monsoro-Burq, 2020).
The interaction of Eya and Six proteins causes a change in the function of the Six transcription factor with DNA (Patrick et al., 2013). FGF signaling induces PPR formation, while deletion of FGF signaling can increase or decrease PPR through the presence of Wnt and BMP signaling. In addition, the presence of FGF signaling is necessary for the expression of genes such as Six and Eya (Fig. 2) (Hintze et al., 2017, Litsiou et al., 2005).
A set of gene expressions including FoxD3, Snai1/2, Twist, Sox10, and Ets1 in the inner part of NPB causes the formation of neural crest cells (Fig. 2) (Barembaum and Bronner, 2013, Simões-Costa et al., 2012). Factors such as FoxD3, Pax3/7, and Msx1 determine the identity of neural crest cells in the head and trunk region (Simões-Costa et al., 2012). Pax3/7 interacts directly with Zic1, which, depending on the species, eventually leads to the activation of Snai1/2 and the expression of Ets1 (Plouhinec et al., 2014, Simões-Costa et al., 2014, Milet et al., 2013).
Pattern of the neural plate border formation
As soon as the neural tube is closed, the neural crest cells, which are located on the dorsal midline of the neural tube, begin to migrate. Therefore, under the EMT influence, the neural crest cells begin to separate and spread throughout the body of the embryo and differentiate. At this stage, neural crest cells begin to express genes involved in migration such as Sox10, Sox9, FoxD3, and Ets1, and on the contrary, they reduce or silence the expression of mesenchymal to epithelial state markers such as N-cadherin, E-cadherin, Cadherin11 and Cadherin7 (Simões-Costa and Bronner, 2016, Kashef et al., 2009).
During gastrulation, molecular asymmetry occurs along the rostrocaudal axis. In the epiblast region, Otx2 and Gbx2 genes are expressed in the anterior and posterior axes, respectively. Maintenance of this pattern occurs through bilateral suppression as the neural plate is induced and formed. At this stage, the border between the midbrain and the hindbrain was determined (Fig. 2). Pax6 and Pax2 gene expression occur in the anterior region of the forebrain and midbrain, respectively, while Six1 and Irx3 are exclusively expressed in the forebrain. The formation pattern of anterior-posterior PPR also occurs similarly. Hence, the expression of Otx2 and Gbx2 genes in these areas is necessary for developing the trigeminal and otic placode, respectively (Steventon et al., 2012). Pax family genes such as Pax6, Pax3, and Pax2/8 are expressed along the anterior-posterior axis which is involved in the developmental stages of placodal derivatives (Saint-Jeannet and Moody, 2014, Koontz et al., 2023).
On the contrary, Hox family genes are expressed in the neural plate region posterior to rhombomere 1 of the hindbrain, while no expression of them is seen in the pre-placodal ectoderm region. Since the neural crest cells located in the hindbrain begin to migrate and enter the pharyngeal arches, they do not necessarily express Hox family genes in the pharyngeal arches. For example, neural crest cells located in the first pharyngeal arch do not express any Hox family genes, while cells located in the second pharyngeal arch express Hoxa2 and Hoxb2 genes (Parker et al., 2018). Reports have shown that cultured migrating neural crest cells have the ability to express Hox family genes, while the factors and signals that caused their non-expression in the pre-placodal ectoderm region have not yet been identified (Trainor and Krumlauf, 2000). However, the investigation of factors affecting the expression of Hox genes in neural crest cells continues, and understanding how the expression of these genes is silenced in the placodal region is welcomed by researchers (Trainor and Krumlauf, 2000).
Researchers have been trying to understand why neural crest cells are not formed in the more anterior regions of the neural plate. Studies have shown that olfactory placode cells derived from the anterior neural fold can differentiate into the epidermis, olfactory placode, olfactory bulb, and forebrain, but not into neural crest cells (Parker et al., 2018). Furthermore, studies have shown that the anterior neural fold can differentiate into some neural crest cells when transplanted to the rostral hindbrain (Torres-Paz et al., 2021, Ezin et al., 2014). However, the induction signals for placodal formation and the lack of neural crest cells in the anterior region of the neural plate have not been fully described. Researchers suggest that signals such as Wnt, BMP, and retinoic acid may play an essential role in this pathway (Villanueva et al., 2002). Some studies have also shown that inactivating the Wnt antagonist gene expression, Dkk, and some members of Tcf/Lef in the anterior region of the epiblast can induce neural crest cells (Mašek et al., 2016). The visualization of the NPB formation can be achieved through techniques such as immunostaining or in situ hybridization, which utilize markers specific to different border derivatives. These techniques reveal that at initial stages the boundaries between cells differentiating into various derivatives are imprecise. However, over time, these boundaries become more distinct and form clear domains. This process of self-organization and refinement has been observed not only in the NPB but also in micropatterned cultures of embryonic stem cells (Pla and Monsoro-Burq, 2018). With the help of Hybridization Chain Reaction and in vivo transcriptomic techniques, such as Multiplexed Error-Robust Fluorescence in situ Hybridization (MERFISH), visualization of border genes in response to high concentrations of activin is becoming more feasible (Pajanoja et al., 2023, Choi et al., 2018).
With the advent of single cell technologies, it is now possible to compare the epigenetic and transcriptional states of stem cells as they transition into neural crest cells. This will help to determine which GRNs governing pluripotency are present in pre-migratory and migratory neural crest cells, and even neural crest-like peripheral glia stem cells (Erickson et al., 2023). The NPB region of the chordates has only a few cellular thickness, and techniques like Slide-seq and MERFISH may provide higher resolution spatial information on the cellular transcriptome during border development. The former technique transfers the single cell thick tissue onto a sequencing grid to analyze single cell RNA while the latter method uses a multichannel in situ hybridization (ISH) technique to probe the same sections for thousands of RNA transcripts. Both methods provide a spatial context to the transcriptomic profiles. These techniques can be used to identify the cellular identities at the NPB region where the four ectodermal lineages are intermingled over just a few cell diameters, depending on their cellular resolution (Thawani and Groves, 2020, Yao et al., 2023). Single-cell RNA sequencing techniques can provide more detailed information on the levels of signaling and expression of downstream effectors, allowing for a deeper understanding of the interactions in signaling pathways at the NPB. Techniques such as multiple annealing and dC-tailing-based quantitative single-cell RNA-seq (MATQ-seq) and SMARTer single cell total RNA sequencing (SMARTer-seq) are highly sensitive but have lower throughput, making them ideal for a more detailed analysis of individual cells. For example, recent studies have used SMART-seq2 to understand the fate programs of neural crest cells and detected over 7,000 genes per cell, providing a better understanding of the transcriptomic decisions made by pre-migratory/migratory crest cells as they proceed towards sensory, glial, or mesenchymal fates. In addition to the transcriptomic status of the cells in developing NPB region it remains to be elucidated when the cells are fully committed to a lineage are fully committed to a particular lineage (Jovic et al., 2022). Epigenomic sequencing analysis, such as Assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq), can identify accessible genomic loci that are available for transcriptional activity (Buenrostro et al., 2015). Single cell ATAC-seq is now available and can be combined with scRNA-seq to identify relevant enhancers for lineage-specific transcription factors, evaluate plasticity of the cells and determine whether trans-differentiation is feasible from one ectodermal path to another (Jiang et al., 2023). For instance, Lukoseviciute et al., used single cell RNA-seq, ATAC-seq, and ChIP-seq data to identify a bimodal function for FoxD3, a key transcription factor that plays a crucial role in neural crest specification and differentiation. Their results revealed that FoxD3 activates cis-regulatory elements for neural crest specifier genes as an activator and represses mesenchymal and migratory programs at later stages to prevent premature differentiation (Lukoseviciute et al., 2018).
Over-expression and knockdown studies in various species have demonstrated cross-repressive interactions between transcription factors expressed in the early non-neural ectoderm and those expressed in the definitive neural plate. For instance, the over-expression of Dlx, Gata, Msx, Foxi, and Ap2 factors repress neural markers like Sox2, while knockdown of these genes expands the neural plate at the expense of non-neural ectoderm. Conversely, positive auto-regulatory interactions between non-neural genes can sharpen the boundary between neural and non-neural domains. Examples of such genes include Ap2c, Foxi1, and Gata2 (Lau et al., 2023). In experiments conducted on amphibian blastula, it was observed that knockdown or over-expression of certain neural crest transcriptional effectors, such as Snail or Sox5, led to a depletion of pluripotency-associated genes and reduced the cells' ability to form mesoderm. On the other hand, over-expression of transcription factors that induced a neural crest or NPB state, extended the cells' competence to form mesoderm and endoderm (Schock et al., 2023). It was also observed that NPB tissue could be induced to form endoderm. The potential for development of cells is regulated by FGF/MAPK signaling and a transition to PI3K/Akt signaling, along with the replacement of SoxB effectors with SoxE effectors leads to a more restricted developmental state (Pla and Monsoro-Burq, 2018).
Signaling pathways related to neural plate border formation
It seems that the differentiation of NPB cells is influenced by various signals which affect several transcription factors. According to research, the Tfap2a and Gbx2 genes are crucial in inducing and differentiating neural crest cells by triggering the expression of genes like msx1, pax3, and hes4 (de Crozé et al., 2011). Additionally, Tfap2a is essential for the formation of placode progenitors through Six1/Eya1 induction and neural crest via FoxD3 gene, while Gbx2 enables differentiation towards neural crest by inhibiting the expression of Six1 (Maharana and Schlosser, 2018). On the other hand, Gata2/3 and Foxi TFs induce differentiation towards placode progenitors by regulating the expression of Dlx3/5 and affecting the Six1 gene (Li et al., 2009). Studies have shown that Dlx3 and Dlx5 play a direct role in inducing the formation of placode progenitors by affecting Six1 (Hintze et al., 2017). In vivo studies have shown that Dlx3 and Dlx5 play a direct role in inducing the formation of placode progenitors by affecting Six1. Interestingly, Msx1 represses Six1 expression, thus inhibiting placode progenitor’s formation and inducing neural crest formation (Maharana and Schlosser, 2018). However, a recent study has reported that the expression of Six1/Eya1 genes requires the expression of Msx1, indicating a direct relationship between them, while the lack of control of Msx1 expression causes aberrant expression of Six1/Eya1 (Rothstein and Simões-Costa, 2020). Reports have also shown that the expression of genes such as Tfap2a and msx1 is necessary for the subsequent stages of neural crest development. Although these results may seem contradictory, they can be attributed to different stages of differentiation. In confirmation of this, reports have shown that the expression of genes such as Tfap2a and Msx1 is necessary in the next stages of neural crest development (Sato et al., 2010).
Pax3 and the more anteriorly localized Zic1 factor are considered to be the most important factors involved in the induction of neural crest and placode progenitors (Plouhinec et al., 2014, Bae et al., 2014). It has been reported in several studies that high levels of Pax3 and Zic1 expression cause the differentiation of ectodermal cells into glandular and placode progenitors, respectively, while the expression of both of them leads to differentiation into the neural crest. Zic1 interacts with Dlx3 to differentiate ectodermal cells into placode progenitors, while Pax3 expression directly results in the lack of expression of six1/eya1 (Maharana and Schlosser, 2018). Pax3/Zic1 in interaction with each other directly affects the expression of specific genes of neural crest cells (Plouhinec et al., 2014, Simões-Costa et al., 2014). Various studies have reported that in the gastrulation stage in the anterior neural border region, the expression of Zic1-positive/Pax3-negative causes the formation of placode progenitors, while the overlapping of Pax3 and Zic1 causes the formation of neural crest cells. In addition, there is a slight overlap between pax3/7-negative and Six1/Eya1-positive, which raises the question of how cells in these regions differentiate into different cell types (Roellig et al., 2017). Such questions can be solved with more studies in the future and taking into account temporal, morphogenetic, and species differences in the neurulation stage.
Several transcription factors that are involved in cell differentiation into neural crest/placode progenitors have been identified. Studies conducted on Xenopus have shown that the expression of Hes4 (hairy2b) and znf703 genes in the neural border region is necessary for differentiation into the neural crest. Hes4 controls the increased expression level of FoxD3, which induces pluripotency in neural crest cells. This mode of action activates the Notch/Delta signaling pathway and triggers Id3, which finally leads to the differentiation of neural crest cells (de Crozé et al., 2011, Nichane et al., 2008). Znf703 is a gene necessary for neural crest differentiation, targeting Pax3 and Zic1 genes (Hong and Saint‐Jeannet, 2017, Janesick et al., 2019). Several in vivo studies have demonstrated that the expression of Axud1, Pax7, and Msx1 is crucial for the formation of neural crest (Simões-Costa and Bronner, 2015, Azambuja and Simões-Costa, 2021). On the other hand, Znf462 and Pdlim4 have a direct impact on the expression of Foxi3 and Dlx5, respectively, which leads to the development of placode progenitors (Mohammadparast and Chang, 2022).
According to a recent study, it was found that Ash2L and Dpy30 are crucial in the development of neural crest. The study also revealed that Dpy30/Ash2L has a direct correlation with NPB transcription factors such as Msx1 and Tfap2a. These transcription factors activate/deactivate a series of molecular signals for their differentiation through the induction of Dpy30 and Ash2L in the defining regions of the neural crest (Mohammadparast and Chang, 2022). Another research team has reported that the transcription factor zinc finger of the cerebellum 1 (ZIC1) determines the fate of neural crest cells in the NPB. ZIC2, ZIC3, and ZIC5 transcription factors work together with FoxD3 to increase conventional Wnt activity at the border of the rodent neural plate. The function of ZIC proteins is greatly improved by SUMOylation. Conversely, the presence of basal ZIC proteins in the lateral regions of the neuroectoderm (a region of low canonical Wnt activity) represses Wnt/TCF-mediated transcription factors. Therefore, Wnt signaling and SUMOylation play an important role in the induction and differentiation of neural crest cells in the NPB (Bellchambers et al., 2021).
Cranial neural crest cells are another derivative of NPB. The development and differentiation of these cells depend on the crucial role played by Twist1 and Irf6. β/δ-catenins interaction with Twist1 leads to neural tube closure, while Irf6 determines the boundary of neural folds by limiting AP2α expression. Twist1 is also involved in the EMT and migration of cranial neural crest cells by repressing Irf6 and other factors. If Twist1 is suppressed, it prevents the migration of these cells and increases cell adhesion (Bertol et al., 2022).
According to a recent report, the cell population in the NPB is uniform during the gastrulation stage. However, this uniformity is disrupted during the neurulation stage, resulting in heterogeneity on the outer side of the border. This heterogeneity is caused by the expression of Pax7, which initiates cell differentiation (Williams et al., 2022). The report also suggests that knocking out Pax3/7 can lead to defects in neural tube closure and damage to Motor Ganglion neuron specification (Kim et al., 2022).
Folate, which is the precursor of S-adenosylmethionine, plays a crucial role in determining the fate of NPB cells. Since this type of folate has a significant impact on DNA methylation. The methylation changes that occur in specific genomic regions due to Folate Carrier 1 (RFC1) deficiency can disrupt early developmental pathways such as Notch1 and BMP4 signaling according to recent reports in this field. These interactions have a direct effect on the establishment and connection of progenitor cells in the neural plate and NPB (Alata Jimenez and Strobl-Mazzulla, 2022). In confirmation of this result, a recent report has demonstrated that BMP signaling and its antagonists, Noggin and Chordin, play a crucial role in the development of cranial neural placodes in the anterior NPB. This is because the expression of Foxg, Six1/2, and Zf220 requires BMP signaling in this region (Liu et al., 2023).
It has been reported that different molecules are involved in the signaling pathways related to neural tube closure, proliferation, and migration of cranial neural crest cells. One such molecule is Adam11, which is a non-proteolytic ADAM and a possible tumor suppressor. Adam11 has the ability to bind to proteins involved in both the Wnt and BMP4 signaling pathways. It acts by upregulating BMP4 signaling and downregulating β-catenin. Through its modulation of these signaling pathways, Adam11 plays a critical role in regulating neural tube closure time, as well as the proliferation and migration of cranial neural crest cells (Pandey et al., 2023).
Recent advances in transgenic animal models, CRISPR technology, and high-resolution live imaging of fluorescent reporters have provided us with new tools to visualize signaling dynamics in space and time. For instance, we can use these techniques to investigate whether the developing epiblast contains intermediate cell types that have the potential to give rise to both neural crest cells and placodes. This can help us test the "binary competence model" of ectodermal patterning. Despite the limitations of these technologies, we can integrate data from published studies to trace cell lineage along the developmental timeline (Huang et al., 2023).
The most important signaling factors in the formation of the neural plate border
Wnt signaling pathway
The NPB is initially induced by external signaling molecules such as Wnts, FGFs, BMPs, and Notch. These molecules activate specific pathways that lead to the expression of genes that specify the NPB (Stuhlmiller and García-Castro, 2012a). After activation of these signaling pathways, a group of molecular inducers and downstream signaling pathways cause neural crest cell formation, differentiation, and migration (Simões-Costa and Bronner, 2015).
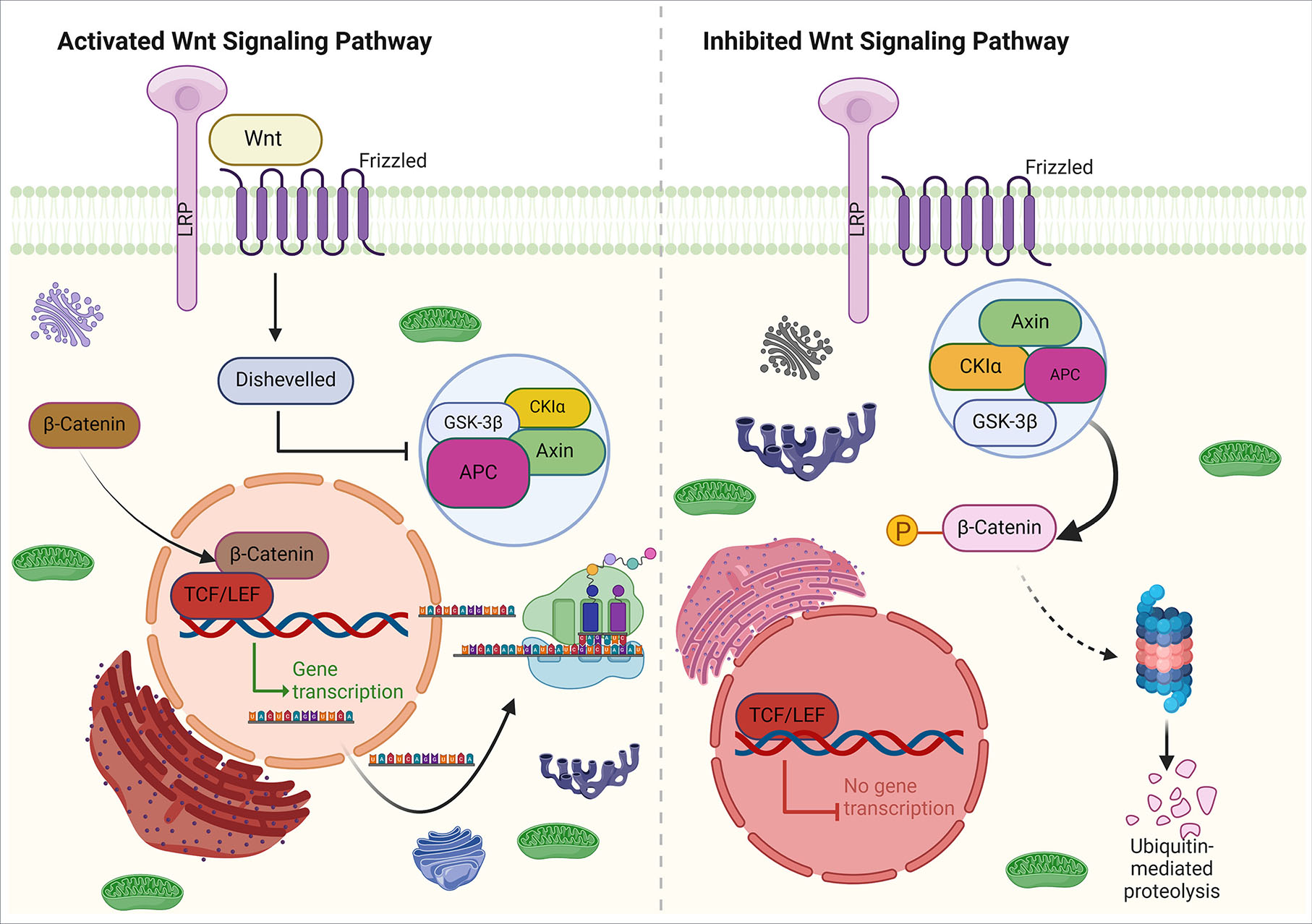
The Wnt signaling pathway is a group of secreted molecules that bind to receptors on the cell membrane. This binding triggers a variety of responses within the cell. Wnt signaling has been shown to play a critical role in various developmental stages, diseases, and the progression of cancer (Akoumianakis et al., 2022, Zhang and Wang, 2020). In the process of forming the NPB, the Wnt pathway plays an important role in two ways: canonical and non-canonical. The canonical pathway is more commonly observed in the formation of the NPB and it acts on molecules involved in cytoskeletal organization and transcriptional regulation, such as β-catenin. In the absence of ligand, β-catenin is phosphorylated and marked for degradation by glycogen synthase kinase 3 (GSK3). However, in the presence of the Wnt ligand, it binds to the Frizzled (Fzd) / Lrp receptor, which activates the Disheveled (DSH) protein and prevents the formation of the degradation complex. This leads to the stabilization of β-catenin, which is then transferred to the nucleus. Together with T-cell factor/lymphoid enhancer factor (TCF/LEF) proteins, β-catenin activates genes transcription involved in the NPB formation (Fig. 3) (Steinhart and Angers, 2018, Ali et al., 2021).
Fig. 3. Canonical Wnt signaling.
In the absence of a Wnt ligand, β-catenin phosphorylation by forming a degradation complex (consisting of Auxin, APC, CK1, and GSK3β) causes its ubiquitination and is ready for degradation by the proteasome. The absence of β-catenin in the nucleus leads to the binding of the repressor complex containing TCF/LEF to the target gene, thereby suppressing its activity. As a result of the Wnt ligand binding to the Frizzled receptor and co-receptor LRP (right), the β-catenin degradation complex is deactivated, which leads to the accumulation of β-catenin in the cytoplasm and its transport to the nucleus, where it forms a complex with TCF/LEF and transcribes the target genes.
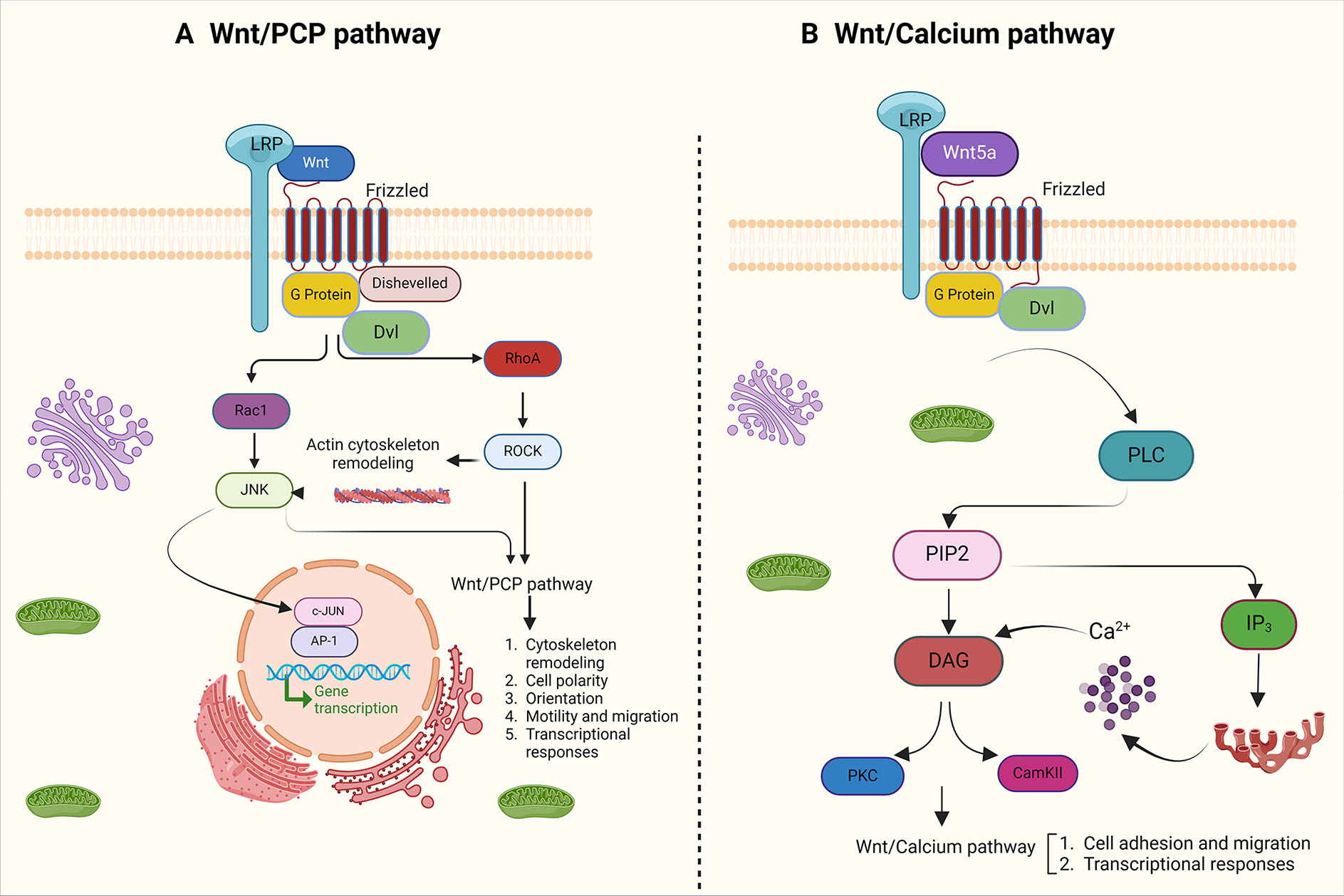
One of the canonical Wnt signaling pathways is the planar cell polarity (PCP) pathway, which triggers asymmetric cytoskeletal organization (Humphries and Mlodzik, 2018). Therefore, the Wnt/PCP pathway is crucial in regulating tissue patterns and cell migration. When Wnt binds to Fzd receptors, it can recruit DSH to the cell membrane, resulting in the formation of a complex with Dvl-associated activator of morphogenesis 1 (Daam1) (Mayor and Theveneau, 2014). Following the formation of the complex, Rho GTPases become activated and subsequently lead to the activation of Rho-associated kinase (Rock) in this pathway. This, in turn, affects the arrangement of the cytoskeleton and cell migration (Ridley, 2011). The non-canonical Wnt-cGMP/Ca2+ signaling pathway is another pathway that can affect the amount of Ca2+ inside the cell. When Wnt binds to Fzd, this mechanism leads to the production of IP3 and DAG inside the cell. IP3 triggers the endoplasmic reticulum (ER) to release Ca2+. This increased Ca2+ secretion results in the release of DAG, which then activates protein kinase C (PKC) and calcium/calmodulin-dependent protein kinase II (CamKII). Ultimately, these factors stimulate transcription factors inside the nucleus (De, 2011).
Fig. 4. Non-canonical Wnt pathways.
(A) In the Wnt/PCP pathway, distribution of FZD receptors causes cell polarity and activation of RhoA/Rock GTPases and JNK through Dsh and DAAM1. This mechanism affects cytoskeleton arrangement and cell migration. (B) In the the Wnt/calcium pathway, as a result of Wnt-Fz binding, PLC is activated, which then hydrolyzes PIP2 and produces IP3 and DAG. IP3 induces the release of calcium from the endoplasmic reticulum, which is a stimulus for the activation of calcium/calmodulin-dependent protein kinase II (CamKII) and PKC.
Several in vivo studies have shown that the expression of canonical ligand genes Wnt1 and Wnt3a occurs in the NPB and the dorsal neural tube (as depicted in Fig. 5A). Elimination of the expression of these genes results in the lack of NPB formation and defects in the differentiation and formation of neural crest cells. Furthermore, these studies have reported that retinoic acid (RA) plays a significant role in the expression of Wnt3a (Ribes et al., 2009). Similar results have also shown that deleting enzymes of the RA synthesis pathway has a direct impact on the expression of Wnt3a and reduces the expression of NPB determinants, such as Msx1 and Pax3 (Duester, 2008, Ribes et al., 2009). Unlike Wnt1 and Wnt3a, Wnt8 is expressed in the paraxial mesoderm, as opposed to the NPB (Fig. 5A).
In Xenopus, Fgf8 is the most important inducing factor for Wnt8 expression and NPB formation in paraxial mesoderm (Fig. 5B) (Hong et al., 2008). When the Wnt/β-catenin signaling pathway is activated, it triggers the activation of a transcription factor called Tcf7l1. If Tcf7l1 fails to bind with β-catenin, then the NPB formation (Msx1) is not formed, and instead neural crest induction (Snai2, and Sox9) occurs (Heeg-Truesdell and LaBonne, 2006). Gbx2 is a transcription factor involved in Wnt/β-catenin pathway. expression and functional defect in Tcf7l1 negatively activates Gbx2 (Li et al., 2009). Gbx2 plays a crucial role in the development of neural folds and NPB by affecting the expression of Zic1, Pax3, and Msx1. The activity level of Gbx2 determines whether neural crest cells will differentiate into pre-placodal cells or not, by influencing the expression of Zic1 (Li et al., 2009).
Studies conducted in vivo have shown that conventional Wnt/β-catenin signaling is involved in the expression of Apoc1, which plays a role in the formation and translocation of lipids (Bolanos-Garcia and Miguel, 2003). Also, another recent result showed that Apoc1 protein levels were affected by the expression of genes such as Msx1, Pax3, and Zic1, which caused impairment in the formation of the NPB. Additionally, genes like c-Myc, Sox9, Snai2, Twist1, and Id3 affected the formation of neural crest cells, resulting in its disruption (Yokota et al., 2017). In addition, Wnt signaling also affects Sp5 (a transcription factor). Fgf8a or Wnt8 signals can induce neural crest formation through Sp5 (Park et al., 2013). Sp5 controls the progression of NPB formation by directly affecting Msx1 and Pax3, and induces neural crest cells by affecting Zic1 (Park et al., 2013). In addition to its other functions, Wnt signals have an impact on various kinases, including Awp1. Furthermore, they regulate the expression of several genes, such as Msx1, Pax3, Sox10, and Snai2 that play a crucial role in the formation and induction of the NPB and neural crest (Seo et al., 2013).
Research in the field of NPB formation has demonstrated the significant role played by non-conventional Wnt signaling. In vivo experiments have revealed that the expression of genes related to the formation of NPB and neural crest is facilitated by the involvement of Dvl and Ror2 in conjunction with Wnt5a and Wnt11. The function of Par1 in the expression of genes Sox8, Foxd3, and Snai2, which are involved in neural crest development, has been established. The effect of Wnt5/Wnt11 signaling on Par1 triggers a series of enzymatic reactions that ultimately control Pax3 expression (Ossipova and Sokol, 2011). Ror2 plays a crucial role in determining the polarity of neuroectodermal cells and forming the NPB. If Ror2 is not functioning properly, it can lead to a decrease in the expression of genes involved in the formation of NPB and neural crest (Schille et al., 2016).
BMP signaling pathway
BMP molecules play a crucial role in various developmental processes and belong to TGF-β family. When BMP secretory proteins bind to their receptors, they cause the activation of transcription factors like Smad1/5/8 through phosphorylation. It has been proven that BMP signaling is involved in the development and its defective function leads to several abnormalities and diseases (Liu et al., 2023). The formation of neural crest cells has been shown to be influenced by BMP signaling. Several hypotheses have been put forth to explain how BMP works, including the gradient model. In this model, different levels of BMP concentration lead to the formation of different cells. Specifically, high concentrations of BMP lead to the formation of the epidermis, medium concentrations lead to the formation of the neural crest, and low concentrations lead to the formation of the neural plate (as shown in Fig. 5A). Another hypothesis suggests that the attenuation of BMP signaling can trigger neural crest formation by creating a competence zone with the help of Wnt and FGF signaling (Steventon et al., 2009). In the next stage of development, BMP signaling is responsible for the expression of NPB and neural crest genes in the corresponding region. Recent studies have revealed that molecules such as SNW1 are responsible for maintaining BMP levels. This molecule acts upstream of BMP signaling in the NPB and restricts the scope of BMP activity in this area (Wu et al., 2011). In addition, a recent report has shown that CKIP-1/Smurf1 modulates the precise level of phospho-Smad1/5/8 to induce neural crest cells at the NPB (Piacentino and Bronner, 2018).
Special NPB genes, namely Msx1, Msx2, Dlx5, and Dlx6 genes are expressed under the control of BMP. It's interesting to note that BMP concentration level for induction the expression of these genes is also different, which means that on the lateral side of the NPB, its high concentration causes the expression of dlx3 compared to other genes in this region (Tríbulo et al., 2003). In other investigations, it has been proven that the expression of tfap2a in the NPB is dependent on BMP concentration. (Nordin and LaBonne, 2014). Several in vitro studies have also demonstrated that BMP is required for the formation of the NPB (Wu et al., 2011).
The role of fibroblast growth factor in the formation of neural plate border
The FGF signaling pathway has a significant impact on various differentiation, migration, and patterning processes (Dorey and Amaya, 2010). Studies have shown that mesoderm is the main target of the FGF signaling pathway in the induction of neural crest cells (Monsoro-Burq et al., 2003). In vivo studies have revealed the occurrence of BMPs, FGF and Wnts signaling pathways in the lateral epiblast, while the FGF signaling pathway is present only in the medial epiblast (Wilson et al., 2001). In the early stages of gastrulation, FGFs, and Chordin are expressed in the primitive node, while Wnts and FGFs are expressed in the primitive streak (Chapman et al., 2004). During gastrulation, NPB is determined by FGFs which originate from paraxial mesoderm (Streit and Stern, 1999). It has been reported that disrupting FGF (dnFgfr1/Mkp3) signaling in the NPB region during gastrulation inhibits Pax7 and Snai2 expression (Stuhlmiller and García-Castro, 2012b). The results obtained suggest that FGF/MAPK signaling plays a direct role in the development of NPB cells. However, it is important to note that the independent role of mesoderm in FGF signaling cannot be overlooked, as FGFR1/4 is only expressed in the NPB and not in the mesoderm (Stuhlmiller and García-Castro, 2012b). In confirmation of these findings, a recently published report indicates that the expression of specific neural crest genes (Pax7 and Sox10) does not occur by FGF inhibition (Betters et al., 2018). In another recently published report, it has been demonstrated that FGF affects the transcription factor Foxg in the anterior NPB regions, leading to the development of placodes and telencephalon (Liu and Satou, 2019).
The role of Notch signaling in the formation of neural plate border
As a result of the Notch receptor binding to its ligand, a series of proteolytic reactions takes place within the cell. Once this proteolytic cascade is activated, the Notch intracellular domain (NICD) is released. Ultimately, NICD is transported to the nucleus where it functions as a transcription factor (Kopan and Ilagan, 2009). In vivo studies have shown that Notch signaling in the BMP4 upstream induces neural crest cells during gastrulation (Endo et al., 2002). In addition, recent studies have revealed that the activation of Notch signaling pathway is critical in the formation of neural crest cells, but is only limited to certain regions along the neural tube (Hernandez-Lagunas et al., 2011). However, the role of Notch signaling in the formation of the NPB remains unclear in many species. Nonetheless, it is known that Notch signaling influences various processes such as growth, differentiation, and migration of nerve cells. During the early stages of NPB formation, Notch signaling affects the expression of hairy2. It is required for the development of neural crest cells in the initial and final stages of gastrulation. However, for the formation of the NPB, hairy2 expression depends on BMP, FGF, and Wnt factors but independent of Notch (Cornell and Eisen, 2005).
Conclusion
Despite numerous studies over the past few decades, there is still a need for further research to uncover convincing answers to questions regarding the formation of the NPB and provide a clear understanding of how progenitor cells differentiate, form and migrate from this region. The lack of detailed descriptions of the processes involved in this phenomenon could be attributed to the one-dimensional nature of studies on transcription, gene regulation, and molecular pathways at the cellular level. Comprehensive analysis of all the factors that affect different parts of the NPB and the interactions between cells and adjacent tissues is essential to answer these questions. It is important to note that there is a close relationship between Wnt signals, FGF, BMP signaling, Notch, and many other factors. These factors affect the activation or repression of genes and molecular cascades in this field (Fig. 6). Additionally, time, place, and concentration of these factors play an important role in the nearby structures. By studying these pathways and factors and their interactions with adjacent cells and structures, we can improve our understanding and potentially develop better treatments for diseases originated from defect in NPB development.
Fig. 6. The outline of a gene regulatory network (GRN) that controls the formation of neural crest cells.
During embryonic development, various inductive signals pattern the embryonic ectoderm and induce the expression of neural plate border specifier genes. These genes work together to define the neural plate border territory, and also drive neural crest specification by activating the neural crest specifier genes. This neural crest specification program leads to the activation of the EMT machinery, which allows the neural crest cells to become migratory. The migratory neural crest cells express specific regulators that provide them with motility and the ability to initiate various differentiation programs.
Acknowledgements
The authors thank Gerash University of Medical Sciences, Gerash, Shiraz, Iran.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Competing interests
The authors declare that no competing interests exist.
References
Akoumianakis I., Polkinghorne M., Antoniades C. (2022). Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications. Nature Reviews Cardiology 19: 783-797.
Alasaadi D. N., Alvizi L., Hartmann J., Stillman N., Moghe P., Hiiragi T., Mayor R. (2024). Competence for neural crest induction is controlled by hydrostatic pressure through Yap. Nature Cell Biology 26: 530-541.
Alata Jimenez N., Strobl-Mazzulla P. H. (2022). Folate Carrier Deficiency Drives Differential Methylation and Enhanced Cellular Potency in the Neural Plate Border. Frontiers in Cell and Developmental Biology 10: 834625.
Albazerchi A., Stern C. D. (2007). A role for the hypoblast (AVE) in the initiation of neural induction, independent of its ability to position the primitive streak. Developmental Biology 301: 489-503.
Ali R. G., Bellchambers H. M., Warr N., Ahmed J. N., Barratt K. S., Neill K., Diamand K. E. M., Arkell R. M. (2021). WNT-responsive SUMOylation of ZIC5 promotes murine neural crest cell development, having multiple effects on transcription. Journal of Cell Science 134: jcs256792.
Azambuja A. P., Simões-Costa M. (2021). A regulatory sub-circuit downstream of Wnt signaling controls developmental transitions in neural crest formation. PLOS Genetics 17: e1009296.
Bae C.J., Park B.Y., Lee Y.H., Tobias J. W., Hong C.S., Saint-Jeannet J.P. (2014). Identification of Pax3 and Zic1 targets in the developing neural crest. Developmental Biology 386: 473-483.
Bally-Cuif L., Gulisano M., Broccoli V., Boncinelli E. (1995). c-otx2 is expressed in two different phases of gastrulation and is sensitive to retinoic acid treatment in chick embryo. Mechanisms of Development 49: 49-63.
Barembaum M., Bronner M. E. (2013). Identification and dissection of a key enhancer mediating cranial neural crest specific expression of transcription factor, Ets-1. Developmental Biology 382: 567-575.
Bellchambers H. M., Barratt K. S., Diamand K. E. M., Arkell R. M. (2021). SUMOylation Potentiates ZIC Protein Activity to Influence Murine Neural Crest Cell Specification. International Journal of Molecular Sciences 22: 10437.
Ben Amar D., Thoinet K., Villalard B., Imbaud O., Costechareyre C., Jarrosson L., Reynaud F., Novion Ducassou J., Couté Y., Brunet J.F., Combaret V., Corradini N., Delloye-Bourgeois C., Castellani V. (2022). Environmental cues from neural crest derivatives act as metastatic triggers in an embryonic neuroblastoma model. Nature Communications 13: 2549.
Bertol J. W., Johnston S., Ahmed R., Xie V. K., Hubka K. M., Cruz L., Nitschke L., Stetsiv M., Goering J. P., Nistor P., Lowell S., Hoskens H., Claes P., Weinberg S. M., Saadi I., Farach-Carson M. C., Fakhouri W. D. (2022). TWIST1 interacts with β/δ-catenins during neural tube development and regulates fate transition in cranial neural crest cells. Development 149: dev200068.
Betters E., Charney R. M., Garcia-Castro M. I. (2018). Early specification and development of rabbit neural crest cells. Developmental Biology 444: S181-S192.
Bolanos-Garcia V. M., Miguel R. N. (2003). On the structure and function of apolipoproteins: more than a family of lipid-binding proteins. Progress in Biophysics and Molecular Biology 83: 47-68.
Brugmann S. A., Pandur P. D., Kenyon K. L., Pignoni F., Moody S. A. (2004). Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131: 5871-5881.
Buenrostro J. D., Wu B., Chang H. Y., Greenleaf W. J. (2015). ATAC‐seq: A Method for Assaying Chromatin Accessibility Genome‐Wide. Current Protocols in Molecular Biology 109: 21.29.1-21.29.9.
Catala M., (2020). Development of the Central Nervous System. In Textbook of Pediatric Neurosurgery. (Ed. Di Rocco C., Pang D., Rutka J. T., ) Springer International Publishing.
Chapman S. C., Brown R., Lees L., Schoenwolf G. C., Lumsden A. (2004). Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Developmental Dynamics 229: 668-676.
Cornell R. A., Eisen J. S. (2005). Notch in the pathway: The roles of Notch signaling in neural crest development. Seminars in Cell & Developmental Biology 16: 663-672.
De A. (2011). Wnt/Ca<sup>2+</sup> signaling pathway: a brief overview. Acta Biochimica et Biophysica Sinica 43: 745-756.
de Crozé N., Maczkowiak F., Monsoro-Burq A. H. (2011). Reiterative AP2a activity controls sequential steps in the neural crest gene regulatory network. Proceedings of the National Academy of Sciences 108: 155-160.
Dorey K., Amaya E. (2010). FGF signalling: diverse roles during early vertebrate embryogenesis. Development 137: 3731-3742.
Duester G. (2008). Retinoic Acid Synthesis and Signaling during Early Organogenesis. Cell 134: 921-931.
Endo Y., Osumi N., Wakamatsu Y. (2002). Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development 129: 863-873.
Erickson A. G., Kameneva P., Adameyko I. (2023). The transcriptional portraits of the neural crest at the individual cell level. Seminars in Cell & Developmental Biology 138: 68-80.
Ezin M., Barembaum M., Bronner M. E. (2014). Stage-dependent plasticity of the anterior neural folds to form neural crest. Differentiation 88: 42-50.
Flierman S., Tijsterman M., Rousian M., de Bakker B. S. (2023). Discrepancies in Embryonic Staging: Towards a Gold Standard. Life 13: 1084.
Gandhi S., Ezin M., Bronner M. E. (2020). Reprogramming Axial Level Identity to Rescue Neural-Crest-Related Congenital Heart Defects. Developmental Cell 53: 300-315.e4.
Garnett A. T., Square T. A., Medeiros D. M. (2012). BMP, Wnt and FGF signals are integrated through evolutionarily conserved enhancers to achieve robust expression of Pax3 and Zic genes at the zebrafish neural plate border. Development 139: 4220-4231.
Gouignard N., Rouvière C., Theveneau E. (2021). Using Xenopus Neural Crest Explants to Study Epithelial-Mesenchymal Transition. In The Epithelial-to Mesenchymal Transition. (Ed. Campbell Kyra, Theveneau Eric) Springer US, New York, NY.
Grocott T., Tambalo M., Streit A. (2012). The peripheral sensory nervous system in the vertebrate head: A gene regulatory perspective. Developmental Biology 370: 3-23.
Groves A. K., LaBonne C. (2014). Setting appropriate boundaries: Fate, patterning and competence at the neural plate border. Developmental Biology 389: 2-12.
Hans S., Christison J., Liu D., Westerfield M. (2007). Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Developmental Biology 7: 5.
Heeg-Truesdell E., LaBonne C. (2006). Neural induction in Xenopus requires inhibition of Wnt-β-catenin signaling. Developmental Biology 298: 71-86.
Hernandez-Lagunas L., Powell D. R., Law J., Grant K. A., Artinger K. B. (2011). prdm1a and olig4 act downstream of Notch signaling to regulate cell fate at the neural plate border. Developmental Biology 356: 496-505.
Hintze M., Prajapati R. S., Tambalo M., Christophorou N. A. D., Anwar M., Grocott T., Streit A. (2017). Cell interactions, signals and transcriptional hierarchy governing placode progenitor induction. Development 144: 2810-2823.
Hoffman T. L., Javier A. L., Campeau S. A., Knight R. D., Schilling T. F. (2007). Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 308B: 679-691.
Hong C.S., Park B.Y., Saint-Jeannet J.P. (2008). Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 135: 3903-3910.
Hong C.S., Saint-Jeannet J.P. (2007). The Activity of Pax3 and Zic1 Regulates Three Distinct Cell Fates at the Neural Plate Border. Molecular Biology of the Cell 18: 2192-2202.
Hong C.S., Saint‐Jeannet J.P. (2017). Znf703, a novel target of Pax3 and Zic1, regulates hindbrain and neural crest development in Xenopus . genesis 55: e23082.
Huang S., Dai R., Zhang Z., Zhang H., Zhang M., Li Z., Zhao K., Xiong W., Cheng S., Wang B., Wan Y. (2023). CRISPR/Cas-Based Techniques for Live-Cell Imaging and Bioanalysis. International Journal of Molecular Sciences 24: 13447.
Humphries A. C., Mlodzik M. (2018). From instruction to output: Wnt/PCP signaling in development and cancer. Current Opinion in Cell Biology 51: 110-116.
Janesick A., Tang W., Ampig K., Blumberg B. (2019). Znf703 is a novel RA target in the neural plate border. Scientific Reports 9: 8275.
Jiang S., Huang Z., Li Y., Yu C., Yu H., Ke Y., Jiang L., Liu J. (2023). Single-cell chromatin accessibility and transcriptome atlas of mouse embryos. Cell Reports 42: 112210.
Jovic D., Liang X., Zeng H., Lin L., Xu F., Luo Y. (2022). Single‐cell RNA sequencing technologies and applications: A brief overview. Clinical and Translational Medicine 12: e694.
Kashef J., Köhler A., Kuriyama S., Alfandari D., Mayor R., Wedlich D. (2009). Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases . Genes & Development 23: 1393-1398.
Kelsh R. N. (2006). Sorting out Sox10 functions in neural crest development . BioEssays 28: 788-798.
Kim K., Orvis J., Stolfi A. (2022). Pax3/7 regulates neural tube closure and patterning in a non-vertebrate chordate. Frontiers in Cell and Developmental Biology 10: 999511.
Koontz A., Urrutia H. A., Bronner M. E. (2023). Making a head: Neural crest and ectodermal placodes in cranial sensory development. Seminars in Cell & Developmental Biology 138: 15-27.
Kopan R., Ilagan M. X. G. (2009). The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 137: 216-233.
Lau M. S., Hu Z., Zhao X., Tan Y. S., Liu J., Huang H., Yeo C. J., Leong H. F., Grinchuk O. V., Chan J. K., Yan J., Tee W.W. (2023). Transcriptional repression by a secondary DNA binding surface of DNA topoisomerase I safeguards against hypertranscription. Nature Communications 14: 6464.
Li B., Kuriyama S., Moreno M., Mayor R. (2009). The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction . Development 136: 3267-3278.
Li J., Perfetto M., Materna C., Li R., Thi Tran H., Vleminckx K., Duncan M. K., Wei S. (2019). A new transgenic reporter line reveals Wnt-dependent Snai2 re-expression and cranial neural crest differentiation in Xenopus. Scientific Reports 9: 11191.
Li W., Cornell R. A. (2007). Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Developmental Biology 304: 338-354.
Litsiou A., Hanson S., Streit A. (2005). A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132: 4051-4062.
Liu B., Ren X., Satou Y. (2023). BMP signaling is required to form the anterior neural plate border in ascidian embryos. Development Genes and Evolution 233: 13-23.
Liu B., Satou Y. (2019). Foxg specifies sensory neurons in the anterior neural plate border of the ascidian embryo. Nature Communications 10: 4911.
Lukoseviciute M., Gavriouchkina D., Williams R. M., Hochgreb-Hagele T., Senanayake U., Chong-Morrison V., Thongjuea S., Repapi E., Mead A., Sauka-Spengler T. (2018). From Pioneer to Repressor: Bimodal foxd3 Activity Dynamically Remodels Neural Crest Regulatory Landscape In Vivo. Developmental Cell 47: 608-628.e6.
Maharana S. K., Schlosser G. (2018). A gene regulatory network underlying the formation of pre-placodal ectoderm in Xenopus laevis. BMC Biology 16: 79.
Mašek J., Machoň O., Kořínek V., Taketo M. M., Kozmik Z. (2016). Tcf7l1 protects the anterior neural fold from adopting the neural crest fate. Development 143: 2206-2216.
Mayor R., Theveneau E. (2014). The role of the non-canonical Wnt–planar cell polarity pathway in neural crest migration. Biochemical Journal 457: 19-26.
Méndez-Maldonado K., Vega-López G. A., Aybar M. J., Velasco I. (2020). Neurogenesis From Neural Crest Cells: Molecular Mechanisms in the Formation of Cranial Nerves and Ganglia. Frontiers in Cell and Developmental Biology 8: 635.
Meulemans D., Bronner-Fraser M. (2002). Amphioxus and lamprey AP-2 genes: implications for neural crest evolution and migration patterns. Development 129: 4953-4962.
Milet C., Maczkowiak F., Roche D. D., Monsoro-Burq A. H. (2013). Pax3 and Zic1 drive induction and differentiation of multipotent, migratory, and functional neural crest in Xenopus embryos . Proceedings of the National Academy of Sciences 110: 5528-5533.
Mirdass C., Catala M., Bocel M., Nedelec S., Ribes V. (2023). Stem cell-derived models of spinal neurulation. Emerging Topics in Life Sciences 7: 423-437.
Mohammadparast S., Chang C. (2022). Ash2l, an obligatory component of H3K4 methylation complexes, regulates neural crest development. Developmental Biology 492: 14-24.
Monsoro-Burq A.H., Fletcher R. B., Harland R. M. (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals . Development 130: 3111-3124.
Moody S. A., LaMantia A.S. (2015). Transcriptional Regulation of Cranial Sensory Placode Development. In Neural Crest and Placodes. (Ed. Trainor P.) Elsevier.
Nichane M., de Crozé N., Ren X., Souopgui J., Monsoro-Burq A. H., Bellefroid E. J. (2008). Hairy2–Id3 interactions play an essential role in Xenopus neural crest progenitor specification. Developmental Biology 322: 355-367.
Nordin K., LaBonne C. (2014). Sox5 Is a DNA-Binding Cofactor for BMP R-Smads that Directs Target Specificity during Patterning of the Early Ectoderm. Developmental Cell 31: 374-382.
Ossipova O., Sokol S. Y. (2011). Neural crest specification by noncanonical Wnt signaling and PAR-1. Development 138: 5441-5450.
Pandey A., Cousin H., Horr B., Alfandari D., (2023). ADAM11 a novel regulator of Wnt and BMP4 signaling in neural crest and cancer. Frontiers in cell and developmental biology 11: 1271178.
Park B.Y., Saint-Jeannet J.P. (2008). Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Developmental Biology 324: 108-121.
Park D.S., Seo J.H., Hong M., Bang W., Han J.K., Choi S.C. (2013). Role of Sp5 as an essential early regulator of neural crest specification in xenopus . Developmental Dynamics 242: 1382-1394.
Parker H. J., Pushel I., Krumlauf R. (2018). Coupling the roles of Hox genes to regulatory networks patterning cranial neural crest. Developmental Biology 444: S67-S78.
Patrick A. N., Cabrera J. H., Smith A. L., Chen X. S., Ford H. L., Zhao R. (2013). Structure-function analyses of the human SIX1–EYA2 complex reveal insights into metastasis and BOR syndrome. Nature Structural & Molecular Biology 20: 447-453.
Phillips B. T., Kwon H.J., Melton C., Houghtaling P., Fritz A., Riley B. B. (2006). Zebrafish msxB, msxC and msxE function together to refine the neural–nonneural border and regulate cranial placodes and neural crest development. Developmental Biology 294: 376-390.
Piacentino M. L., Bronner M. E. (2018). Intracellular attenuation of BMP signaling via CKIP-1/Smurf1 is essential during neural crest induction. PLOS Biology 16: e2004425.
Pieper M., Ahrens K., Rink E., Peter A., Schlosser G. (2012). Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development 139: 1175-1187.
Pla P., Monsoro-Burq A. H. (2018). The neural border: Induction, specification and maturation of the territory that generates neural crest cells. Developmental Biology 444: S36-S46.
Plouhinec J.L., Roche D. D., Pegoraro C., Figueiredo A. L., Maczkowiak F., Brunet L. J., Milet C., Vert J.P., Pollet N., Harland R. M., Monsoro-Burq A. H. (2014). Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Developmental Biology 386: 461-472.
Ponzoni M., Bachetti T., Corrias M. V., Brignole C., Pastorino F., Calarco E., Bensa V., Giusto E., Ceccherini I., Perri P. (2022). Recent advances in the developmental origin of neuroblastoma: an overview. Journal of Experimental & Clinical Cancer Research 41: 92.
Prasad M. S., Charney R. M., García‐Castro M. I. (2019). Specification and formation of the neural crest: Perspectives on lineage segregation. genesis 57: e23276.
Ravi K. S., Divasha , Hassan S. B., Pasi R., Mittra S., Kumar R. (2021). Neural tube defects: Different types and brief review of neurulation process and its clinical implication. Journal of Family Medicine and Primary Care 10: 4383-4390.
Rex M., Orme A., Uwanogho D., Tointon K., Wigmore P. M., Sharpe P. T., Scotting P. J. (1997). Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Developmental Dynamics 209: 323-332.
Ribes V., Le Roux I., Rhinn M., Schuhbaur B., Dollé P. (2009). Early mouse caudal development relies on crosstalk between retinoic acid,Shh and Fgf signalling pathways. Development 136: 665-676.
Richard A., Boullu L., Herbach U., Bonnafoux A., Morin V., Vallin E., Guillemin A., Papili Gao N., Gunawan R., Cosette J., Arnaud O., Kupiec J.J., Espinasse T., Gonin-Giraud S., Gandrillon O. (2016). Single-Cell-Based Analysis Highlights a Surge in Cell-to-Cell Molecular Variability Preceding Irreversible Commitment in a Differentiation Process. PLOS Biology 14: e1002585.
Ridley A. J. (2011). Life at the Leading Edge. Cell 145: 1012-1022.
Rocha M., Singh N., Ahsan K., Beiriger A., Prince V. E. (2020). Neural crest development: insights from the zebrafish. Developmental Dynamics 249: 88-111.
Rodrigues-Da-Silva M. A., de Espindola da Silveira G., Taufer C. R., Calloni G. W. (2022). The mesenchymal potential of trunk neural crest cells. The International Journal of Developmental Biology 66: 317-331.
Roellig D., Tan-Cabugao J., Esaian S., Bronner M. E. (2017). Dynamic transcriptional signature and cell fate analysis reveals plasticity of individual neural plate border cells. eLife 6: e21620.
Rogers C. D., Ferzli G. S., Casey E. S. (2011). The response of early neural genes to FGF signaling or inhibition of BMP indicate the absence of a conserved neural induction module. BMC Developmental Biology 11: 74.
Rothstein M., Simões-Costa M. (2020). Heterodimerization of TFAP2 pioneer factors drives epigenomic remodeling during neural crest specification. Genome Research 30: 35-48.
Roure A., Chowdhury R., Darras S. (2023). Regulation of anterior neurectoderm specification and differentiation by BMP signaling in ascidians. Development 150: dev201575.
Saint-Jeannet J.P., Moody S. A. (2014). Establishing the pre-placodal region and breaking it into placodes with distinct identities. Developmental Biology 389: 13-27.
Sato S., Ikeda K., Shioi G., Ochi H., Ogino H., Yajima H., Kawakami K. (2010). Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Developmental Biology 344: 158-171.
Sauka-Spengler T., Bronner-Fraser M. (2008a). A gene regulatory network orchestrates neural crest formation. Nature Reviews Molecular Cell Biology 9: 557-568.
Sauka-Spengler T., Bronner‐Fraser M. (2008b). Evolution of the neural crest viewed from a gene regulatory perspective. genesis 46: 673-682.
Schille C., Bayerlová M., Bleckmann A., Schambony A. (2016). Ror2 signaling is required for local upregulation of GDF6 and activation of BMP signaling at the neural plate border. Development 143: 3182-3194.
Schille C., Schambony A. (2017). Signaling pathways and tissue interactions in neural plate border formation. Neurogenesis 4: e1292783.
Schock E. N., York J. R., LaBonne C. (2023). The developmental and evolutionary origins of cellular pluripotency in the vertebrate neural crest. Seminars in Cell & Developmental Biology 138: 36-44.
Seal S., Monsoro-Burq A. H. (2020). Insights Into the Early Gene Regulatory Network Controlling Neural Crest and Placode Fate Choices at the Neural Border. Frontiers in Physiology 11: 608812.
Selleck M. A. J., Bronner-Fraser M. (1995). Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development 121: 525-538.
Seo J.H., Park D.S., Hong M., Chang E.J., Choi S.C. (2013). Essential role of AWP1 in neural crest specification in Xenopus. The International Journal of Developmental Biology 57: 829-836.
Siismets E. M., Hatch N. E. (2020). Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis. Journal of Developmental Biology 8: 18.
Simões-Costa M., Bronner M. E. (2016). Reprogramming of avian neural crest axial identity and cell fate. Science 352: 1570-1573.
Simões-Costa M., Bronner M. E. (2015). Establishing neural crest identity: a gene regulatory recipe. Development 142: 242-257.
Simões-Costa M., Tan-Cabugao J., Antoshechkin I., Sauka-Spengler T., Bronner M. E. (2014). Transcriptome analysis reveals novel players in the cranial neural crest gene regulatory network. Genome Research 24: 281-290.
Simões-Costa M. S., McKeown S. J., Tan-Cabugao J., Sauka-Spengler T., Bronner M. E. (2012). Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome. PLoS Genetics 8: e1003142.
Steinhart Z., Angers S. (2018). Wnt signaling in development and tissue homeostasis. Development 145: dev146589.
Stern C. D., Downs K. M. (2012). The hypoblast (visceral endoderm): an evo-devo perspective. Development 139: 1059-1069.
Steventon B., Araya C., Linker C., Kuriyama S., Mayor R. (2009). Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 136: 771-779.
Steventon B., Mayor R., Streit A. (2012). Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning. Developmental Biology 367: 55-65.
Streit A., Stern C. D. (1999). Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mechanisms of Development 82: 51-66.
Stuhlmiller T. J., García-Castro M. I. (2012a). Current perspectives of the signaling pathways directing neural crest induction. Cellular and Molecular Life Sciences 69: 3715-3737.
Stuhlmiller T. J., García-Castro M. I. (2012b). FGF/MAPK signaling is required in the gastrula epiblast for avian neural crest induction. Development 139: 289-300.
Sullivan C. H., Majumdar H. D., Neilson K. M., Moody S. A. (2019). Six1 and Irx1 have reciprocal interactions during cranial placode and otic vesicle formation. Developmental Biology 446: 68-79.
Tang P.C., Chen L., Singh S., Groves A. K., Koehler K. R., Liu X. Z., Nelson R. F. (2023). Early Wnt Signaling Activation Promotes Inner Ear Differentiation via Cell Caudalization in Mouse Stem Cell-Derived Organoids. Stem Cells 41: 26-38.
Thawani A., Groves A. K. (2020). Building the Border: Development of the Chordate Neural Plate Border Region and Its Derivatives. Frontiers in Physiology 11: 608880.
Torres-Paz J., Tine E. M., Whitlock K. E. (2021). Dissecting the neural divide: a continuous neurectoderm gives rise to the olfactory placode and bulb. The International Journal of Developmental Biology 65: 275-287.
Trainor P. A., Krumlauf R. (2000). Patterning the cranial neural crest: Hinbrain segmentation and hox gene plasticity. Nature Reviews Neuroscience 1: 116-124.
Tríbulo C., Aybar M. J., Nguyen V. H., Mullins M. C., Mayor R. (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130: 6441-6452.
Villanueva S., Glavic A., Ruiz P., Mayor R. (2002). Posteriorization by FGF, Wnt, and Retinoic Acid Is Required for Neural Crest Induction. Developmental Biology 241: 289-301.
Williams R. M., Lukoseviciute M., Sauka-Spengler T., Bronner M. E. (2022). Single-cell atlas of early chick development reveals gradual segregation of neural crest lineage from the neural plate border during neurulation. eLife 11: e74464.
Wilson S., Rydström A., Trimborn T., Willert K., Nusse R., Jessell T. M., Edlund T. (2001). The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411: 325-330.
Wu M. Y., Ramel M.C., Howell M., Hill C. S. (2011). SNW1 Is a Critical Regulator of Spatial BMP Activity, Neural Plate Border Formation, and Neural Crest Specification in Vertebrate Embryos. PLoS Biology 9: e1000593.
Yao Z., van Velthoven C. T. J., Kunst M., Zhang M., McMillen D., Lee C., Jung W., Goldy J., Abdelhak A., Aitken M., Baker K., Baker P., Barkan E., Bertagnolli D., Bhandiwad A., Bielstein C., Bishwakarma P., Campos J., Carey D., Casper T., Chakka A. B., Chakrabarty R., Chavan S., Chen M., Clark M., Close J., Crichton K., Daniel S., DiValentin P., Dolbeare T., Ellingwood L., Fiabane E., Fliss T., Gee J., Gerstenberger J., Glandon A., Gloe J., Gould J., Gray J., Guilford N., Guzman J., Hirschstein D., Ho W., Hooper M., Huang M., Hupp M., Jin K., Kroll M., Lathia K., Leon A., Li S., Long B., Madigan Z., Malloy J., Malone J., Maltzer Z., Martin N., McCue R., McGinty R., Mei N., Melchor J., Meyerdierks E., Mollenkopf T., Moonsman S., Nguyen T. N., Otto S., Pham T., Rimorin C., Ruiz A., Sanchez R., Sawyer L., Shapovalova N., Shepard N., Slaughterbeck C., Sulc J., Tieu M., Torkelson A., Tung H., Valera Cuevas N., Vance S., Wadhwani K., Ward K., Levi B., Farrell C., Young R., Staats B., Wang M.Q. M., Thompson C. L., Mufti S., Pagan C. M., Kruse L., Dee N., Sunkin S. M., Esposito L., Hawrylycz M. J., Waters J., Ng L., Smith K., Tasic B., Zhuang X., Zeng H. (2023). A high-resolution transcriptomic and spatial atlas of cell types in the whole mouse brain. Nature 624: 317-332.
Yardley N., García-Castro M. I. (2012). FGF signaling transforms non-neural ectoderm into neural crest. Developmental Biology 372: 166-177.
Yokota C., Åstrand C., Takahashi S., Hagey D. W., Stenman J. M. (2017). Apolipoprotein C-I mediates Wnt/Ctnnb1 signaling during neural border formation and is required for neural crest development. The International Journal of Developmental Biology 61: 415-425.
Zhang Y., Wang X. (2020). Targeting the Wnt/β-catenin signaling pathway in cancer. Journal of Hematology & Oncology 13: 165.