Int. J. Dev. Biol. 67: 91 - 100 (2023)
Evaluation of changes of apelin and apelin receptor (APJ) expression in cervix-uterus and placental axis in an LPS-induced preterm labor model
Original Article | Published: 16 October 2023
Abstract
Although preterm birth is among the preventable causes of maternal and infant death, its mechanism has not yet been clarified. When evaluated in terms of the results, the psycho-social burden of mother-infant losses and the costs of rehabilitation, care, and treatment for postpartum sequelae are high. When evaluated in terms of its causes, infection/inflammation has an important place. Therefore, it is essential to understand the role of pro- and anti-inflammatory proteins in the process. In our study, apelin and apelin receptor (APJ) expression in the cervix-uterus and placental axis were evaluated at tissue and protein levels in pregnant and non-pregnant control, sham, PBS, and LPS groups in the infection model in which LPS induction was performed by midline laparotomy, in CD-1 mice. The evaluation of this axis regarding apelin and apelin receptor in the preterm birth model is new in the literature. Apelin is expressed more intensely in uterine epithelial cells than in the cervix. In the placenta, expression is more intense in the junctional zone compared to other zones. Apelin protein levels decrease significantly in the cervix and placenta whereas it increases in the uterus. While no change was observed in the expression of the apelin receptor at the tissue and protein level in the cervix and uterus, it increased in both aspects in the placenta in the invasive procedure groups. We propose that the decrease in apelin protein due to LPS in the preterm delivery model may be related to the effort to compensate for the balance deteriorated in the pro-inflammatory direction with post-transitional modification at the tissue level. The tendency of apelin to increase with pregnancy has led to the conclusion that it is necessary for a healthy pregnancy. Although the apelin receptor does not change with inflammation, it is necessary to investigate the mechanisms associated with its stress and trauma-induced increase, since it increases in the invasive procedure group.
Keywords
LPS, inflammation, preterm birth, apelin, apelin receptor, uterus, cervix, placenta
Introduction
Considering the delivery process, the most critical complication contributing to pregnancy and newborn outcomes is preterm birth, which accounts for 75% of perinatal mortality and more than 50% of long-term morbidity (Callaghan et al., 2006). The World Health Organization estimates the incidence of preterm birth to be between 4% and 16% (WHO, 2023). Premature birth is one of the leading causes of death in childhood, accounting for 18% of all deaths among children under 5 and 35% of all deaths in newborns (less than 28 days old) (Walani, 2020). Considering the care costs of babies affected by preterm birth complications, intensive care units and health systems pose a severe financial and psychological burden on families and society (Moster et al., 2008).
Preterm labor is the onset of labor before 37 weeks. When divided into sub-categories, defined as <28 weeks severely preterm, 28 to 32 weeks very early, and 32 to 37 weeks moderate to late preterm (Nour, 2012). Many antecedents are associated with spontaneous preterm birth. Infection/inflammation, multiple pregnancies, intrauterine bleeding, hormonal stress, etc., although there are some of these, it can be said that infection in the pregnancy compartment causes 50% or more of preterm births (Klein and Gibbs, 2004). The most common possible mechanism underlying preterm labor due to infection is increased colonization of vaginal pathogens in the gestational cavity (Goldenberg et al., 2008). It has also been shown that vaginal pathogens can reach the amniotic membrane (Romero et al., 2014).
Pregnancy is a dynamic period that includes a series of processes necessary for the proper development of the fetus. This process, which starts from fertilization, continues with the embryo's implantation into the uterine wall, followed by the development of unique structures such as the placenta (Erlebacher and Fisher, 2017). The placenta is essential for the continuation of a healthy pregnancy, and functional dysfunction of the placenta is associated with many pregnancy pathologies today (Tsai et al., 2021). Fetal placental membranes provide a physical barrier against LPS administration and microbial agents (Kim et al., 2002).
The mature mouse placenta consists of four separate layers: the decidua, the junctional zone, the labyrinth zone, and the chorionic plate. Parietal trophoblast giant cells separate the decidua and the junctional zone (Isaac et al., 2014). In mid-pregnancy, the mouse placenta produces blood cells, and the labyrinth zone promotes the differentiation of fetal erythrocytes. On the other hand, the junctional zone regulates erythropoietic differentiation (Azevedo Portilho and Pelajo-Machado, 2018, Azevedo Portilho et al., 2016). In the placenta, trophoblast giant cells regulate uterine decidualization in early pregnancy and support temporary placental formation by anastomosis with maternal blood vessels. Spongiotrophoblast and trophoblast glycogen cells comprise most of the endocrine structure associated with hormone regulation (Hemberger et al., 2020, Hu and Cross, 2010).
Apelin (APLN) is a 36 amino acid peptide isolated from the bovine stomach and identified as the Apelin receptor (APJ; angiotensin II protein J receptor (Li et al., 2022) ligand, a member of the G protein-coupled receptor family (Antushevich and Wojcik, 2018, Tatemoto et al., 1998). There are many final forms of apelin, each descending from a common precursor, a pre-propeptide of 77 amino acids. After post-translational modification, pre-proapelin is converted to endogenous isoforms such as apelin-36, apelin-17, apelin-13, apelin-16, or exogenous apelin-12. Interestingly, the apelin variations mentioned above differ in the polypeptide chain length. The researchers proved that longer chains of this protein are characterized by lower biological activity and converted to more active short-chain forms (Chen et al., 2003). Endogenous apelin and its long- or short-acting forms interact differently with APJ. APLN-13 exhibits the most robust binding to APJ in between all isoforms (Kawamata et al., 2001, Xiong et al., 2017).
Apelin is an adipokine with pleiotropic effects thought to be involved in regulating many metabolic functions. It is well known that adipokines are endocrine-acting factors secreted mainly by white adipose tissue. In addition, studies have shown that they have a central role in energy metabolism (Mercati et al., 2019, Zhou et al., 2018).
It is widely believed that apelin may have antioxidant and anti-inflammatory effects (Ishimaru et al., 2017). Basic and preliminary research has shown that the APLN/APJ system plays a role in many diseases, such as cardiovascular system diseases, liver and kidney diseases, neurological diseases, inflammatory bowel diseases, pancreatitis, lung damage, aging, and obesity. Moreover, deficiency or excess of apelin may exacerbate disease states as inflammation is an essential physiological defense mechanism and a potential mediator of organ damage. Therefore, recent developments in APLN/APJ system research indicate that apelin and APJ may be potential targets for treating diseases mediated or exacerbated by inflammation. (Wang et al., 2022).
Our study aimed to determine apelin and APJ protein changes in the cervix-uterus and placental axis during LPS-induced preterm labor. Our hypothesis is that apelin, which has anti-inflammatory activity, will decrease the inflammatory process. In addition, the occurrence of the APJ-related apelin response will also be evaluated. In the literature, no study was found to evaluate the expression status of apelin and APJ together in the cervix, uterus, and placenta in the LPS-induced preterm labor model.
Results
In the LPS-only group, 85-100% complete uterine emptying was observed within 24 hours. No preterm birth was observed in the other experimental groups. Here, we present the results obtained for Apelin and Apelin receptor (APJ) in immunohistochemical staining and western blot analysis.
Immunohistochemical apelin staining in cervical orifice and cervix/uterine transition regions
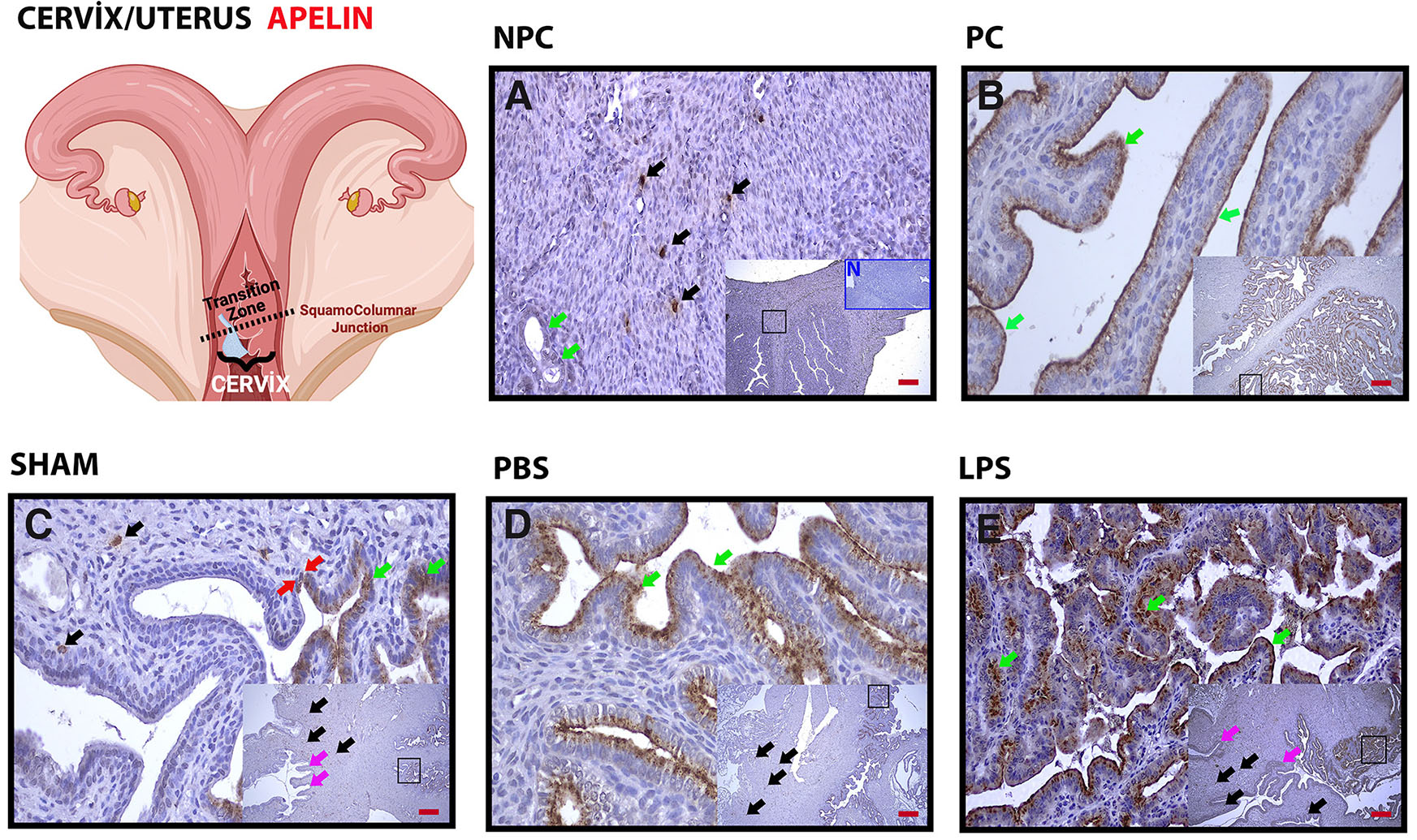
When the cervix tissues were examined in the non-pregnant group, it was observed that some specific cells performed apelin expression at the cervix (Fig. 1A, black arrows). Expression in the epithelium was relatively weak. Expression in epithelial tissue increased with pregnancy in the uterus (Fig. 1. B-D, green arrows). Expression increased significantly from a certain point in the transition area from the cervix to the uterus (Fig. 1C, red arrows). In the group given LPS, expression in the epithelium was more intense compared to the other groups (Fig. 1E, green arrows). Staining in the vaginal fornix and external os epithelium, especially in the basal area, was increased in the LPS group compared to the control groups (Fig. 1. A-E, pink arrows) (Table 1).
Table 1
Comparison of apelin expression in the uterus, cervix and placenta in LPS-induced preterm labor
| Apelin / H-Score | NPC | PC | Sham | PBS | LPS | |
|---|---|---|---|---|---|---|
| Uterus | Epithelium | (-)/(+) | (+) | (+) | (+) | (++) |
| Muscle | (-) | (-) | (-) | (-) | (-) | |
| Gland | (-)/(+) | (+) | (+) | (+) | (+) | |
| Stroma | (+) | (+) | (+) | (+) | (+) | |
| Cervix | Epithelium | (-)/(+) | (-)/(+) | (-)/(+) | (-)/(+) | (++) |
| Stroma | (+) | (+) | (+) | (+) | (+) | |
| Placental Zones | Decidua | (+) | (+) | (+) | (+) | |
| Junctional | (++) | (++) | (++) | (+) | ||
| Labyrinth | (+) | (+) | (+) | (+) | ||
| Chorion | (++) | (++) | (++) | (+) | ||
H-Score Analysis. NPC, non-pregnant control; PC, pregnant control; Sham, surgical control; PBS, phosphate buffer solution, solvent control; LPS, lipopolysaccharide, 25 µg/100 µl. Abbreviations: ++, strongly positive; +, moderately positive; -/+, positive; -, negative or weakly positive.
Fig. 1. Apelin (APLN) immunohistochemical staining in the cervical irifice and cervix/uterine transition Regions.
Apelin expression is increased at the tissue level in the cervix orifice and uterus in LPS-induced preterm labor. The change is especially intense in epithelial tissue (A) Non-Pregnant Control (NPC); (B) Pregnant Control (PC); (C) Sham Surgical control; (D) Phosphate Buffer Solution (PBS) solvent control; (E) Lipopolysaccharide (LPS), 25 µg/100 µl. Black arrowheads, positive staining stromal cells; green arrows, positive staining uterine epithelium; pink arrows, negative staining epithelium, Red Arrows: Transition zone from stratified squamous epithelium to columnar epithelium. Each large image is an enlargement of the black frame within the thumbnail. N in (A), negative control (blue frame); magnification, 5X, 20X; Scale bars, 50 µm.
Immunohistochemical apelin staining in placental zones and placental cell structures
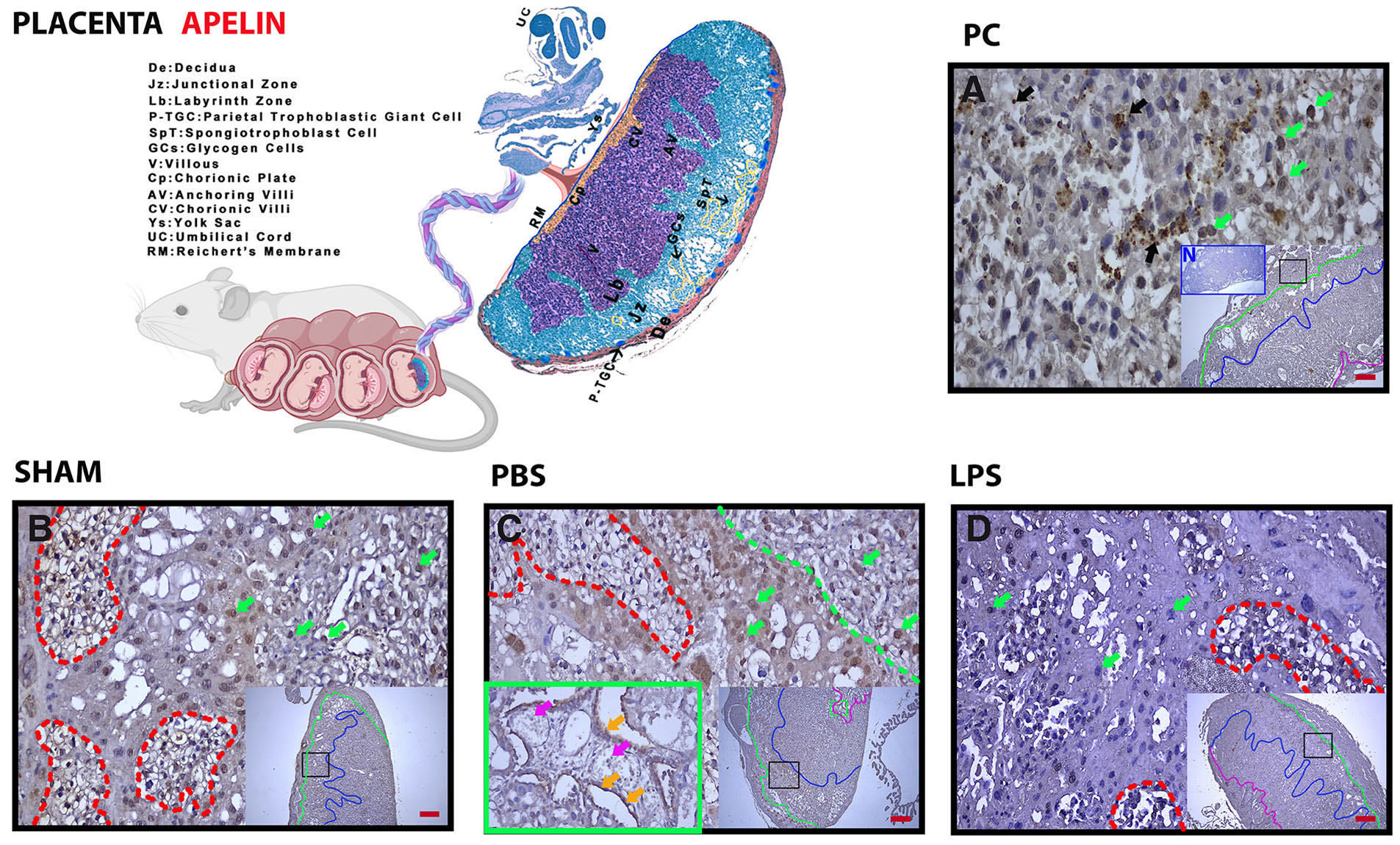
Placental apelin expression is decreased in LPS-induced preterm labor. The decrease is evident in glycogen islets and spongiotrophoblasts in the junctional region. Expression of placental apelin is concentrated in glycogen islets and spongiotrophoblasts in the junctional zone (Fig. 2 A-C, red dashed line and green arrows). In the group given LPS, the decrease in this zone was especially evident in spongiotrophoblasts (Fig. 2D, red dashed line and green arrows). Expression was also positive in granular structures (probable mast cell granules) in the decidua (Fig. 2A, black arrows) (Table 1).
Fig. 2. Apelin immunohistochemistry in placental zones and placental cell structures.
(A) Pregnant Control (PC); (B) Sham, surgical control; (C) Phosphate Buffer Solution (PBS), solvent control; (D) Lipopolysaccharide (LPS), 25 µg/100 µl. Above the green line is the Decidual Zone (DZ); above the blue line is the Junctional Zone (JZ); blue dashed bottom, Labyrinth Zone (LZ); below the pink line: Chorionic Zone (CZ); and Red Dashed Line, glycogen islets. Green arrows, spongiotrophoblasts; black arrows, positive staining granular structures. Each large image is an enlargement of the frame shown in the smaller image. N in (A), negative control (blue frame); magnification: 5X, 20X; scale bar, 50 µm.
Apelin receptor (APJ) immunohistochemistry in cervical orifice and cervix/uterine transition regions
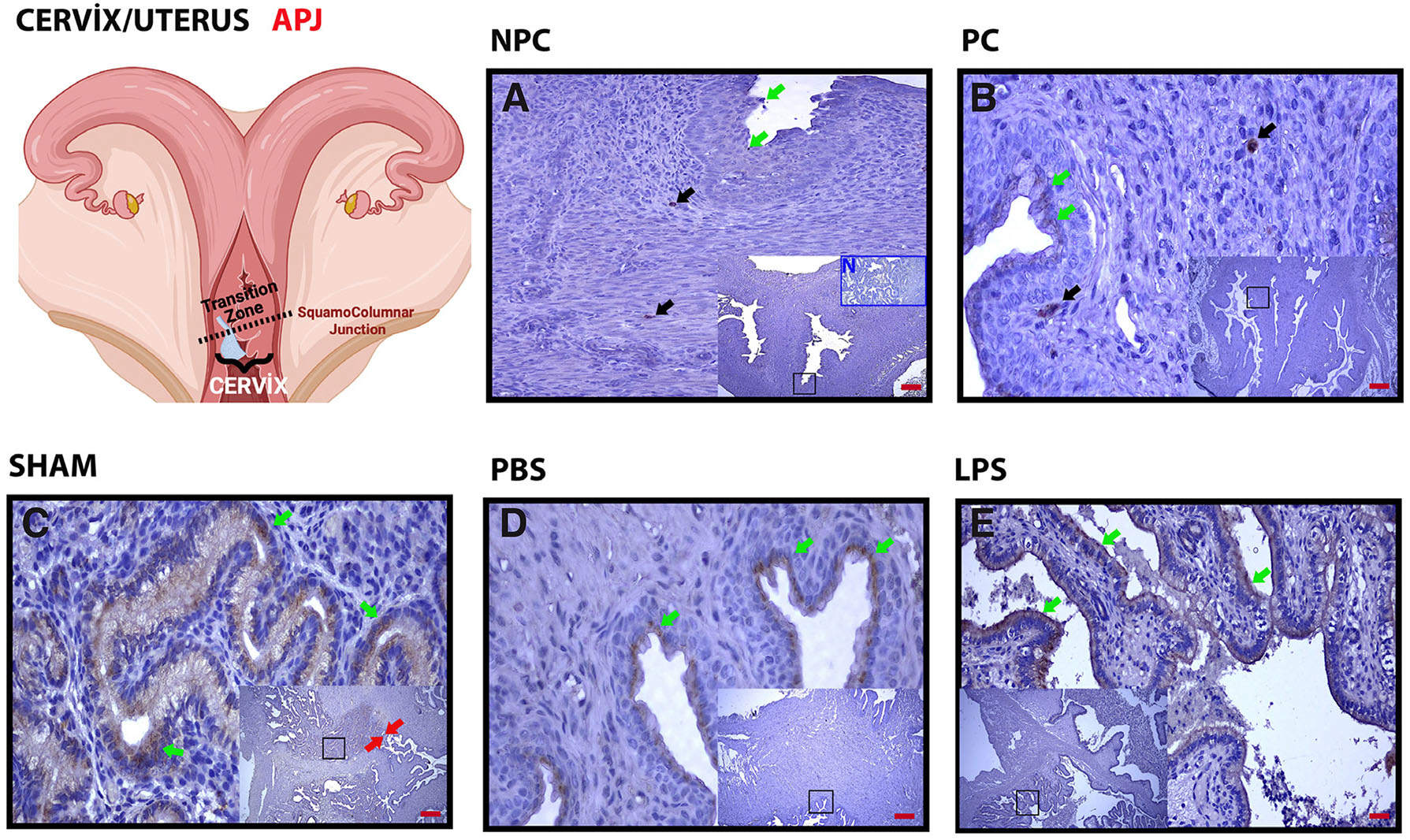
APJ labeling was very weak in the epithelium (Fig. 3A, green arrows) in the cervix in the non-pregnant group and positive in a few cells in the stroma in the internal os area (Fig. 3A, black arrows). With pregnancy, especially in the transformation zone, which is the cervix-uterine transition area, expression was intense in the epithelium from a certain border (Fig. 3C, red arrows; Fig. 3 B-D, green arrows). In the LPS group, expression increased, especially in the apical region compared to the basal epithelium (Fig. 3E, green arrows) (Table 2).
Table 2
Comparison of apelin receptor expression in the uterus, cervix and placenta in LPS-induced preterm labor
| Apelin / H-Score | NPC | PC | Sham | PBS | LPS | |
|---|---|---|---|---|---|---|
| Uterus | Epithelium | (-)/(+) | (+) | (+) | (+) | (+) |
| Muscle | (-) | (-) | (-) | (-) | (-) | |
| Gland | (-) | (-) | (-) | (-) | (-) | |
| Stroma | (+) | (+) | (+) | (+) | (+) | |
| Cervix | Epithelium | (-)/(+) | (-)/(+) | (-)/(+) | (-)/(+) | (-)/(+) |
| Stroma | (+) | (+) | (+) | (+) | (+) | |
| Placental Zones | Decidua | (+) | (+) | (+) | (+) | |
| Junctional | (+) | (++) | (++) | (+) | ||
| Labyrinth | (+) | (+) | (+) | (+) | ||
| Chorion | (+) | (+) | (+) | (+) | ||
H-Score Analysis. NPC, non-pregnant control; PC, pregnant control; Sham, surgical control; PBS, phosphate buffer solution, solvent control; LPS, lipopolysaccharide, 25 µg/100 µl. Abbreviations: ++, strongly positive; +, moderately positive; -/+, positive; -, negative or weakly positive.
Fig. 3. Basal APJ expression observed in the uterine epithelium with LPS application is concentrated in the apical area.
(A) Non-Pregnant Control (NPC); (B) Pregnant Control (PC); (C) Sham, surgical control; (D) Phosphate Buffer Solution (PBS), solvent control; (E) Lipopolysaccharide (LPS), 25 µg/100 µl. Black arrowheads, positive staining stromal cells; green arrowheads, positive staining uterine epithelium; red arrowheads, transition zone from stratified squamous epithelium to columnar epithelium. Each large image is an enlargement of the black frame within the thumbnail. N in (A), negative control (blue frame); magnification, 5X, 20X; scale bar, 50 µm.
Apelin receptor (APJ) immunohistochemistry of placental zones and placental cell structures
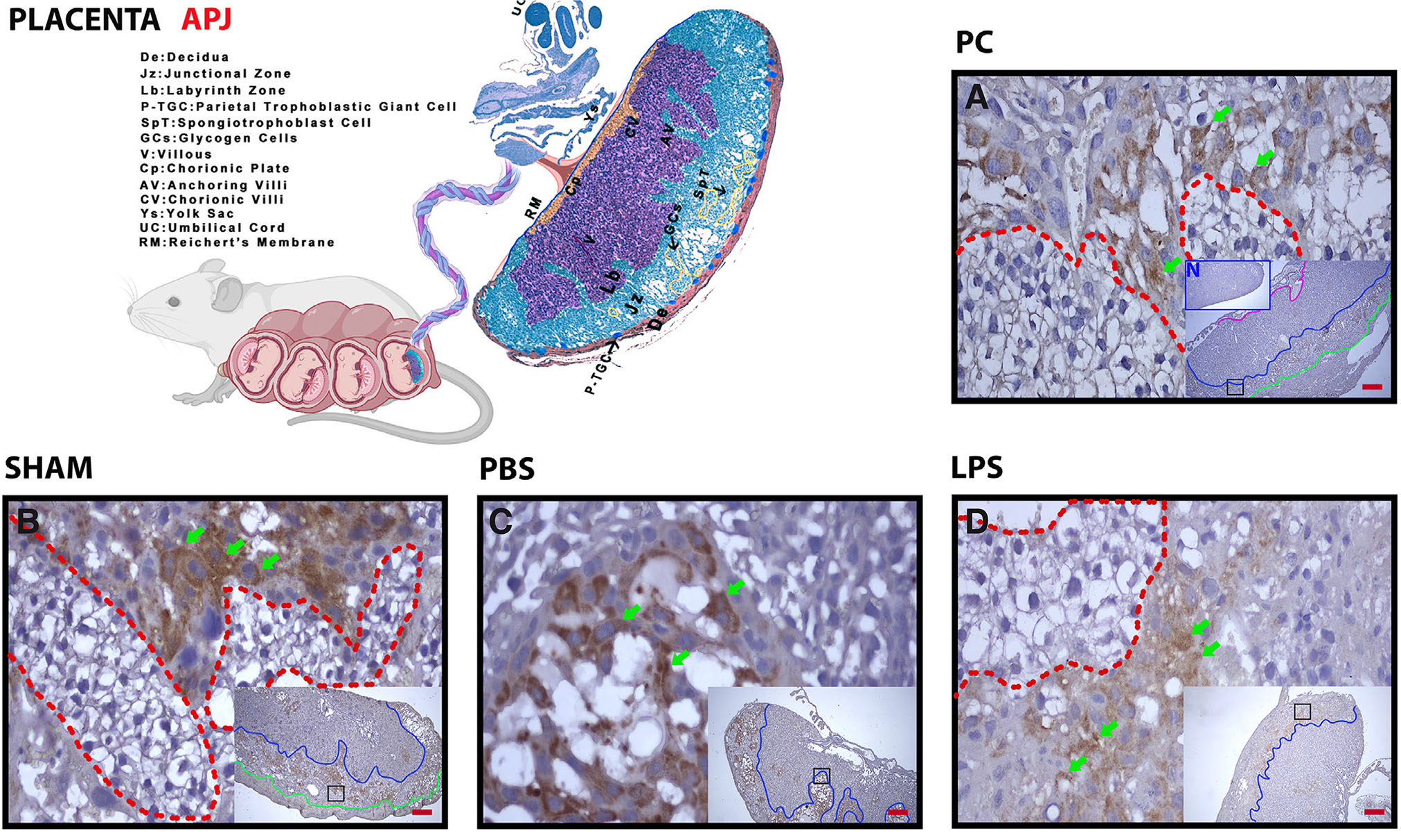
When the placenta was examined, APJ expression was intense in the junctional zone and positive in the labyrinth zone. In the junctional zone, especially spongiotrophoblasts were intensely positive (Fig. 4 A-C, green arrows), while glycogen islets were negative (Fig. 4 A-C, red dashed line). Expression was weak in the junctional zone in the group given LPS (Fig. 4D, above the blue line). Expression in glycogen islets was similar to other groups (Fig. 4D, red dashed line) (Table 2).
Fig. 4. The expression of placental APJ is decreased by LPS administration, especially in spongiotrophoblast.
(A) Pregnant Control (PC); (B) sham, surgical control; (C) Phosphate Buffer Solution (PBS), solvent control; (D) Lipopolysaccharide (LPS), 25 µg/100 µl. Above the green line, Decidual Zone (DZ); above the blue line: Junctional Zone (JZ); blue dashed bottom, labyrinth zone (LZ); red dashed line, glycogen islets; green arrows, spongiotrophoblasts. Each large image is an enlargement of the black frame within the thumbnail. N in (A), negative control (blue frame); magnification, 5X, 20X; scale bar, 50 µm.
Apelin protein levels in cervix, uterus and placental tissues
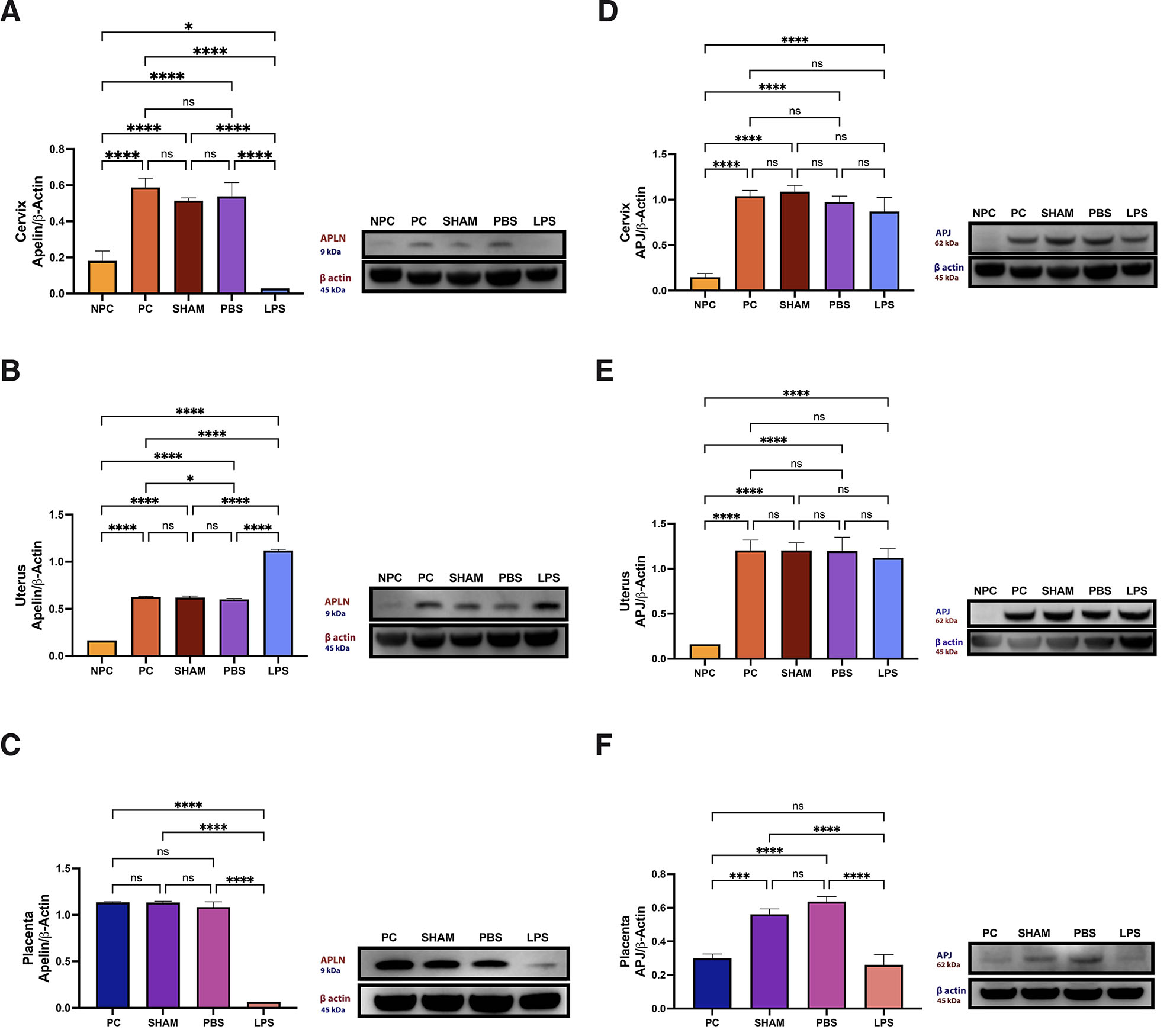
While the protein level decreased significantly in the LPS group in the cervix compared to the other groups (Fig. 5A), it increased in the uterus (Fig. 5B) (p<0.05). In general, apelin protein level increased significantly with pregnancy when compared to the control (p<0.05). Thus, apelin expression is increased at the tissue level in the cervix orifice and uterus in LPS-induced preterm labor. The change is especially intense in epithelial tissue. Apelin protein, which was detected at the basal level in the placenta in pregnant and non-pregnant groups, decreased significantly in the LPS group (Fig. 5C) (p<0.05).
Fig. 5. Protein levels of apelin.
[cervix (A), uterus (B), placenta (C)] and apelin receptor [cervix (D), uterus (E), placenta (F)]. Abbreviations: LPS, lipopolysaccharide, 25 µg/100 µl; NPC, Non-Pregnant Control; PBS, phosphate buffer solution, solvent control; PC, Pregnant Control; Sham, surgical control.
APJ protein levels in cervix, uterus and placental tissues
APJ protein levels increased in the cervix and uterus tissues with pregnancy (Fig. 5 D,E) (p<0.05). There was no significant difference in protein levels between the LPS-applied group and the other pregnant groups (Fig. 5, D,E). Although the amount of APJ protein is determined at a certain level with pregnancy, it increases in groups with any surgical procedure or incision (p<0.05). In contrast, it is similar to the control in the LPS group (Fig. 5F).
Discussion
When the results were evaluated in general, it was seen that APLN/APJ expression started after a certain point in the transition area from the cervix to the uterus. The placenta, especially the junctional region, had an important role in expression. To draw attention to the regional response and better evaluate it, the cervix/uterus and placenta were treated as separate sections.
Developing a healthy ovary and uterus is essential for successful female fertility. For this reason, it is vital to investigate the factors responsible for regulating ovarian and uterine functions that occur in the pre-pubertal period. Apelin, an adipokine, and its receptor APJ are found in female reproductive organs. The developmental localization of apelin and APJ in different compartments of the ovary and uterus is thought to be related to their physiological role (Anima et al., 2023).
It has been shown that apelin and APJ are also expressed in ovarian follicles and granulosa cells. Apelin and APJ are increased in polycystic ovary syndrome (PCOS), associated with abnormal ovarian hormone level and function. This situation draws attention to the place of apelin and APJ in menstrual cycle disorders and the anovulatory cycle (Liu et al., 2020). Some studies have reported that with the activation of the APJ receptor, Apelin causes a statistically significant increase in progesterone (P4) and Estradiol (E2) secretion. In vitro studies show that apelin can regulate steroidogenesis in ovarian cells (Kurowska et al., 2018, Roche et al., 2017). E2 can reach supraphysiological levels during controlled ovarian hyperstimulation. Although E2 is necessary for successful implantation, prolonged exposure to high E2 may cause cellular and textural changes in the oocyte and endometrium, and the endometrial response may be impaired depending on the difference in the E2/P4 ratio. This may result in an unsuccessful implantation (Joo et al., 2010). In a study examining apelin and APJ receptor change in the uterus in an experimental model mimicking the IVF process, the apelin and APJ levels were lower in the pregnant control group than in the PMSG+hCG group. In the study, it was emphasized that the increase in APLN/APJ protein expression due to superovulation, especially in uterine glands containing E2 and P4 receptors, uterine epithelium, and stromal cells may be related to the effort to balance the increased E2 and/or P4 levels in the blood after ovulation induction (Avci S and Celik Ozenci C, 2023).
APLN/APJ functions are not yet evident in the literature. There is evidence to suggest that apelin also affects the process of cell proliferation, inflammatory processes, and angiogenesis. Apelin reduces the production of pro-inflammatory cytokines by blocking the nuclear factor kappa light chain enhancer of the activated B cell (NF-κB)/natural killer (NK) signaling pathway responsible for inflammation (Chen et al., 2015, Yang et al., 2015). Accumulating evidence indicates that the functions of the APLN-APJ may vary depending on its target organs and physiological or pathophysiological conditions. While it was seen that APJ alone may have an inhibitory function independent of apelin, it was shown in a different study that apelin could inhibit uterine contraction. These findings suggest that APLN/APJ may display dual properties (Hehir and Morrison, 2012, Sun et al., 2011).
Our study data shows a basal expression of apelin and APJ in the cervix and uterine area at 14.5 days of gestation. Considering that it has a place in the E2/P4 balance, as mentioned above, it is predicted that this axis should be protected to maintain a healthy pregnancy. While apelin is lower in pre-pregnancy control tissues, APJ is close to negative this confirms that there may be expression differences in different tissues and even in other cells within the same tissue, as reported in the literature. The increase in expression in the cervical epithelium in the LPS group is thought to be an effort to compensate for the decrease in protein level with post-transitional modification. In addition, although no study was found on the expression pattern in the cervix, it was predicted that the decrease might have triggered cervical maturation for the evacuation of the pups. Apelin also showed increased tissue and protein levels in the uterus. It is reported that apelin is required to reduce proinflammatory cytokine production. Therefore, it was hypothesized that the uterus supports an upregulated cellular response to maintain balance due to LPS-induced depression of apelin. Moreover, Although APJ expression increased with pregnancy in the cervix and uterus tissues, LPS application did not make a significant difference. This response suggests that apelin may act independently of APJ or bind to a different receptor in the inflammatory process.
Apelin and APJ are known to be expressed in the human placenta (Mlyczyńska et al., 2021). The presence of apelin in the human placenta indicates the importance of this peptide for pregnancy (Cobellis et al., 2007, Telejko et al., 2010). Studies have shown the positive effects of apelin on pre-eclampsia (PE). Chronic administration of apelin has been shown to reduce maternal hypertension and proteinuria and improve fetal growth in a rat model of PE (Wang et al., 2017).
When we examine the studies in terms of preterm birth, Lim et al., examined the placenta and fetal membranes obtained from women with preterm delivery. They detected significantly lower apelin expression in preterm birth fetal membranes. However, they emphasized that the role of the apelin/APJ system in placental endothelial and vascular function should be confirmed in future studies (Lim et al., 2013). Moreover, the necessity of examining the APLN/APJ axis, especially in the first, second, and third trimesters, with a focus on temporal change, is also emphasized in the literature (Alizadeh Pahlavani, 2022). Studies in mice have shown that apelin can increase glucose utilization (Dray et al., 2008). During pregnancy, an impaired/altered insulin sensitivity may be encountered, which is a necessary adaptation to meet the glucose requirement of the fetus (Paradisi et al., 2002, Ramos et al., 2003).
The majority of the endocrine components of the mouse placenta are composed of spongiotrophoblasts and trophoblast glycogen cells located in the junctional zone (Hemberger et al., 2020). It has been reported that apelin expression decreases during pregnancy in cytotrophoblast and syncytiotrophoblast, whereas APJ expression increases in cytotrophoblast and endothelial cells (Cobellis et al., 2007). Apelin also has a positive effect on the survival of trophoblasts by inhibiting apoptosis (Dawid et al., 2021).
Studies report that apelin expression can be approximately 20 times higher in the placenta than in adipose tissue (Vaughan et al., 2019). In pregnant women, the APLN/APJ system can promote changes such as fatty acid oxidation, glucose uptake, angiogenesis, vasodilation, hypotension, myocardial contraction, diuresis, and stress response. Apelin affects the placental vascular tonus and is associated with exchanging oxygen and nutrients between mother and fetus. Moreover, she reported that apelin is crucial for the fetal cardiovascular system and early placental development, increased fetal angiogenesis, and energy homeostasis in mice (Eberlé et al., 2019).
Plasma apelin and insulin levels are significantly higher in obese patients, and their expression is strongly inhibited by fasting in adipose cells and is recovered after feeding. Also, the lack of insulin increases its concentration, and apelin can act like insülin (Alipour et al., 2017, Boucher et al., 2005). Van Mieghem et al., showed that the serum level of apelin was 30% lower in women with intrauterine growth retardation compared with uncomplicated pregnancies (Van Mieghem et al., 2016). The findings identify the apelin/APJ axis as a promising therapeutic target to increase reproductive capacity in diabetic patients (Li et al., 2022, Song et al., 2022).
In our study, when apelin and APJ localization and protein levels in the placenta were evaluated, especially in terms of fetal energy requirement, it was observed that glycogen islets and spongiotrophoblasts in the junctional zone, which have endocrine function, made intense apelin expression compared to other compartments. In contrast, protein amount and cellular expression decreased significantly when LPS was given. When cellular expression and severity are evaluated, our results show that expression is positive in glycogen islets in addition to spongiotrophoblasts, and apelin is suppressed during an inflammatory process similar to preeclampsia. APJ was negative in glycogen islets and positive in spongiotrophoblasts and increased with any invasive application. Expression in the LPS-treated group was similar to the control. Combined with the literature, these data confirm that apelin may have a role in insulin and glucose balance. It suggests that the placental increase of APJ due to invasion may be triggered above a certain threshold, and the increase may be caused by stress or trauma.
In conclusion, although preterm birth is among the preventable causes of maternal and infant mortality, the process has not yet been clarified since it is associated with many mechanisms in terms of research dynamics. Our study results show that after LPS application, there is a decrease in apelin expression, especially at protein levels in the cervix and placenta. In this environment, anti-inflammatory activity is decreased in the cervix and placenta while the pro-inflammatory effect is increased. Meanwhile, APJ decreased in the placenta but remained unchanged in the cervix. On the other hand, apelin increased in the uterus while APJ remained unchanged, so the uterus may play a role in balancing the decreased expression.
Again, LPS/inflammation seems to disrupt the energy balance of the fetus by affecting apelin secretion in glycogen islets in the placenta. In this process, it was thought that the uterus primarily tried to compensate for the regression through epithelial cells. Decreased APJ in spongiotrophoblasts may indicate a dysregulation in placental hormone regulation. Since apelin tends to increase during pregnancy, it can also be considered essential for maintaining a healthy pregnancy. The threshold value and possible mechanisms of stress and trauma-induced increase in APJ must be investigated. Although it is evident that a complex process needs to be supported by further studies to illuminate it, the new data that our study results will add to the literature will contribute to progress in this regard and encourage the planning of recent studies.
Materials and Methods
Experimental design
Five-week-old CD-1 female mice in estrus (assessed by smear and appearance of vaginal mucosa) were housed in separate cages with males for mating, and mating plugs were checked the next day. The plaque-positive female mice were considered pregnant = 0.5 days. Experimental groups were arranged as Non-pregnant control (NPC), Pregnant Control (PC), Sham, PBS, and Lipopolysaccharide (LPS). There are n:6 subjects in each group. Before the experiment, the following treatments were performed on pregnant female mice according to their groups;
LPS (L2630; Sigma) was administered as a single dose (25 μg/100 μl in PBS) at 10:00 a.m. The application volumes in all groups are 100 µl. All surgical procedures were performed at 14.5 dpc (days after coitus). A midline laparotomy was performed during the surgery, and applications were made between the two uterine sacs closest to the cervix. The sacrificed subjects' cervix, uterus, and placenta tissues were removed at 15.5 dpc. Localization of apelin and APJ in tissue was determined by immunohistochemistry, and protein amounts were evaluated by western blot.
Surgical procedures
Mid-line laparotomy was performed by anesthetizing mice with 0.017 ml/kg Avertin (2.5% tribromoethanol T48402; Sigma, and 2.5% tert-amyl alcohol 240486; Sigma) at 14.5 days of gestation. In the right uterine horn, a 27-gauge needle was inserted between the 1st and 2nd sacs, and LPS or PBS was injected according to the group in which the subject was included. The peritoneum was closed with 5-0 Vicryl sutures. Fluid and food intakes were ad libitum. The postoperative observation was performed at least twice on the day of surgery. Subjects carrying <3 fetuses in the uterine horn (1 subject) or experiencing severe stress after administration were euthanized and excluded from the experiment. The cervical dislocation was used for euthanasia in the experimental groups.
Division of tissue into regions
When we evaluate the cervix/uterus and placenta findings separately in terms of the literature; our previous study revealed that the expression power of proteins can change in the cervix/uterus area, and different responses can be produced during the transition from the cervix to the uterus (Avci et al., 2022b). Therefore, in this study, care was taken to include the transition areas of the uterus and cervix and mark these areas. However, it is not easy to distinguish the cervix-uteri region as a clear line in protein-level examinations. It is known that the placenta consists of different areas and cell components that can produce different responses (Avci et al., 2022a, Simmons et al., 2007). For this reason, it was tried to determine the states of cells and placental zones separately, as in the cervix and uterus, regarding the markers examined in evaluating proteins' localizations and cellular expression status.
Immunohistochemistry
After the tissues were removed, 10% formalin fixative (818708; Merck) was prepared, and the cervix, uterus, and placenta tissues were kept in the solution for 24 hours. Following incubation, tissues were washed with tap water for three hours and then kept in a series of 70%, 80%, and 90% ethanol (459844; Sigma-Aldrich), respectively, for 24 hours. Tissues were incubated in 100% ethanol for three hours and then cleared in xylene (534056; Sigma-Aldrich) for approximately 5 minutes. After passing through paraffin again and removing xylene, it was embedded in clean paraffin and cut to a thickness of five micrometers. Sections were incubated overnight at 60°C, passed through xylene and a decreasing alcohol series, and then 1X PBS (P4417-100TAB; Sigma-Aldrich) solution was used to wash the sections. Before staining, sections were treated with Citric Acid (100244; Merck) buffer. 3% H2O2 Solution (106009; Merck, 18312; Sigma) was used to eliminate endogenous peroxidase activity, followed by Ultra V Block (TA-125-UB Thermo Scientific).
Anti-Apelin (PA5-114860; Thermo, 1/100, 9 kDa), Anti-Apelin Receptor (ABD43; Sigma, 1/200, 62 kDa), and Goat Anti-Rabbit IgG Antibody (H+L), Biotinylated secondary antibody (Vector; BA-1000, 1/500) were used. The sections were incubated with HRP-Conjugated Streptavidin Peroxidase (TS-125 HR, Thermo) at room temperature for 20 minutes and then washed with PBS. Diaminobenzidine tablet (D4168; Sigma) was used as chromogen, and Mayer's hemalum solution (109249, Merck) was used for contrast staining. Protein expression levels were evaluated with Image J (1.52 R, National Institutes of Health, USA) software, and statistical analysis was performed. In the statistical evaluations, images of 3 different areas from 3 randomly selected sections of 6 experimental animals were analyzed for each marker in all experimental groups.
Histological H-Score evaluation
Immunohistochemical staining intensity was evaluated with H-Score for each experimental group, considering the tissue-specific compartments. For the cervix; the transitional region, endo, and ecto cervical sections, and in the placenta; chorion, labyrinth, junctional zone, and decidual areas were examined. Uterus, cervix, and placenta tissues were scored regarding epithelium, stroma, and muscle and vascular structures in these regions. The H-Score analysis examined the areas according to the staining intensity by giving numerical values between (0-3).
Protein extraction and Western Blot analysis
To prepare cervix, uterus, and placenta tissue lysates, using lysis buffer solution (Tris, 108387; Merck) and protease inhibitor cocktail (P8340; Sigma), the amount of protein present in the lysates was measured with the BCA protein assay kit (23225; Pierce). SDS-Polyacrylamide gel electrophoresis was performed by loading 50 µg of protein for each well. For this process, 120 V 90 minutes of electrophoresis was applied using a 4-12% Bolt Bis-Tris Plus Gel system (NW04120BOX; Invitrogen). Then, after 7 min of blotting with the iBlot Dry gel transfer system (IB1001; Invitrogen), the membranes were blocked at room temperature using 5% nonfat dry milk powder (Blotting Grade Blocker, 1706404; Bio-Rad). The imaging step was started after the primary antibodies and membranes were incubated overnight on a shaker at +4°C. Primary antibodies are as follows:
Primary antibodies: Anti-Apelin (PA5-114860; Thermo, 1/100, 9 kDa), Anti-Apelin Receptor (US43, Sigma 1/200, 62 kDa). Goat Anti-Rabbit (ab205718) was used as the secondary antibody. Internal control is beta-actin (Cell Signaling, 13E5, 1/2000, 45 kDa).
After incubation with the primers, they were washed with Tris-buffered saline-TBS-T (1% Tween-20, 822184; Merck), followed by incubation of the membranes with secondary antibodies for 45 minutes at room temperature. Membranes washed with TBS-T were incubated with Super Signal Chemiluminescence (CL)-HRP (A45918; Thermo Scientific) and imaged with Azure Imaging Biosystem (Color C 600).
Statistical analysis
After protein and beta-actin band measurements were made using the Image J program for western blot analysis, protein/beta-actin values were evaluated statistically using the GraphPad Prism v9 program. The homogeneity of the obtained data was assessed with the Brown-Forsythe test.
Holm-Sidak's multiple comparisons test was used to evaluate the difference between groups using one-way analysis of variance (ANOVA) for parametric data. The statistical significance limit was accepted as p<0.05. The difference between the groups is marked with asterisks in the columns. BioRender Scientific Image and Illustration Software was used for the illustrations.
Declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
S.A.: Design, Surgical Procedures, Funding, Analysis, Data Collection, Processing and Analysis, Writing, Literature Review, Interpretation, E.G: Data Collection, Processing and Analysis, N.A: Funding, Literature Review, Interpretation.
Funding
The project was supported by the Scientific and Technological Research Council of Turkey (1002/A-222S696).
Availability of data and material
All data generated or analyzed during this study are included in this published article, and all raw data will be provided by the corresponding author upon request.
Ethical Approval
Approval was obtained from the ethics commYttee of Akdeniz University Animal Experiments Local Ethics Committee/02.01.2023-5. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
References
Alipour F. G., Ashoori M. R., Pilehvar-Soltanahmadi Y., Zarghami N. (2017). An overview on biological functions and emerging therapeutic roles of apelin in diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 11: S919-S923.
Alizadeh Pahlavani H. (2022). Possible roles of exercise and apelin against pregnancy complications. Frontiers in Endocrinology 13: 965167.
Anima B., Guruswami G., Roy V. K. (2023). Postnatal developmental expression and localization of apelin and apelin receptor protein in the ovary and uterus of mice. Molecular Reproduction and Development 90: 42-52.
Antushevich H., Wójcik M. (2018). Review: Apelin in disease. Clinica Chimica Acta 483: 241-248.
Avci S., Celik-Ozenci C. (2023). Controlled ovarian hyperstimulation increases the expression of apelin and apelin receptor in uterus. Akdeniz Medical Journal 9: -186.
Avci S., Kuscu N., Durkut B., Kilinc L., Ustunel I., Celik-Ozenci C. (2022a). Altered expression of Notch signaling, Tlr receptors, and surfactant protein expression after prostaglandin inhibition may be associated with the delayed labor in LPS-induced mice. Journal of Assisted Reproduction and Genetics 39: 1531-1544.
Avci S., Kuscu N., Kilinc L., Ustunel I. (2022b). Relationship of Notch Signal, Surfactant Protein A, and Indomethacin in Cervix During Preterm Birth: Mast Cell and Jagged-2 May Be Key in Understanding Infection-mediated Preterm Birth. Journal of Histochemistry & Cytochemistry 70: 121-138.
Azevedo Portilho N., Pelajo-Machado M. (2018). Mechanism of hematopoiesis and vasculogenesis in mouse placenta. Placenta 69: 140-145.
Azevedo Portilho N., Tavares Guedes P., Croy B. A., Pelajo-Machado M. (2016). Localization of transient immature hematopoietic cells to two distinct, potential niches in the developing mouse placenta. Placenta 47: 1-11.
Boucher J., Masri B., Daviaud D., Gesta S., Guigné C., Mazzucotelli A., Castan-Laurell I., Tack I., Knibiehler B., Carpéné C., Audigier Y., Saulnier-Blache J.S., Valet P. (2005). Apelin, a Newly Identified Adipokine Up-Regulated by Insulin and Obesity. Endocrinology 146: 1764-1771.
Callaghan W. M., MacDorman M. F., Rasmussen S. A., Qin C., Lackritz E. M. (2006). The Contribution of Preterm Birth to Infant Mortality Rates in the United States. Pediatrics 118: 1566-1573.
Chen L., Tao Y., Feng J., Jiang Y. R. (2015). Apelin Protects Primary Rat Retinal Pericytes from Chemical Hypoxia-Induced Apoptosis. Journal of Ophthalmology 2015: 1-14.
Chen M. M., Ashley E. A., Deng D. X.F., Tsalenko A., Deng A., Tabibiazar R., Ben-Dor A., Fenster B., Yang E., King J. Y., Fowler M., Robbins R., Johnson F. L., Bruhn L., McDonagh T., Dargie H., Yakhini Z., Tsao P. S., Quertermous T. (2003). Novel Role for the Potent Endogenous Inotrope Apelin in Human Cardiac Dysfunction. Circulation 108: 1432-1439.
Cobellis L., De falco M., Mastrogiacomo A., Giraldi D., Dattilo D., Scaffa C., Colacurci N., De Luca A., (2007). Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histology and histopathology 22: 1-8.
Dawid M., Mlyczyńska E., Jurek M., Respekta N., Pich K., Kurowska P., Gieras W., Milewicz T., Kotula-Balak M., Rak A. (2021). Apelin, APJ, and ELABELA: Role in Placental Function, Pregnancy, and Foetal Development—An Overview. Cells 11: 99.
Dray C., Knauf C., Daviaud D., Waget A., Boucher J., Buléon M., Cani P. D., Attané C., Guigné C., Carpéné C., Burcelin R., Castan-Laurell I., Valet P. (2008). Apelin Stimulates Glucose Utilization in Normal and Obese Insulin-Resistant Mice. Cell Metabolism 8: 437-445.
Eberlé D., Marousez L., Hanssens S., Knauf C., Breton C., Deruelle P., Lesage J. (2019). Elabela and Apelin actions in healthy and pathological pregnancies. Cytokine & Growth Factor Reviews 46: 45-53.
Erlebacher A., Fisher S. J. (2017). Baby's First Organ. Scientific American 317: 46-53.
Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. (2008). Epidemiology and causes of preterm birth. The Lancet 371: 75-84.
Hehir M. P., Morrison J. J. (2012). The adipokine apelin and human uterine contractility. American Journal of Obstetrics and Gynecology 206: 359.e1-359.e5.
Hemberger M., Hanna C. W., Dean W. (2020). Mechanisms of early placental development in mouse and humans. Nature Reviews Genetics 21: 27-43.
Hu D., Cross J. C. (2010). Development and function of trophoblast giant cells in the rodent placenta. The International Journal of Developmental Biology 54: 341-354.
Isaac S. M., Langford M. B., Simmons D. G., Adamson S. L., (2014). Anatomy of the mouse placenta throughout gestation, in the guide to investigation of mouse pregnancy. Academic Press, London.
Ishimaru Y., Sumino A., Kajioka D., Shibagaki F., Yamamuro A., Yoshioka Y., Maeda S. (2017). Apelin protects against NMDA-induced retinal neuronal death via an APJ receptor by activating Akt and ERK1/2, and suppressing TNF-α expression in mice. Journal of Pharmacological Sciences 133: 34-41.
Joo B. S., Park S. H., An B. M., Kim K. S., Moon S. E., Moon H. S. (2010). Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertility and Sterility 93: 442-446.
Kawamata Y., Habata Y., Fukusumi S., Hosoya M., Fujii R., Hinuma S., Nishizawa N., Kitada C., Onda H., Nishimura O., Fujino M. (2001). Molecular properties of apelin: tissue distribution and receptor binding. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1538: 162-171.
Kim H. S., Cho J. H., Park H. W., Yoon H., Kim M. S., Kim S. C. (2002). Endotoxin-Neutralizing Antimicrobial Proteins of the Human Placenta. The Journal of Immunology 168: 2356-2364.
Klein L. L., Gibbs R. S. (2004). Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. American Journal of Obstetrics and Gynecology 190: 1493-1502.
Kurowska P., Barbe A., Różycka M., Chmielińska J., Dupont J., Rak A. (2018). Apelin in Reproductive Physiology and Pathology of Different Species: A Critical Review. International Journal of Endocrinology 2018: 1-12.
Li C., Cheng H., Adhikari B. K., Wang S., Yang N., Liu W., Sun J., Wang Y. (2022). The Role of Apelin–APJ System in Diabetes and Obesity. Frontiers in Endocrinology 13: 820002.
Lim R., Barker G., Riley C., Lappas M. (2013). Apelin Is Decreased With Human Preterm and Term Labor and Regulates Prolabor Mediators in Human Primary Amnion Cells. Reproductive Sciences 20: 957-967.
Liu Q., Jiang J., Shi Y., Mo Z., Li M. (2020). Apelin/Apelin receptor: A new therapeutic target in Polycystic Ovary Syndrome. Life Sciences 260: 118310.
Mercati F., Scocco P., Maranesi M., Acuti G., Petrucci L., Cocci P., Renzi A., De Felice E., Dall’Aglio C. (2019). Apelin system detection in the reproductive apparatus of ewes grazing on semi-natural pasture. Theriogenology 139: 156-166.
Mlyczyńska E., Myszka M., Kurowska P., Dawid M., Milewicz T., Bałajewicz-Nowak M., Kowalczyk P., Rak A. (2021). Anti-Apoptotic Effect of Apelin in Human Placenta: Studies on BeWo Cells and Villous Explants from Third-Trimester Human Pregnancy. International Journal of Molecular Sciences 22: 2760.
Moster D., Lie R. T., Markestad T. (2008). Long-Term Medical and Social Consequences of Preterm Birth. New England Journal of Medicine 359: 262-273.
Nour N. M., (2012). Premature delivery and the millennium development goal. Reviews in obstetrics & gynecology 5: 100-105.
Paradisi G., Biaggi A., Ferrazzani S., De Carolis S., Caruso A. (2002). Abnormal Carbohydrate Metabolism During Pregnancy. Diabetes Care 25: 560-564.
Ramos M. P., Crespo-Solans M. D., del Campo S., Cacho J., Herrera E. (2003). Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. American Journal of Physiology-Endocrinology and Metabolism 285: E318-E328.
Roche J., Ramé C., Reverchon M., Mellouk N., Rak A., Froment P., Dupont J. (2017). Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reproduction 153: 589-603.
Romero R., Dey S. K., Fisher S. J. (2014). Preterm labor: One syndrome, many causes. Science 345: 760-765.
Simmons D. G., Fortier A. L., Cross J. C. (2007). Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Developmental Biology 304: 567-578.
Song K., Yang X., An G., Xia X., Zhao J., Xu X., Wan C., Liu T., Zheng Y., Ren S., Wang M., Chang G., Cronin S. J. F., Penninger J. M., Jing T., Ou X., Rao S., Liu Z., Zhao X.Y. (2022). Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models. Nature Communications 13: 7335.
Sun X., Iida S., Yoshikawa A., Senbonmatsu R., Imanaka K., Maruyama K., Nishimura S., Inagami T., Senbonmatsu T. (2011). Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertension Research 34: 701-706.
Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., Fujino M. (1998). Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochemical and Biophysical Research Communications 251: 471-476.
Telejko B., Kuzmicki M., Wawrusiewicz-Kurylonek N., Szamatowicz J., Nikolajuk A., Zonenberg A., Zwierz-Gugala D., Jelski W., Laudański P., Wilczynski J., Kretowski A., Gorska M. (2010). Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Research and Clinical Practice 87: 176-183.
Tsai K., Tullis B., Jensen T., Graff T., Reynolds P., Arroyo J. (2021). Differential expression of mTOR related molecules in the placenta from gestational diabetes mellitus (GDM), intrauterine growth restriction (IUGR) and preeclampsia patients. Reproductive Biology 21: 100503.
Van Mieghem T., Doherty A., Baczyk D., Drewlo S., Baud D., Carvalho J., Kingdom J. (2016). Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reproductive Sciences 23: 1037-1043.
Vaughan O.R., Powell T.L., Jansson T. (2019). Apelin is a novel regulator of human trophoblast amino acid transport. American Journal of Physiology-Endocrinology and Metabolism 316: E810-E816.
Walani S. R. (2020). Global burden of preterm birth. International Journal of Gynecology & Obstetrics 150: 31-33.
Wang C., Liu X., Kong D., Qin X., Li Y., Teng X., Huang X. (2017). Apelin as a novel drug for treating preeclampsia. Experimental and Therapeutic Medicine 14: 5917-5923.
Wang X., Zhang L., Li P., Zheng Y., Yang Y., Ji S. (2022). Apelin/APJ system in inflammation. International Immunopharmacology 109: 108822.
- WHO , (2023). Born Too Soon: The Global Action Report on Preterm Birth World Health Organization Geneva
Xiong Q., He W., Wang H., Zhou J., Zhang Y., He J., Yang C., Zhang B. (2017). Effect of the spinal apelin-APJ system on the pathogenesis of chronic constriction injury-induced neuropathic pain in rats. Molecular Medicine Reports 16: 1223-1231.
Yang F., Bai Y., Jiang Y. (2015). Effects of Apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides 69: 133-143.
Zhou H., Yang R., Wang W., Xu F., Xi Y., Brown R. A., Zhang H., Shi L., Zhu D., Gong D.W. (2018). Fc-apelin fusion protein attenuates lipopolysaccharide-induced liver injury in mice. Scientific Reports 8: 11428.