Int. J. Dev. Biol. 66: 333 - 347 (2022)
Intraflagellar transport 20 cilia-dependent and cilia-independent signaling pathways in cell development and tissue homeostasis
Open Access | Review | Published: 17 August 2022
Abstract
Intraflagellar transport (IFT) is an essential condition for ciliogenesis. The primary cilia protrude like antennae and act as chemical or mechanical sensory organelles that coordinate specific receptor localization and signal transduction. IFT20 is the smallest molecule in IFT complex B, which is located in both the cilia and the Golgi complex. Recent studies have shown that IFT20 is a key molecule in multiple signaling pathways. Importantly, in the function of IFT20, signal transduction is not restricted to cilia, but is also involved in non-ciliary functions. Here we summarize current knowledge regarding IFT20-mediated signaling pathways and their relationship with cell development and tissue homeostasis, and analyse the cilia-dependent and cilia-independent mechanisms of IFT20 coordinated signaling pathways and potential crosstalk between the mechanisms. This review provides a comprehensive perspective on IFT20 coordinates signaling mechanisms in cell development and tissue homeostasis.
Keywords
primary cilia, Intraflagellar transport (IFT)20, cilia-dependent signaling pathways, cilia-independent signaling pathway
Introduction
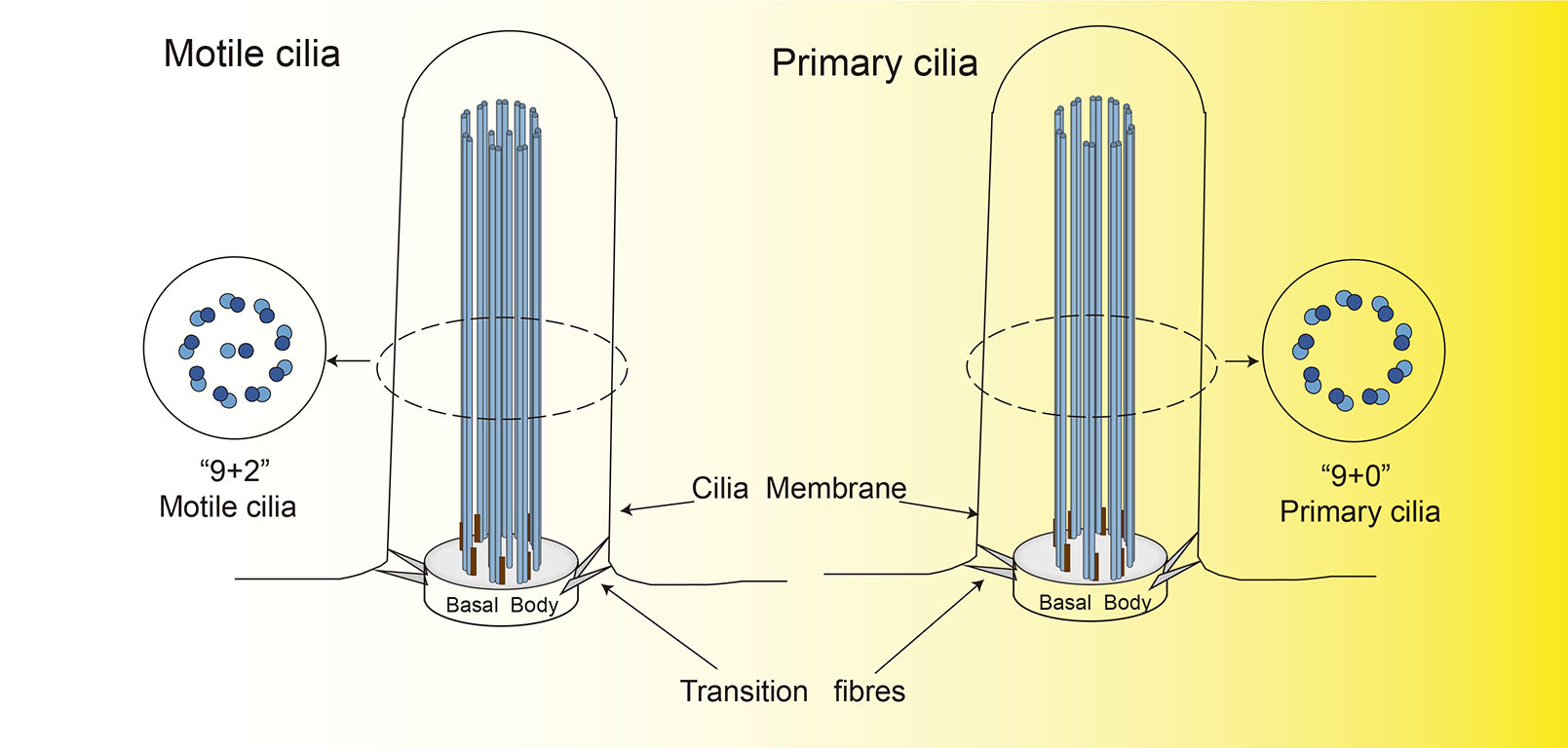
Cilia and flagella are microtubule (MT)-based and membrane-bound organelles that project from the cell surface and serve both motile and sensory functions. The core MT structures, called axonemes, originate in and grow from modified centrioles to form structures that become the ciliary basal body. The axonemes of microtubules are generally composed of one of two common patterns. The 9 + 2 MT structure consists of nine pairs of peripheral double MTs surrounded by a pair of central single MTs, and the 9 + 0 pattern lacks the central pair and thus only has the nine pairs of peripheral double MTs (Łysyganicz et al., 2021). Mammalian cilia are typically classified into two types: motile cilia and primary cilia. The motile cilia tend to possess the 9 + 2 MT structure (Zhao et al., 2020), and the primary cilia tend to have the 9 + 0 pattern (Fig. 1). Several exceptions to these patterns exist, as motile 9 + 0 cilia and immotile 9 + 2 cilia can be formed (Takeda and Narita, 2012). Moreover, recent studies have shown that the double microtubule structure of the 9 + 0 pattern is only present at the basal level of mammalian primary cilia; as the microtubule extends to the top, this structure gradually transforms into one consisting of actin filaments associated with unstructured microtubules decorated with end-binding 1 protein (Kiesel et al., 2020). This structure is clearly different from the more stable 9 + 2 MT structure of motile cilia. Motile cilia make cells move, for example in the case of sperm motility (Leung et al., 2021), and they are involved in the movement of fluids through a tissue, such as in the clearance of mucus (Bustamante-Marin et al., 2019), the flow of cerebrospinal fluid (Thouvenin et al., 2020), and leftward flow in the embryonic node (Schweickert et al., 2007). In addition, both motile cilia and primary cilia perform sensory functions (Pigino, 2021). Primary cilia in particular function as sensors of changes in the extracellular environment, and they transmit this information through signaling pathways to regulate cell development and other physiological processes (Cai et al., 2017). Because of the diverse roles of primary cilia, defects in these structures are associated with multiple ciliopathies(Zhou et al., 2020), including polycystic kidney disease (Ma et al., 2013), Bardet-Biedl syndrome (Kanie and Jackson, 2021) and Meckel-Gruber syndrome (Zhang et al., 2021).
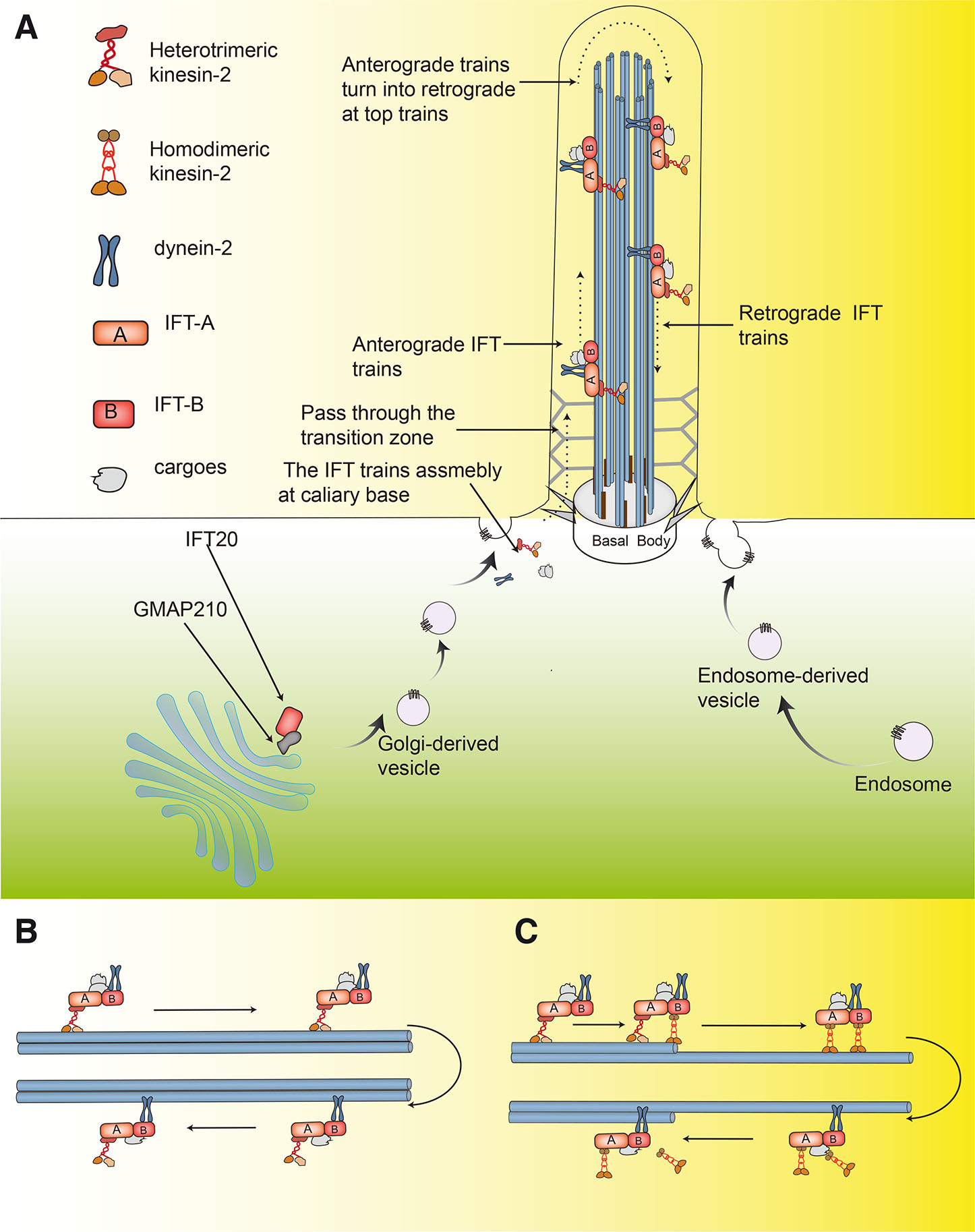
The biogenesis, function and structural maintenance of cilia rely on vesicular and IFT pathways. Pathways associated with vesicular trafficking mainly involve the transport of specific signaling molecules or receptors from Golgi or endosomes to the ciliary base, which guides the early steps of ciliogenesis (Sung and Leroux, 2013). Because the cilia themselves cannot synthesize protein, the further assembly and maturation of cilia require specialized IFT pathways to transport building blocks and signaling molecules. Movement in IFT is powered by motor proteins that are directed both toward and away from the cilia. Kinesin-2 (KIF3 in mammals) (Engelke et al., 2019) and cytoplasmic dynein-2 (Toropova et al., 2019) drive anterograde and retrograde IFT movement, respectively, and the transport is mediated by two complexes, namely IFT-A and IFT-B(Wei et al., 2012). The IFT-A complex contains known 6 proteins. IFT-B can be further divided into two sub-complexes, IFT-B1 and IFT-B2, which contain 10 and 6 known proteins, respectively (Taschner et al., 2016). The structures that mediate IFT are often referred to as IFT trains. In anterograde IFT, these trains are assembled in the basal body of the cilia as the IFT complex interacts with cargoes and kinesin-2 (Zhu et al., 2021). These combined structures then traverse the transition zone (Scheidel and Blacque, 2018), a Y-type diffusion barrier between the microtubule doublets and ciliary membrane that controls the entrance and exit of ciliary proteins (Webb et al., 2020). The structure then moves to the top of the cilia along the B-tubule of microtubule doublets or the A-tubule of primary cilia distal singlet axonemal microtubules (Kiesel et al., 2020), up to the ciliated tip. At the tip, anterograde trains turn into retrograde trains that return to ciliary base along the A-tubule under the power of dynein-2 (Fig. 2) (Webb et al., 2020; Wingfield et al., 2021).
One protein in the IFT-B complex, IFT20, is not only located in the cilia and the centrosome pool, but is also found anchored to the Golgi complex by the Golgi microtubule-associated protein 210 (GMAP-210) (Follit et al., 2008). In ciliated cells, on the one hand, IFT20 located in the cilia participates in the transport of IFT trains. On the other hand, IFT20 located in the Golgi complex moves rapidly between the Golgi complex and the cilia, facilitating the trafficking of ciliary membrane proteins from the Golgi complex to the basal body of cilia and playing an important role in the assembly and function of cilia (Follit et al., 2006). IFT20 also possesses cilia-independent functions. For example, IFT20 mediates T cell antigen receptor (TCR) signaling in the activation of T cells in immune synapses (Galgano et al., 2017). It also influences tumor invasiveness via its regulation of Golgi structure and transport by mediating receptor tyrosine kinase-like orphan receptors 2 (ROR2)-dependent signaling (Nishita et al., 2017). Increasing evidence indicates that cell type-specific of IFT20 emerges in different cell lines (Yang et al., 2021). This diverse functioning of IFT20 may help to explain the differential reception and transmission of signals in various cell types, and it allows IFT20 to mediate a variety of cell behaviors. This review summarizes current research on the mechanisms of IFT20 multiple signaling pathways in cell development and tissue homeostasis.
Fig. 2. Overview of primary cilia, IFT20 and IFT trains.
(A) IFT20 anchored to the Golgi complex by GMAP210, where it regulates trafficking of ciliary membrane proteins from the Golgi complex to the cilia. IFT trains assembled in the basal body of cilia by the IFT complex interact with cargoes and kinesin-2, then pass through the transition zone. They move to the top of the cilia along the B-tubule of microtubule doublets or A-tubule of primary cilia distal singlet axonemal microtubules, up to the tip of the cilia. Anterograde trains then turn into retrograde trains that return to the ciliary base along the A-tubule under the power of dynein-2; (B) In IFT trains, heterotrimeric kinesin-2 and dynein-2 drive anterograde and retrograde movement, respectively, in most organisms, such as Chlamydomonas; (C) IFT is driven by two different types of kinesin-2 motors in Caenorhabditis elegans: heterotrimeric kinesin-2 and homodimeric kinesin-2. Heterotrimeric kinesin-2 drives IFT trains through the transition zone and proximal cilia, then they are gradually replaced by homodimeric kinesin-2, which drives the trains along the distal segment to the top of the cilia, while dynein-2 drives retrograde IFT transport.
Hedgehog signaling
Hedgehog signaling plays a crucial role in the development of mammalian tissues and organs, homeostasis of internal environments, and repair and regeneration following injury (Rohatgi et al., 2007). In mammals, there are three hedgehog homologous (HH) genes that encode three HH proteins: sonic hedgehog (SHH); desert hedgehog (DHH); and Indian hedgehog (IHH). HH proteins are the ligands for two cell surface receptors, patched (PTCH1) and smoothened (SMO), which jointly control the activation and inactivation of the zinc finger transcription factor glioblastoma/cubitus interruptus (GLI/CI) (Russell et al., 2007; Hynes et al., 1997). The GLI gene family consists of GLI1, GLI2 and GLI3. The product of the GLI1 gene only acts as a transcriptional activator (Hynes et al., 1997), while the GLI2 and GLI3 gene products have both transcriptional activating and transcriptional repressing activity. When the three transcription factors are full-length, they all show transcriptional activation activity (Hui and Angers, 2011).
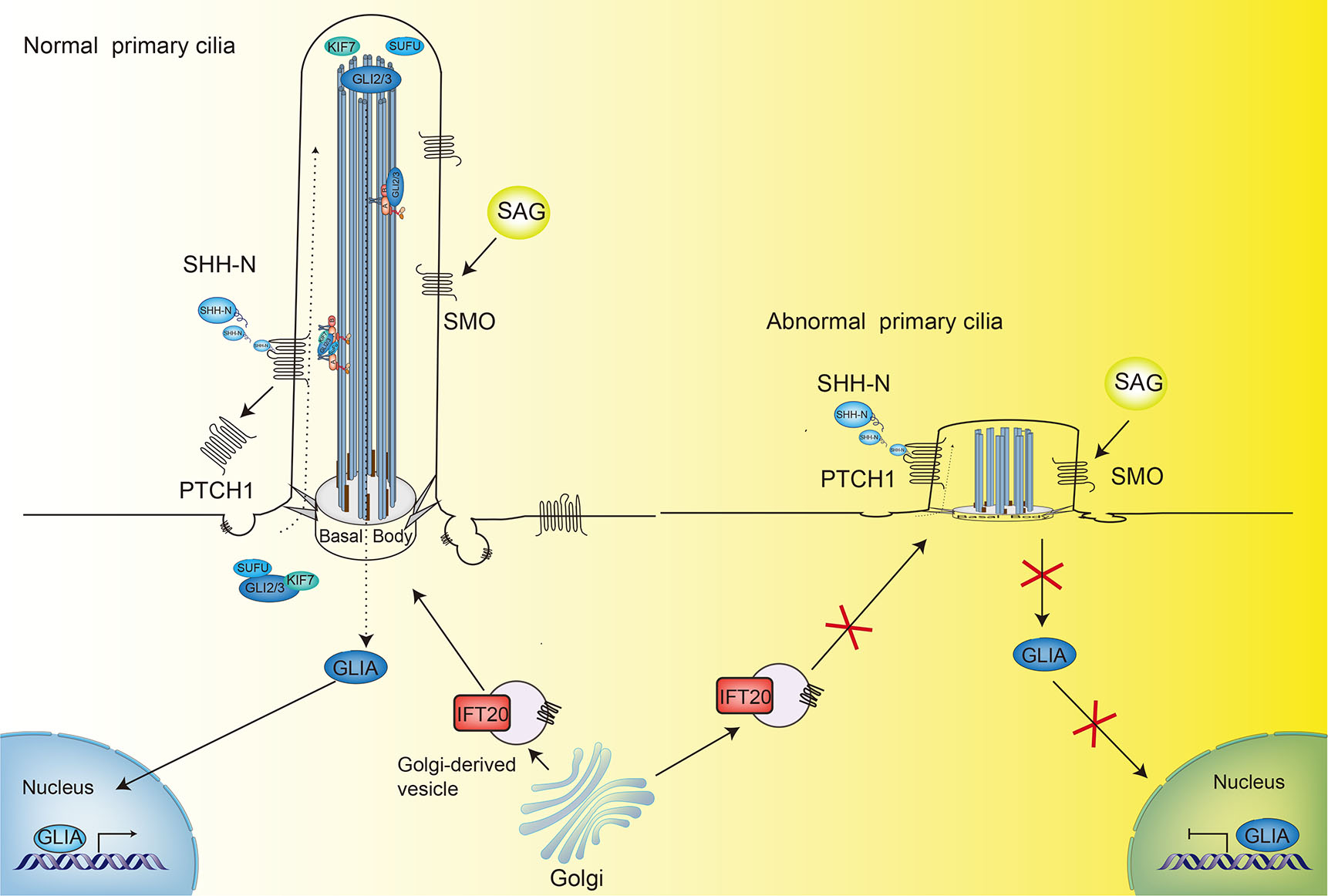
The mechanism by which PTCH1 regulates SMO remains poorly defined, but it is clear that the cilium is intimately involved. In the absence of HH, PTCH1 is localized in the primary cilia and inhibits the activity of SMO by preventing the accumulation of SMO. Upon phosphorylation of full-length GLI (GLI2/3-FL) by protein kinase A, glycogen synthase kinase 3 or casein kinase 1, proteasome-mediated hydrolysis of GLI2/3-FL leads to production of a repressor form of the protein (GLI2/3-R). GLI2/3-R then enters the nucleus and blocks the transcription of downstream target genes. HH binding to PTCH1 relieves the inhibition of SMO, and PTCH1 moves out of the primary cilia and SMO moves into the primary cilia through a process mediated by oxysterols (Rohatgi et al., 2007). KIF7, suppressor of fused (SUFU), and GLI also accumulates in the primary cilia; activated SMO promotes the dissociation of the SUFU-GLI2/3-KIF7 complex and generates activated GLI (GLI-A). Activated GLI-A moves into the nucleus and induces the transcription of downstream target genes (Fig. 3) (Hu and Song, 2019).
The primary cilia represent the key location of reception and transmission of HH signaling. The effective activation of the SHH signaling pathway in particular depends on the normal functioning of IFT (Haycraft et al., 2005). Knockdown of IFT20 has been found to block HH signaling specifically by affecting ciliogenesis (Pampliega et al., 2013; Clement et al., 2009). In addition, decreased expression of Ift20 was shown to result in the decreased expression of both Smo and Gli1, which reduced the proliferation and dedifferentiation potential of rat Müller cells mediated by SHH (Ferraro et al., 2015).
In condylar cartilage, IFT20-assembled primary cilia also mediate the ability of the HH pathway to signal for the production of collagen type X, which is essential for the maturation of chondrocytes. The expression levels of GLI1 and PTCH1 were found to be significantly decreased, and there was no SMO accumulation in primary cilia from which IFT20 was deleted in a chondrogenic cell line. Consistent with these in vitro results, cilia-dependent HH signal transduction was found to be severely attenuated in the pleomorphic, flat and hypertrophic layers of Ift20 conditional knockout mice, while it was abundantly present in the condylar cartilage of control mice (Kitami et al., 2019).
Primary cilia assembled by IFT20 are the venue of receiving and transmitting HH signaling in astrocytes. When astrocytes were stimulated by SMO agonist (SAG), the HH signaling pathway was also found to be activated, and this co-activation improved the survival rate of astrocytes under stress conditions. Conversely, when IFT20 was knocked down, the effect of SAG on cell survival disappeared (Yoshimura et al., 2011). In analysis of Down Syndrome, the disruption of IFT20 trafficking from the Golgi to the centrosome and cilia by elevated pericentrin was found to lead to fewer and shorter cilia, accompanied by the failure of SMO transport to the cilia (Fig. 3). These processes reduced ciliary SHH signaling, which may underlie a series of cardiovascular, musculoskeletal and neurological abnormalities (Galati et al., 2018). In summary, IFT20-deficient cells have multiple defects in signaling-dependent transport of a variety of HH components, including PTCH-1, SMO and GLI, and these defects are primarily related to the function of IFT20 in the biogenesis and structure maintenance of cilia.
Fig. 3. IFT20-dependent HH signaling.
IFT20-assembled primary cilia mediate the HH signaling pathway. Upon binding of the SHH ligand to PTCH1, the inhibition of SMO by PTCH1 is relieved, leading to movement of PTCH1 out of the primary cilia and of SMO into the primary cilia. KIF7, SUFU, and GLI also accumulate in the primary cilia, and activated SMO promotes the dissociation of the SUFU-GLI2/3-KIF7 complex and generates activated GLI(GLI-A). The activated GLI-A moves into the nucleus and induces the transcription of downstream target genes; Mutation or disruption of IFT20 blocks trafficking from the Golgi apparatus to the cilia, resulting in shorter and fewer cilia. Accompanying the failure of SMO transport to cilia is inhibition of cilia-dependent SHH signaling.
WNT signaling
WNT signaling plays critical roles in cell proliferation, differentiation and tissue development. In mammals, 19 WNT ligands and 10 families of its receptor, frizzled (FZD), have been identified. WNT ligands induce the activation of canonical and non-canonical WNT/β-catenin signaling pathways through cross reactions with FZD receptors and the co-receptors low-density lipoprotein-related receptor proteins 5 and 6 (LRP5/6) (Janda et al., 2017).
Canonical WNT/β-catenin signaling
β-catenin is a key protein in canonical WNT signaling. The degradation and production of β-catenin determines the initiation and termination of WNT signaling. In the absence of WNT, cytoplasmic β-catenin is phosphorylated by a "destruction complex" to promote its degradation. The destruction complex consists of four proteins, including scaffolding proteins AXIN, adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and glycogen synthase kinase 3β (GSK-3β) (Nong et al., 2021). Phosphorylated β-catenin is ubiquitinated by E3 ubiquitin ligase β-transducin-repeat-containing protein (β-TRCP) and is subsequently degraded by the proteasome (Ci et al., 2018). As a result, the level of β-catenin is kept low in the absence of WNT, and T cell factor (TCF) is able to bind to the repressor Groucho and is in its inactive state in the nucleus (Fig. 4A) (Li et al., 2020).
Non-canonical, β-catenin-independent WNT signaling
Non-canonical signaling downstream of WNT includes WNT/planar cell polarity (PCP) signaling and WNT/Ca2+ signaling. There are six core proteins in the PCP pathway, which mediates cellular polarization. These proteins include the four-pass transmembrane proteins Van Gogh and striabismus, the seven-pass transmembrane proteins FZD and cadherin Flamingo, and the cytoplasmic proteins Prickle, Diego and disheveled(DVL) (Harrison et al., 2020). The interactions of these core proteins in neighboring cells can promote cytoskeleton reorganization via the action of small GTPases and Rho-associated kinase, and can also induce transcription via C-Jun N-terminal kinase (JNK) activation (Strutt et al., 2019; Wang et al., 2019).
In WNT/Ca2+ signaling, when WNT proteins bind to FZD receptors, two pathways are mediated by heterotrimeric G proteins. One pathway involves activation of phosphodiesterase (PDE) and the decrease of cGMP concentrations. The other pathway involves activation of phospholipase C (PLC), which produces inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), leading to the release of intracellular Ca2+ and the activation of protein kinase C (PKC), respectively (Sheldahl et al., 1999). Both of these pathways affect the activity of transcription factors (Zhen et al., 2020; Wang and Malbon, 2003).
Relationship between IFT20 and the WNT pathway and disease
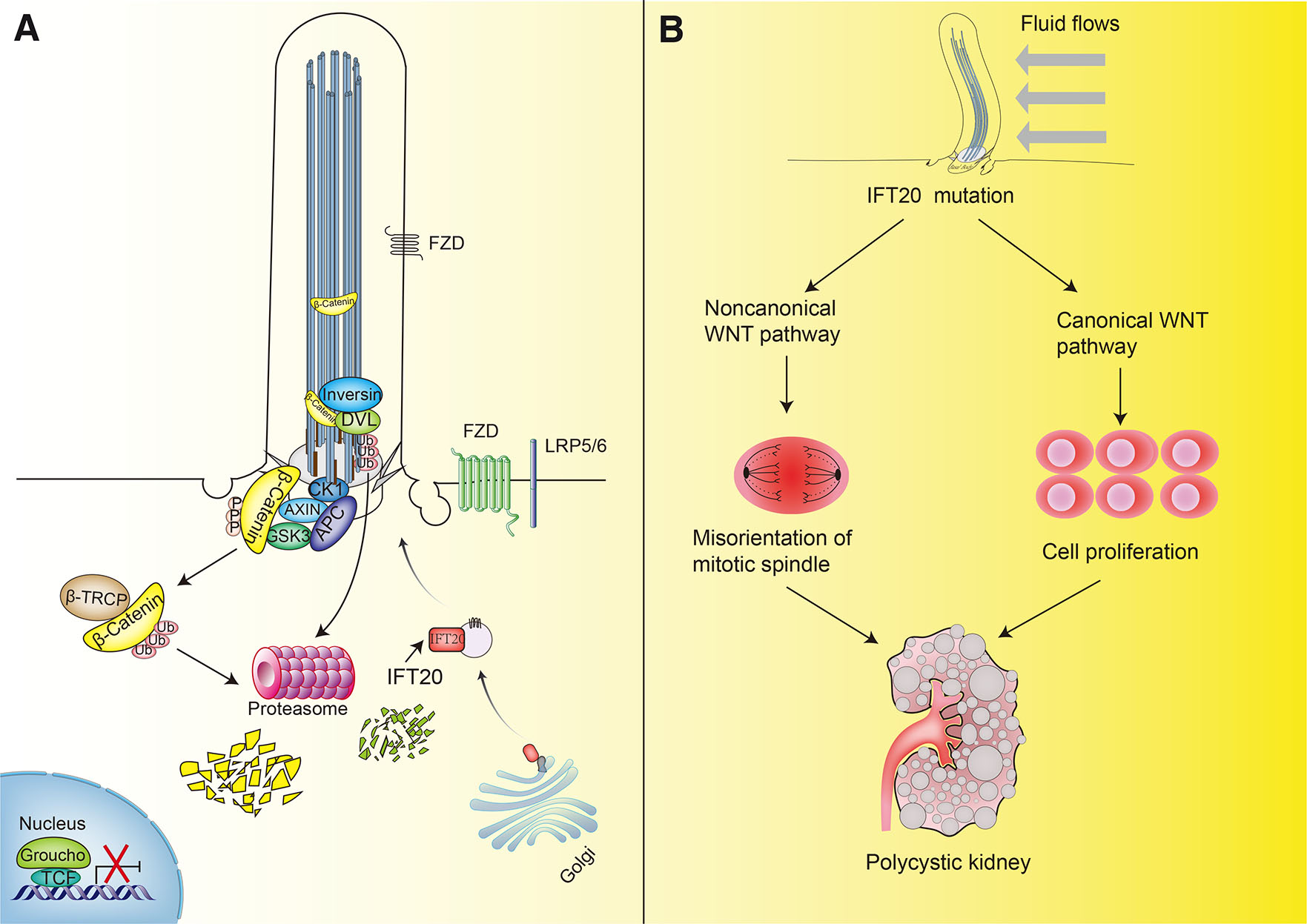
Although primary cilia have been demonstrated to play a negative regulatory role in WNT signaling (Kawata et al., 2021), the links the between primary cilia and canonical WNT/β–catenin or WNT/PCP pathways remain controversial. One of the links between primary cilia and WNT signaling resulted from evidence that the ciliary protein Inversin, which is localized to the primary cilium of tubular epithelial cells, inhibits the canonical WNT pathway through degradation of cytoplasmic β-catenin and DVL by the recruitment of the proteasome (Christian Wigley et al., 1999). Here, Inversin and DVL interact to form a protein complex that increases DVL ubiquitination resulting in proteasome-dependent degradation (Fig. 4A). Accordingly, mutations of the Inversin gene that occur during embryonic development lead to cystic kidney disease (Simons et al., 2005). Another link was indicated by the finding that the loss of primary cilia in mice via the deletion of the ciliogenesis genes Kif3a, Ift88 and Orofacial digital type I syndrome (Ofd1) increases the activation of the canonical WNT pathway (Corbit et al., 2008).
However, some researchers believe that there is no substantial link between primary cilia and WNT signaling. In mouse embryos with mutations in Ift88, Ift72, and Kif3a and the gene encoding dynein-2 (Dync2h1), the activities of the WNT target Axin-2 gene and a transgenic reporter of canonical WNT signaling were both normal. The conversion from canonical WNT signaling to non-canonical WNT signaling has also been found to be normal in embryonic fibroblasts from mice with an IFT mutation (Ocbina et al., 2009).
Research has indicated that IFT20 is also related to WNT signaling regulation. For example, generation of dysfunction of primary cilia by the deletion of Ift20 or Itf88 leads to increased canonical WNT signaling and decreased HH signaling, which decreases branching morphogenesis of mammary glands and other organs (McDermott et al., 2010). Notably, non-canonical WNT pathways control the orientation of the mitotic division (Gong et al., 2004), while the canonical WNT/β-catenin pathway regulates cell proliferation (Aulicino et al., 2020), and one of the functions of primary cilia is to regulate the conversion between canonical and non-canonical WNT signaling when fluid flows through the nephron (Simons et al., 2005). Accordingly, deletion of Ift20 in the collecting ducts of the kidney lead to elevate dephosphorylation of β-catenin and increased expression of genes in the WNT pathway, including those coding for WNT ligands (Wnt7a/b, Wnt10a, Wnt9a, Wnt6, Wnt 4, and Wnt2), WNT-binding proteins (Frz6 and Frzb) and WNT targets (Myc, JNK, Tcf3 and Tcf7). Under these conditions, the expression of both the canonical and non-canonical WNT signaling components increased, eventually causing misorientation of the mitotic spindle and increased proliferation of duct cystic epithelial cells and cyst formation (Fig. 4B) (Jonassen et al., 2008). However, because IFT20 also has important extraciliary functions, the specific mechanisms of cross-reaction between canonical and non-canonical WNT signaling and ciliary or non-ciliary functions of IFT20 merits further study.
Fig. 4. IFT20 modulation of WNT signaling.
(A) IFT20-assembled primary cilia mediate the actions of the ciliary protein Inversin, which inhibits the canonical WNT pathway through degradation of cytoplasmic β-catenin and DVL by proteasome recruitment; (B) When fluid flows through the nephron, IFT20 mutations in the collecting ducts of kidney lead to alterations in non-canonical and canonical WNT signaling, causing misorientation of the mitotic spindle and increased proliferation of duct cystic epithelium, respectively. Eventually, cyst formation occurs.
Platelet-derived growth factor signaling
Platelet-derived growth factor (PDGF) signaling plays an important role in embryonic development and tissue homeostasis. The monomer forms of PDGF are inactive, and these monomers include four different polypeptide chains (PDGF-A, PDGF-B, PDGF-C and PDGF-D). PDGF-A and -B are secreted as active peptides linked by disulfide bonds to form homodimers (PDGF-AA or PDGF-BB) and heterodimers (PDGF-AB), while PDGF-C and -D are secreted as latent homodimers (PDGF-CC or PDGF-DD) (Fredriksson et al., 2004). The PDGF isoforms perform their biological effects by binding to and dimerizing two structurally related tyrosine kinase receptors, PDGFR-α and PDGFR-β(Pahara et al., 2010; Li and Schlessinger, 1991). The receptor interactions lead to autophosphorylation and activation of intracellular tyrosine kinase domains (Kazlauskas and Cooper, 1989).
The autophosphorylation serves two important functions. On the one hand, this phosphorylation releases the inhibitory effect of the carboxy-terminal tail of the conserved tyrosine residue and juxtamembrane domain that were folded over the kinase domain; on the other hand, the phosphorylated tyrosines serve as docking sites for containing Src homology 2 (SH2) domains signal transduction molecules (Songyang and Cantley, 1995). Various SH2-domains signal transduction molecules have been reported to be involved in the connection of the docking sites of PDGFR. Some of the SH2-domain molecules act as enzymes, including PLC, the Src family of tyrosine kinases, a GTPase activating protein (GAP) for RAS, phosphatidylinositol 3-kinase and the tyrosine phosphatase SHP-2, while other SH2-domain molecules act as adaptor proteins, including growth factor receptor-bound protein (GRB)2, GRB7 and CRK (Noguchi et al., 2017; Heldin and Lennartsson, 2013). Upon PDGF receptor activation, various SH2 domain-containing molecules initiate a series of signaling transduction pathways, leading to different cellular responses.
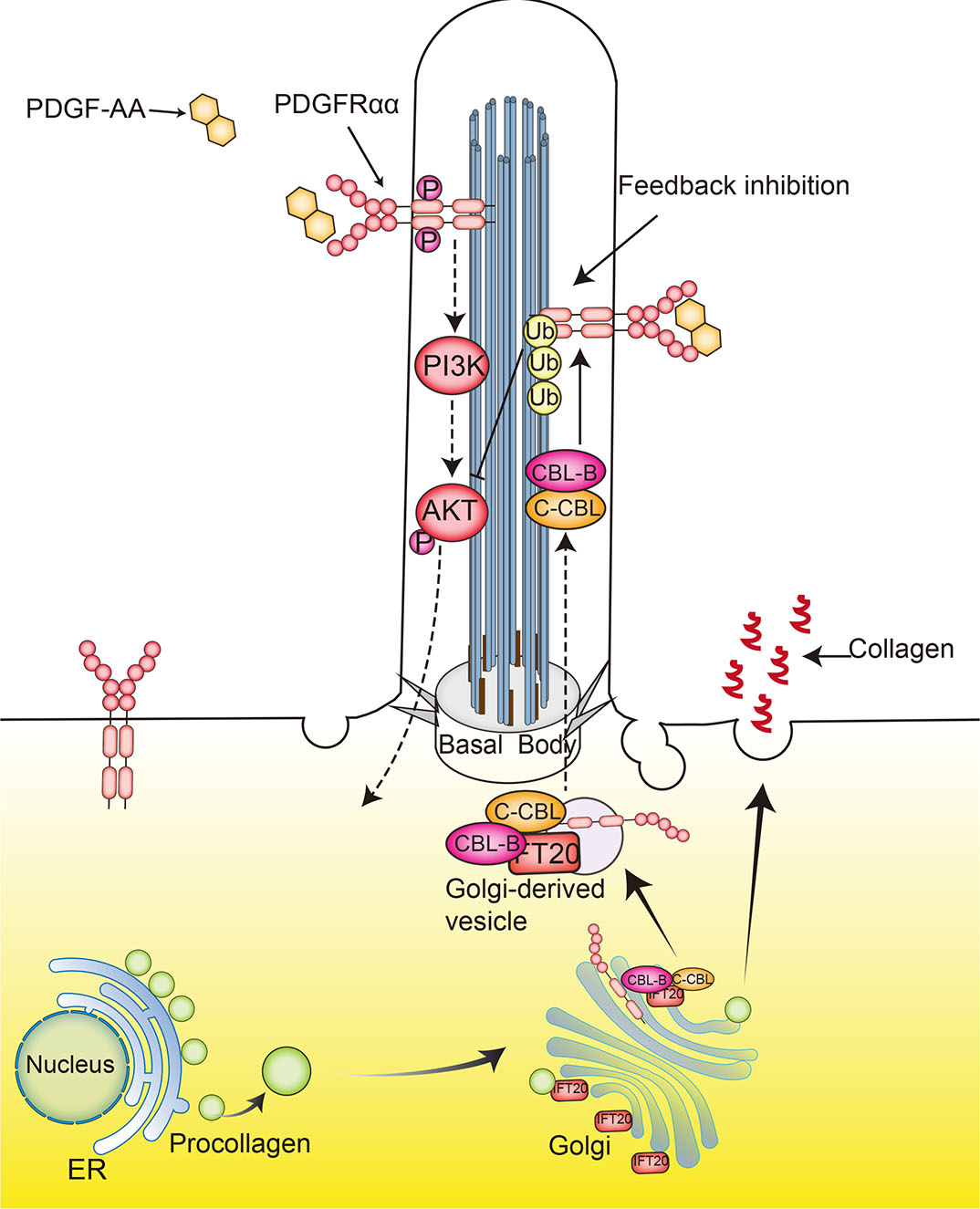
PDGFRα is predominantly localized to the primary cilium, and primary cilium formation is correlated with increases of PDGFRα expression. The signaling mediated by PDGFRα depends on its ciliary localization, while PDGFRβ is primarily localized to the cell surface (Schneider et al., 2005). One connection between the cilia and PDGF signaling involves PDGF-mediated initiation of signaling through phosphatidylinositol 3-kinase (PI3K) and protein kinase B (PKB/AKT), which promotes osteoblastic differentiation and healing of bone fractures (Zhu et al., 2020). IFT20 plays a vital role in craniofacial skeletal development by regulating ciliogenesis and collagen intracellular trafficking PDGFRα activation, and the resulting AKT phosphorylation is mediated through the primary cilia in the regulation of osteogenic proliferation and cell survival. Disruption of IFT20 and the resulting absence of primary cilia decreases PDGFRα localization to the primary cilia. While this receptor is still present on the cell surface under these conditions, osteogenic proliferation was found to have decreased and cell death to have increased. In addition, IFT20 is also indispensable for regulation of secretion of type I collagen by controlling the transport of procollagen from the endoplasmic reticulum (ER) to the Golgi apparatus during skull development (Fig. 5) (Noda et al., 2016). These results indicate that IFT20 mediates the localization of PDGFRα in primary cilia and regulates the activation of PI3K -Akt signaling.
Further studies of the mechanism of interaction between IFT20 and PDGF/PDGFR signaling pathways have indicated that IFT20 stabilizes the Casitas B-lineage lymphoma (CBL) E3 ubiquitin ligases C-CBL and CBL-B, and this stabilization provides a negative feedback mechanism in PDGFRα signaling pathways. When cells are stimulated by PDGF-AA, C-CBL proteins become enriched in the primary cilia, and IFT20 interacts with C-CBL and CBL-B to promote the formation of stable CBL homo- or hetero-dimers (Schmid et al., 2018). Those dimers mediate ubiquitination and internalization of PDGFRα for feedback inhibition of receptor signaling (Fig. 5). In contrast, in the absence of IFT20, PDGFRα mislocalizes to the plasma membrane, where it promotes autoubiquitination and proteasomal degradation of CBL proteins, resulting in overactivation of PDGFRα after PDGF-AA stimulation (Schmid et al., 2018; Anvarian et al., 2019).
The mechanisms of interaction between IFT20 and C-CBL and CBL-B, which play critical negative regulatory roles in cell development, activation, and tolerance induction(Schmid et al., 2018), in the regulation of the localization of PDGFRα on cilia have not been completely clarified. GFP-labeled C-CBL was used to identify potential interaction sites of IFT20 and CBL proteins in Golgi complex and ciliary-base region (Schmid et al., 2018). Contrary to the effects of IFT20 mutations, mutations to IFT72 and IFT88 led to decreased PDGFRα expression, and PDGFRα did not respond to PDGF-AA stimulation in these contexts (Schneider et al., 2005; Schmid et al., 2018; Umberger et al., 2015). Based on these results, a hypothetical model established that IFT20 coordinates CBL protein to mediate the relocalization of PDGFRα to the cilia from the Golgi apparatus (Fig. 5) (Schmid et al., 2018); however, the precise mechanisms require further study.
Fig. 5. IFT20 modulation of PDGF signaling.
A complex containing IFT20 and C-CBL and CBL-B transports PDGFRα to cilia, where it forms homodimer receptors when stimulated by PDGF-AA. The PDGFR dimer leads to autophosphorylation of intracellular tyrosine kinase domains that provides docking sites for signal transduction molecules. An important effect is the activation of the PI3K-AKT signaling pathway, which promotes osteoblastic differentiation and fracture healing. In addition, the interaction of IFT20 with CBL promotes formation of stable CBL homo- or hetero-dimers that mediate ubiquitination and internalization of PDGFRα for feedback inhibition of receptor signaling. IFT20 also trafficks procollagen from the ER to the Golgi in osteoblasts.
Fibroblast growth factor signaling
Signaling involving fibroblast growth factor (FGF) and its receptor (FGFR) play crucial roles in embryonic development, angiogenesis, tissue homeostasis, wound repair, and cancer in various biological models. 22 mammalian FGF proteins have been identified, 18 of which bind to FGF tyrosine kinase receptors and coreceptors. These signaling molecules are grouped into five paracrine subfamilies and one endocrine subfamily based on differences in sequence homology and phylogeny. Four of the FGFs comprise a subfamily of intracellular non-signaling proteins that serve as cofactors for voltage-gated sodium channels and other molecules (Xie et al., 2020). FGFs exert their diverse effects by binding to and activating FGFRs and coreceptors at the cell surface. Coreceptors include heparan sulfate proteoglycans (HSPGs) for paracrine signaling (Jones et al., 2000) and Klotho for endocrine signaling (Goetz et al., 2007).
Activation of the FGFR promotes the phosphorylation of the TK domain, and phosphorylated tyrosines serve as docking sites for downstream signaling molecules, causing a series of signaling cascade reactions. In addition, the activated receptor also catalyzes the phosphorylation of FGFR substrate 2a (FRS2a) (Ong et al., 2000), which activates signaling pathways involving RAS, mitogen-activated protein kinases (MAPKs) (Gotoh et al., 2004), extracellular signal-regulated kinases (ERK), P38, JNKs and PI3K/AKT (Xie et al., 2020; Sacco et al., 2021). The receptor can also recruit and phosphorylate phospholipase Cγ (PLCγ), which activates signaling pathways involving IP3, Ca2+, and DAG and subsequent activation of PKC (Zeng et al., 2020; Landgren et al., 1995; Huang et al., 2016).
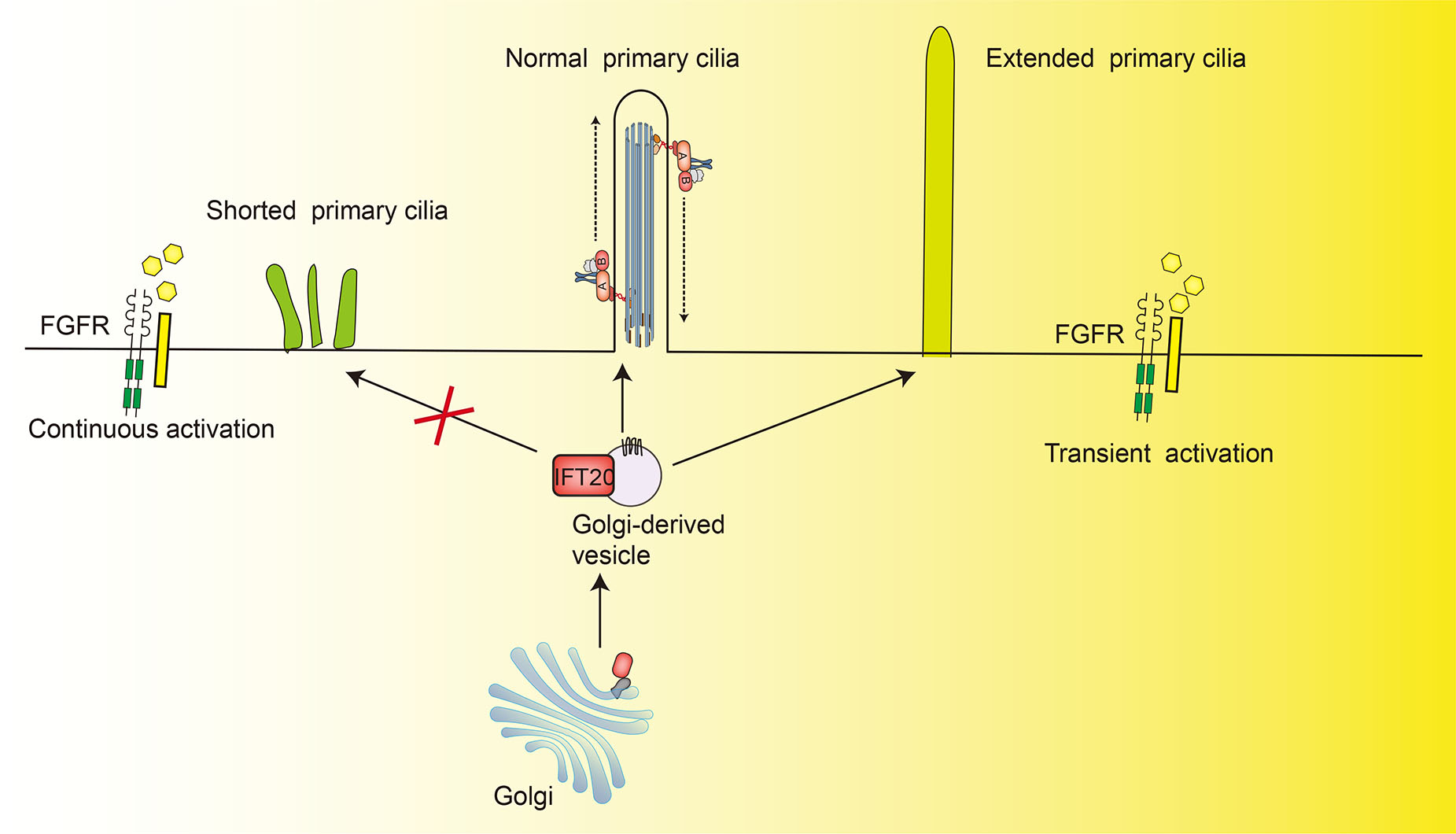
Interactions between cilia and FGF signaling are manifest in part through a series of skeletal ciliopathies that occur due to defects in the biosynthesis or function of primary cilia (Zhang et al., 2018). IFT20 is a key regulator of temporal space FGF signaling required for endochondral osteogenesis. Specific deletion of mice chondrocyte Ift20 increased the expression of Fgf18 in the perichondrium that maintained the expression of Sox9, thus inhibiting endochondral ossification. Inhibition of the enhancing of ERK1/2 activation through phosphorylation can partially rescue defective chondrogenesis in Ift20 deleted cells (Yamaguchi et al., 2022). In addition, continuous signaling caused by gain-of-function mutations of FGFR3 in both bone and cartilage results in shortening of the primary cilia and the induction of achondroplasia and thanatophoric dysplasia (Martin et al., 2018). Constitutively active FGFR3 also affects the length of primary cilia by disrupting the trafficking of IFT20-enriched vesicles from the Golgi complex to the cilia-basal body, and partial depletion of IFT20 resulted in the shortening of the primary cilia (Kim et al., 2011; Stoetzel et al., 2016).
Further research has shown that the molecular mechanisms of interaction may include the activation of TOR signaling pathways (Martin et al., 2018). NIH3T3 fibroblasts transfected with GFP-tagged IFT20 were used to demonstrate that strong and constitutive sustained activation of FGF signaling slowed the speed of both anterograde and retrograde movement of IFT20-GFP, and this deceleration resulted in the shortening of primary cilia. On the contrary, a relatively low level of transient activation of FGF signaling accelerated IFT20-GFP, resulting in the extending of the primary cilia (Fig. 6). These findings indicate that the regulation of the speed of IFT20-mediated transport is a main process by which FGF affects the length of primary cilia (Kunova Bosakova et al., 2018). In addition, the fact that activating FGFR3 mutations decreased both IFT20 speed and primary cilia length suggests that IFT20 plays a vital role in the pathologies of achondroplasia and thanatophoric dysplasia.
Fig. 6. IFT20 modulation of FGF signaling.
Constitutive activation of FGFR3 by gain-of-function mutations results in disrupting the trafficking of IFT20-enriched vesicles from the Golgi complex to the cilia-basal body, and it slows both anterograde and retrograde IFT20-GFP movement, resulting in the shortening of primary cilia. On the contrary, transient (low level and time-restricted) activation of FGF signaling accelerated IFT20-GFP movement, resulting in extending of the primary cilia.
Hippo signaling
The Hippo pathway responds to various upstream stimuli, including biochemical and biomechanical cues; it is an important sensor of cells’ physical environment and integrator of growth control signals. The Hippo pathway has multiple components. The most widely studied are mammalian transcriptional co-activator with a PDZ binding domain (TAZ), and yes-associated protein (YAP), which act as key downstream effectors of the Hippo pathway (Mia et al., 2020). YAP and TAZ are transcriptional coactivators that lack DNA-binding domains and perform their effects through cognate transcription factors (Zhao et al., 2008).
The Hippo kinase cascade is the main regulator of the activities of YAP and TAZ. This cascade inhibits the nuclear activity of the transcriptional coactivators by phosphorylation and thus inhibits the transcription mediated by the associated transcription factors (Zhao et al., 2010). YAP and TAZ therefore serve as integrators of many important signaling pathways, such as Notch signaling, G-protein-coupled receptor signaling, transforming growth factor-β and Bone Morphogenetic Protein (BMP) signaling, and WNT signaling, and they play key roles in controlling cell fate and tissue regeneration (Piccolo et al., 2014).
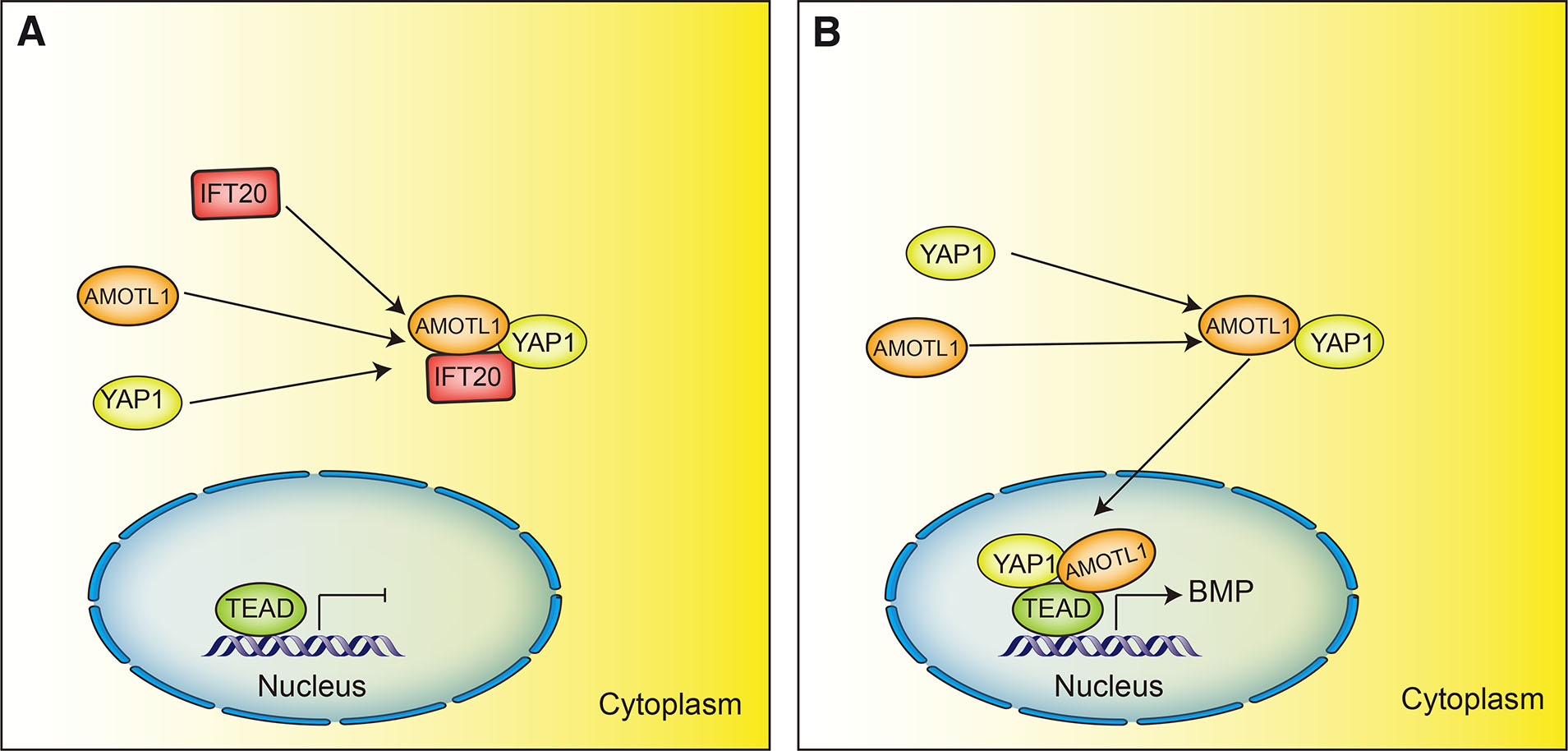
IFT20 regulates the Hippo effector YAP1 independently of its function in cilia signaling to restrict proepicardial and myocardial development in zebrafish and mice. IFT20, YAP1 and the protein angiomotin-like 1 (AMOTL1) form a cytoplasmic regulatory complex that limits the activity of nuclear YAP1 and its associated transcription factor TEA domain family member 1, which restricts the size of the proepicardium and myocardial development by modulating BMP signaling activity (Fig. 7A). In Ift20 knockout zebrafish and mice, YAP1 and AMOTL1 freely interact in the cytoplasm and translocate to the nucleus, where they activate BMP signaling (Fig. 7B). This uncontrolled signaling results in an increased size of the proepicardium. In contrast, overexpression of Ift20 results in decreased nuclear YAP1 activity and restriction of the size of the proepicardium (Peralta et al., 2020). In addition, IFT20 also mediates YAP cilia-dependent signaling during zebrafish kidney development. Joubert syndrome protein ADP ribosylation factor–like GTPase 13B (ARL13B) and IFT88 are both required for ciliogenesis (Seixas et al., 2016; Singh et al., 2020). Genetic analyses indicate that Ift20, Ift88 and the gene encoding Arl13b genetically interact with Yap in pronephric cyst formation, knocking down Yap in zebrafish embryos combined with either knockdown of Ift20, Ift88 or Arl13b, resulting in significant increases in the percentage of pronephric cysts (He et al., 2015). These observations suggest that IFT20 can mediate cilia-dependent and cilia-independent Hippo signaling pathways in different cells and tissues.
Fig. 7. IFT20 modulation of Hippo signaling.
(A) IFT20, YAP1 and AMOTL1 interact in the cytoplasm to form regulatory complexes that limit nuclear YAP1-TEAD activity, which controls the size of the proepicardium; (B) In Ift20 knockout mice, YAP1 and AMOTL1 interact with each other in the cytoplasm and transfer to the nucleus, activating BMP signaling and resulting in increased proepicardium size.
T cell antigen receptor signaling
T cell antigen receptor (TCR) signaling plays a central role in the development, homeostasis, activation, and reactivation of T cells, as well as in the regulation of immune tolerance and immunity (Huang and August, 2015). TCR signaling is activated when TCR binds to peptides presented by major histocompatibility complex (MHC) class I or class II associated with antigen presenting cells. Upon activation, the SRC family protein tyrosine kinase LCK is recruited to the TCR/CD3 complex, where LCK phosphorylates immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic region of the TCR / CD3 complex. This phosphorylation results in the recruitment and activation of zeta chain of TCR-associated protein kinase 70 (ZAP70) (Wardenburg et al., 1996), which phosphorylates linker for activation of T cells (LAT). The phosphorylation of LAT triggers a series of signaling cascades, mainly involving calmodulin, MAPK and nuclear factor-κβ signaling pathways (Huang and August, 2015).
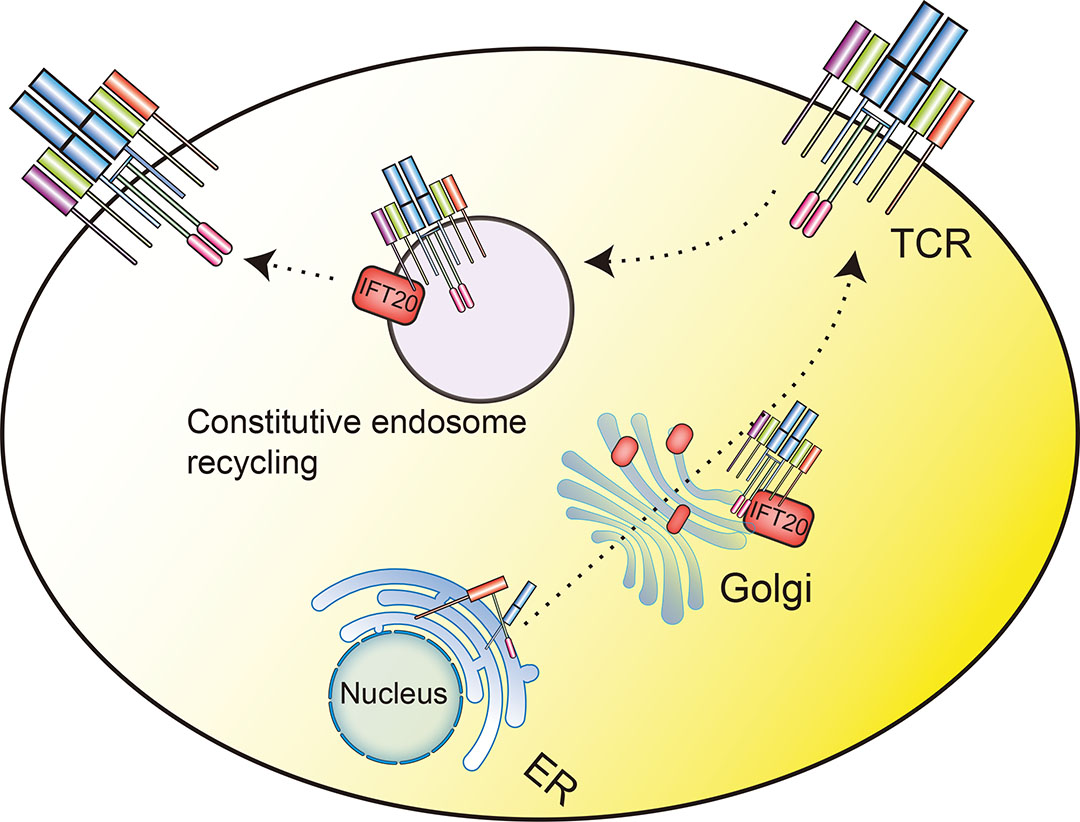
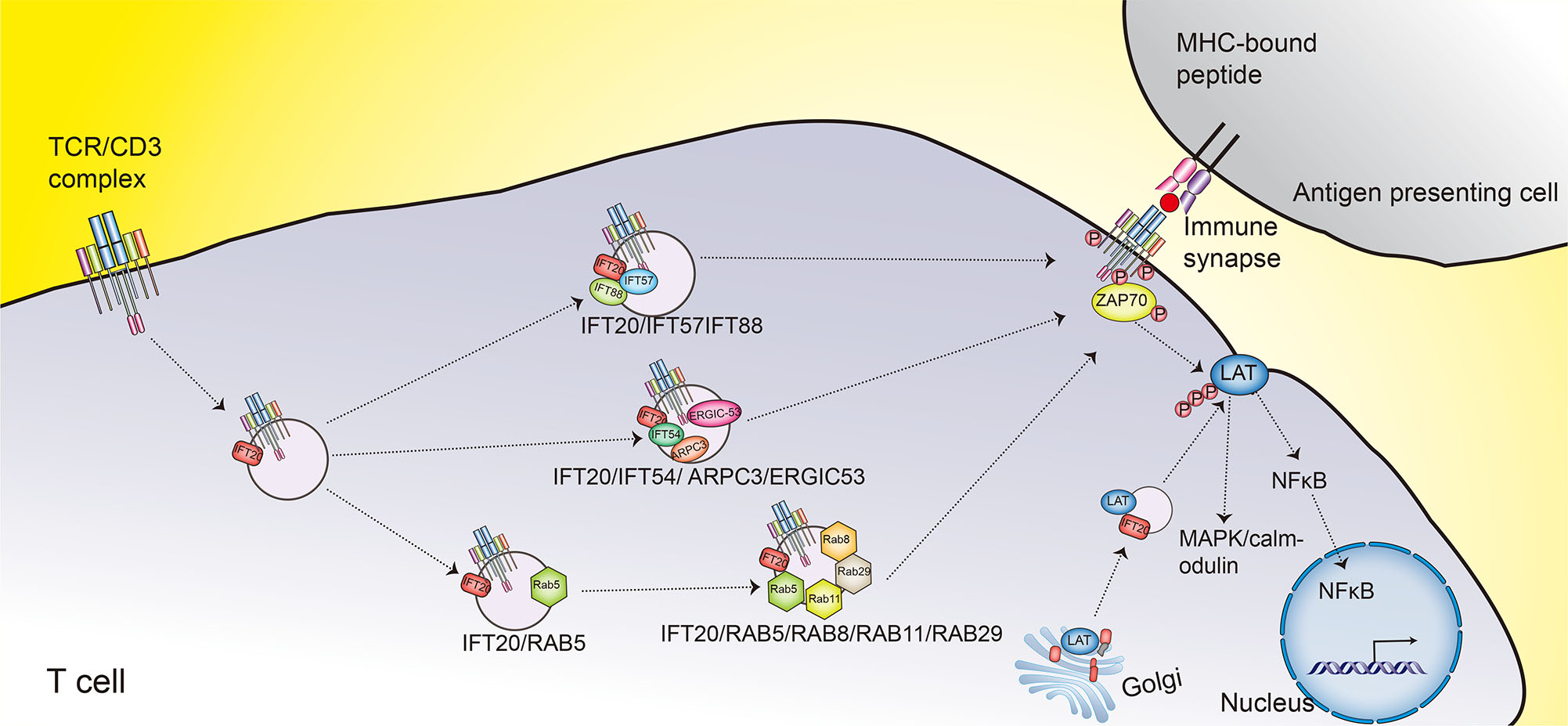
The immune synapse is a specialized structure formed at the site of contact between an antigen presenting cells and a T cell, and it plays an important role in signaling integration, fine-tuning and termination (Čemerski and Shaw, 2006). One indicator of the formation of the immune synapse is targeting of the TCR/CD3 complex to the locus of interaction between the antigen presenting cells and the T cell. This targeting occurs through a combination of lateral diffusion, cytoskeleton-driven movement and polarized endosome recycling (Čemerski and Shaw, 2006; Das et al., 2004). The assembly of the TCR/CD3 complex begins in the ER and is completed in the Golgi apparatus; subsequently, IFT20 regulates the exocytosis of the TCR/CD3 complex to the cell surface through a constitutive recycling pathway in resting cells (Fig. 8) (Finetti et al., 2009).
When the TCR binds to an MHC-bound peptide on the surface of an antigen presenting cell, IFT20 induces IFT88 and IFT57 to bind to the TCR/CD3 complex. This complex formation promotes TCR clustering at immune synapses through polarized endosome recycling, which is required to recruit the tyrosine kinase ZAP70 to activate the TCR signaling (Fig. 8) (Finetti et al., 2009). Accordingly, IFT20 knockdown in Jurkat T-cells resulted in an impairment of both TCR polarized recycling and constitutive recycling that prevented the TCR/CD3 complex from clustering at the immune synapse and the localization of the TCR/CD3 complex on cell surface, respectively (Finetti et al., 2009; Finetti et al., 2014). Moreover, in IFT20-dependent pathway of TCR recycling, IFT20 cooperates with RAB GTPase network proteins RAB5, RAB8, RAB11 and RAB29 to form complexes in Jurkat T cells to control polarized TCR recycling to immune synapses (Finetti et al., 2014; Finetti et al., 2015; Onnis et al., 2015). IFT20 and RAB5 interact on early endosomes (Finetti et al., 2014), and RAB8 regulates the final step of the TCR recycling (Fig. 8) (Finetti et al., 2015).
The recycling pathway responsible for the transport of endosomal TCR to the immune synapse was studied via detailed investigation of five newly discovered binding partners of IFT20. These proteins included IFT54, GMAP-210, actin-related protein2/3 complex subunit-3 (ARPC3), constitutive photomorphogenesis 9 signalosome subunit-1 (CSN1; also known as G protein pathway suppressor 1 (GPS1)) and ER-Golgi intermediate compartment 53 kDa protein (ERGIC53; also known as lectin mannose-binding 1 (LMAN1)). Three of these proteins were found to be involved in the trafficking of the TCR to the immune synapse. Specifically, the research found that IFT20 cooperates with IFT54, ARPC3, and ERGIC53 to regulate polarized TCR and transferrin receptor recycling to the immune synapse (Fig. 8), while GMAP-210 and CSN1 were found to be independent of this process. Confocal microscopic analysis further showed that IFT54 and ERGIC53 could promote the accumulation of phosphorylated ZAP70 at the immune synapse, while ARPC3 had no effect on the accumulation, indicating that ARPC3 was involved in T cell activation downstream of TCR-proximal signal transduction (Galgano et al., 2017). In IFT20 knockout Jurkat T-cells, the basal level of ZAP70 and LAT phosphorylation decreased, and both tonic TCR signaling and mTOR activity were deficient, resulting in disordered autophagic clearance and accumulation of lipid droplets due to defects in the biogenesis and functioning of lysosomes (Finetti et al., 2020).
In order to further clarify the effect of IFT20 on CD4+ T cells in vitro and in vivo, a mouse model with conditionally defective expression of IFT20 on T cells was established. Studies of model mice and of primary cells extracted from these mice showed that IFT20 regulates TCR signaling by controlling the phosphorylation and recruitment of ZAP70 and LAT at the immune synapse, suggesting that IFT20 is involved in the trafficking of vesicular LAT. Confocal imaging also showed that IFT20 and LAT vesicle pool colocalization in Jurkat T-cells (Fig. 8). In the absence of IFT20, recruitment of LAT at the activation sites of TCR was impaired, resulting in deficiencies of CD4+ T cell activation and proliferation. These cells cannot produce effective antigen-specific T cell responses, or induce colitis in the model of T-cell-adoptive transfer (Vivar et al., 2016).
Fig. 9. IFT20 modulation of TCR signaling.
When TCR binds to antigen presenting cells through an MHC-bound peptide on the surface, IFT20 promotes TCR clustering at immune synapses through recycling of polarized endosomes. In addition, IFT20 drives LAT vesicle transport and regulates the TCR signaling pathway by controlling the phosphorylation of LAT and ZAP70 and their recruitment at the immune synapse. The phosphorylation of LAT triggers a series of signaling cascades.
Receptor tyrosine kinase-like orphan receptors 2(ROR2) signaling
ROR2 is a member of the receptor tyrosine kinase (RTK) superfamily. Activation of ROR2 by WNT3A and WNT5A activates canonical WNT/β-catenin and non-canonical β-catenin-independent signaling pathways, respectively. This signaling plays vital roles in polarized cell migration, invasion, and tumor growth (Menck et al., 2021). ROR2 is most typically involved in the activation of non-canonical WNT pathways by WNT5A in various contexts, and this pathway tends to regulate polarity and polarized cell movement of planar cells. WNT5A/ROR2 signaling has been shown to inhibit canonical WNT/β-catenin pathways at the TCF/LEF-mediated transcriptional level (Mikels and Nusse, 2006). The expression of ROR2 is upregulated in many tumors, such as osteosarcomas, renal cell carcinomas, melanomas, and breast cancers (Nishita et al., 2017; Menck et al., 2021).
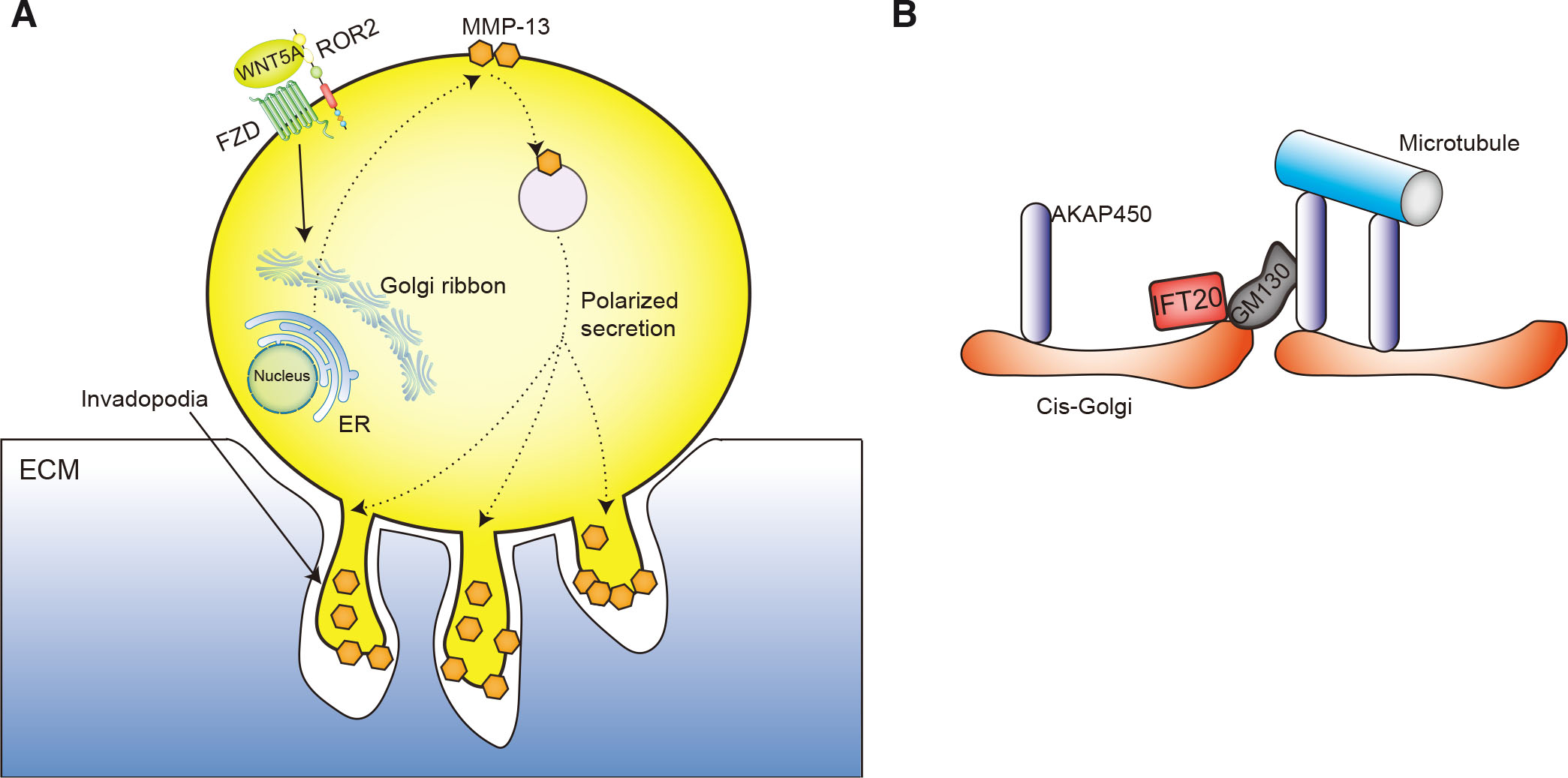
Invasive tumor cells form actin-rich dynamic adhesive structures, called invadopodia, that can degrade the extracellular matrix (Cortesio et al., 2008). The activity of these invadopodia is supported by constitutive activation of WNT5A/ROR2 signaling in osteosarcoma cell lines, as it induces matrix metalloproteinase 13 (MMP-13) expression in a cell-autonomous manner. MMP-13 secreted into the extracellular environment, where it has crucial roles in both invadopodia formation and extracellular matrix degradation (Enomoto et al., 2009; Yamagata et al., 2012). MMPs are targeted for transport to the invadopodia on the surfaces of tumor cells, which is the key site of attachment and degradation of the extracellular matrix. The invadopodia concentrate and target MMPs to specific sites of the extracellular matrix to promote tumor invasion (Kumar et al., 2018). Thus, the polarized secretion of intracellular proteins to the cell surface is necessary for tumors to achieve invasion and migration. The Golgi plays a central role in this polarized secretion, which requires Golgi to use a ribbon-like structure (Fig. 10A) (Nishita et al., 2017; Hurtado et al., 2011).
In ciliated cells, IFT20 located in the Golgi apparatus only associates with the transport of cargoes from to the cilia (Follit et al., 2006), but some tumor cells lose cilia during transformation. In these non-ciliated tumor cells, IFT20 acquires new functions in the regulation of Golgi structure and transport. In particular, IFT20 promotes invasiveness of human osteosarcoma cells through constitutive activation ROR2 signaling. Further studies showed that the regulation of Golgi structure by IFT20-mediated ROR2 signaling is achieved by influencing the assembly of Golgi ribbon rather than the stacking of Golgi cisternae. In addition, through its mediation of ROR2 signaling, IFT20 also promotes the interactions between Golgi matrix protein 130 (GM130) and A kinase anchoring protein 450 (AKAP450) at the cis-Golgi that nucleate Golgi-derived microtubules (Fig. 10B). The formation of these microtubules results in the formation of the Golgi ribbon structure, thus achieving the polarized secretion necessary for cell migration and invasion (Nishita et al., 2017). In colorectal cancer cells, IFT20 plays a vital role in collective invasion by regulating the organization of the direction of growth of Golgi-associated microtubules and polarization of the Golgi apparatus in leader cells. However, this function is independent of the status of expression and function of ROR2. Thus, the role of ROR2 signaling in the impact of IFT20 on invasion may be cancer cell line-dependent (Aoki et al., 2019).
Fig. 10. IFT20 modulation of ROR2 signaling.
(A) IFT20 promotes the assembly of Golgi ribbons through ROR2 signaling, resulting in polarized secretion of MMP-13 to the cell surface, which leads to the degradation of the extracellular matrix (ECM) and the formation of invadopodia in tumor cells; (B) IFT20 promotes the interaction between GM130 and AKAP450 at the cis-Golgi to the nucleate Golgi-derived microtubules by mediating ROR2 signaling, which results in the formation of Golgi ribbons.
Conclusions and Perspectives
As a signal integrator, primary cilia can mediate and integrate various signal pathways to maintain cell development and tissue homeostasis. IFT20, as the only IFT-B complex protein localized to Golgi apparatus except cilia and centrosome, plays a primary cilia-dependent and primary cilia-independent role in signal transduction. Due to the functional specificity of IFT20, we summarized the mechanisms of IFT20 coordinates multiple signaling pathways in cell development and tissue homeostasis, discussed the cross-integration function of various signaling pathways mediated by IFT20, and illustrated the cell, tissue and organ specificity of IFT20 in signal transduction.
IFT20 mutations show a variety of phenotypic characteristics. Although great progress has been made in the study of IFT20-mediated signaling pathways in the manifestation of these phenotypic characteristics, many problems remain to be solved. The questions for continued study include the complete elucidation of the ways that IFT20 mediated signaling pathways in cell development and tissue homeostasis, identification of mechanisms of integration of multiple cilia signaling pathways, the crosstalk between different signaling pathways, and justification of cell, tissue and organ specificity in these pathways. Thus, further research regarding IFT20 is needed.
Acknowledgements
The author thanks all readers who made critical suggestions and verbal modifications to the earlier version of the manuscript.
Abbreviations
AKAP450, A kinase anchoring protein 450; APC, adenomatous polyposis coli; AMOTL1, angiomotin-like 1; ARL13B, ADP ribosylation factor–like GTPase 13B; ARPC3, actin-related protein2/3 complex subunit-3; BMP, Bone Morphogenetic Protein CK1α casein kinase 1α; CBL, Casitas B-lineage lymphoma; CSN1, constitutive photomorphogenesis 9 signalosome subunit-1; DVL, disheveled; DHH, desert hedgehog; DAG, diacylglycerol; ERK, extracellular signal-regulated kinases; ERGIC53, ER-Golgi intermediate compartment 53 kDa protein; ER, endoplasmic reticulum; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; FRS2a, FGFR substrate 2a; GMAP-210, Golgi microtubule-associated protein 210; GLI/CI, glioblastoma/cubitus interruptus; GLI-F, full-length GLI; GAP, GTPase activating protein; GRB, growth factor receptor-bound protein; GM130, Golgi matrix protein 130; GSK-3β, glycogen synthase kinase 3β; HH, hedgehog homologous; HSPGs, heparan sulfate proteoglycans; IHH, Indian hedgehog; ITAMs, immunoreceptor tyrosine-based activation motifs; IP3, inositol 1,4,5-trisphosphate; IFT, Intraflagellar transport; JNK, C-Jun N-terminal kinase; LAT, linker for activation of T cells; LRP5/6, lipoprotein-related receptor proteins 5 and 6 MT microtubule; MAPKs, mitogen-activated protein kinases; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; Ofd1, Orofacial digital type I syndrome; PTCH, patched; PLCγ, phosphorylate phospholipase Cγ; PDGF, Platelet-derived growth factor; PI3K, phosphatidylinositol 3-kinase; PKB/AKT, protein kinase B; PCP, planar cell polarity; PLC, phospholipase C; PKC, protein kinase C; β-TRCP, β-transducin-repeat-containing protein; RTK, receptor tyrosine kinase; ROR2, receptor tyrosine kinase-like orphan receptors 2; SH2, Src homology 2; SHH, sonic hedgehog; SMO, smoothened; SUFU, suppressor of fused; SAG, SMO agonist; TCR T, cell antigen receptor; TCF T, cell factor; TAZ, transcriptional co-activator with PDZ-binding motif; YAP, yes-associated protein; ZAP70, zeta chain of TCR-associated protein kinase 70.Declarations
Conflicts of interest
Fuchang Jin, Ming-hui Zhou, Jingjing Chen, Yi Lin, Zhen-gang Zhang declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Competing interests
The authors declare that no competing interests exist.
Funding
This research was funded by the Health and Family Planning Commission of Hubei Province (WJ2019M133); Ministry of Science and Technology of the People's Republic of China (2018ZX10725506-002).
Compliance with ethics guidelines
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee
References
Anvarian Z., Mykytyn K., Mukhopadhyay S., Pedersen L. B., Christensen S. T. (2019). Cellular signalling by primary cilia in development, organ function and disease. Nature Reviews Nephrology 15: 199-219.
Aoki T., Nishita M., Sonoda J., Ikeda T., Kakeji Y., Minami Y. (2019). Intraflagellar transport 20 promotes collective cancer cell invasion by regulating polarized organization of Golgi‐associated microtubules. Cancer Science 110: 1306-1316.
Aulicino F., Pedone E., Sottile F., Lluis F., Marucci L., Cosma M. P. (2020). Canonical Wnt Pathway Controls mESC Self-Renewal Through Inhibition of Spontaneous Differentiation via β-Catenin/TCF/LEF Functions. Stem Cell Reports 15: 646-661.
Bustamante-Marin X. M., Yin W.N., Sears P. R., Werner M. E., Brotslaw E. J., Mitchell B. J., Jania C. M., Zeman K. L., Rogers T. D., Herring L. E., Refabért L., Thomas L., Amselem S., Escudier E., Legendre M., Grubb B. R., Knowles M. R., Zariwala M. A., Ostrowski L. E. (2019). Lack of GAS2L2 Causes PCD by Impairing Cilia Orientation and Mucociliary Clearance. The American Journal of Human Genetics 104: 229-245.
Cai S., Bodle J. C., Mathieu P. S., Amos A., Hamouda M., Bernacki S., McCarty G., Loboa E. G. (2017). Primary cilia are sensors of electrical field stimulation to induce osteogenesis of human adipose‐derived stem cells. The FASEB Journal 31: 346-355.
Čemerski S., Shaw A. (2006). Immune synapses in T-cell activation. Current Opinion in Immunology 18: 298-304.
Christian Wigley W., Fabunmi R. P., Lee M. G., Marino C. R., Muallem S., DeMartino G. N., Thomas P. J. (1999). Dynamic Association of Proteasomal Machinery with the Centrosome. Journal of Cell Biology 145: 481-490.
Ci Y., Li X., Chen M., Zhong J., North B. J., Inuzuka H., He X., Li Y., Guo J., Dai X. (2018). SCFβ-TRCP E3 ubiquitin ligase targets the tumor suppressor ZNRF3 for ubiquitination and degradation. Protein & Cell 9: 879-889.
Clement C. A., Kristensen S. G., Møllgård K., Pazour G. J., Yoder B. K., Larsen L. A., Christensen S. T. (2009). The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. Journal of Cell Science 122: 3070-3082.
Corbit K. C., Shyer A. E., Dowdle W. E., Gaulden J., Singla V., Reiter J. F. (2008). Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature Cell Biology 10: 70-76.
Cortesio C. L., Chan K. T., Perrin B. J., Burton N. O., Zhang S., Zhang Z.Y., Huttenlocher A. (2008). Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. Journal of Cell Biology 180: 957-971.
Das V., Nal B., Dujeancourt A., Thoulouze M.I., Galli T., Roux P., Dautry-Varsat A., Alcover A. (2004). Activation-Induced Polarized Recycling Targets T Cell Antigen Receptors to the Immunological Synapse. Immunity 20: 577-588.
Engelke M. F., Waas B., Kearns S. E., Suber A., Boss A., Allen B. L., Verhey K. J. (2019). Acute Inhibition of Heterotrimeric Kinesin-2 Function Reveals Mechanisms of Intraflagellar Transport in Mammalian Cilia. Current Biology 29: 1137-1148.e4.
Enomoto M., Hayakawa S., Itsukushima S., Ren D. Y., Matsuo M., Tamada K., Oneyama C., Okada M., Takumi T., Nishita M., Minami Y. (2009). Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 28: 3197-3208.
Ferraro S., Gomez-Montalvo A. I., Olmos R., Ramirez M., Lamas M. (2015). Primary Cilia in Rat Mature Müller Glia: Downregulation of IFT20 Expression Reduces Sonic Hedgehog-Mediated Proliferation and Dedifferentiation Potential of Müller Glia Primary Cultures. Cellular and Molecular Neurobiology 35: 533-542.
Finetti F., Cassioli C., Cianfanelli V., Onnis A., Paccagnini E., Kabanova A., Baldari C. T. (2020). The intraflagellar transport protein IFT20 controls lysosome biogenesis by regulating the post-Golgi transport of acid hydrolases. Cell Death & Differentiation 27: 310-328.
Finetti F., Paccani S. R., Riparbelli M. G., Giacomello E., Perinetti G., Pazour G. J., Rosenbaum J. L., Baldari C. T. (2009). Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature Cell Biology 11: 1332-1339.
Finetti F., Patrussi L., Masi G., Onnis A., Galgano D., Lucherini O. M., Pazour G. J., Baldari C. T., (2014). Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system.. Journal of cell science 127: 1924-1937.
Finetti F., Patrussi L., Galgano D., Cassioli C., Perinetti G., Pazour G. J., Baldari C. T. (2015). The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling TCRs to the immune synapse. Journal of Cell Science 128: 2541-2552.
Follit J. A., San Agustin J. T., Xu F., Jonassen J. A., Samtani R., Lo C. W., Pazour G. J. (2008). The Golgin GMAP210/TRIP11 Anchors IFT20 to the Golgi Complex. PLoS Genetics 4: e1000315.
Follit J. A., Tuft R. A., Fogarty K. E., Pazour G. J. (2006). The Intraflagellar Transport Protein IFT20 Is Associated with the Golgi Complex and Is Required for Cilia Assembly. Molecular Biology of the Cell 17: 3781-3792.
Fredriksson L., Li H., Eriksson U. (2004). The PDGF family: four gene products form five dimeric isoforms. Cytokine & Growth Factor Reviews 15: 197-204.
Galati D. F., Sullivan K. D., Pham A. T., Espinosa J. M., Pearson C. G. (2018). Trisomy 21 Represses Cilia Formation and Function. Developmental Cell 46: 641-650.e6.
Galgano D., Onnis A., Pappalardo E., Galvagni F., Acuto O., Baldari C. T. (2017). The T cell IFT20 interactome reveals new players in immune synapse assembly. Journal of Cell Science 130: 1110-1110.
Goetz R., Beenken A., Ibrahimi O. A., Kalinina J., Olsen S. K., Eliseenkova A. V., Xu C.F., Neubert T. A., Zhang F., Linhardt R. J., Yu X., White K. E., Inagaki T., Kliewer S. A., Yamamoto M., Kurosu H., Ogawa Y., Kuro-o M., Lanske B., Razzaque M. S., Mohammadi M. (2007). Molecular Insights into the Klotho-Dependent, Endocrine Mode of Action of Fibroblast Growth Factor 19 Subfamily Members. Molecular and Cellular Biology 27: 3417-3428.
Gong Y., Mo C., Fraser S. E. (2004). Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430: 689-693.
Gotoh N., Laks S., Nakashima M., Lax I., Schlessinger J. (2004). FRS2 family docking proteins with overlapping roles in activation of MAP kinase have distinct spatial-temporal patterns of expression of their transcripts. FEBS Letters 564: 14-18.
Harrison C., Shao H., Strutt H., Strutt D. (2020). Molecular mechanisms mediating asymmetric subcellular localisation of the core planar polarity pathway proteins. Biochemical Society Transactions 48: 1297-1308.
Haycraft C. J., Banizs B., Aydin-Son Y., Zhang Q., Michaud E. J., Yoder B. K. (2005). Gli2 and Gli3 Localize to Cilia and Require the Intraflagellar Transport Protein Polaris for Processing and Function. PLoS Genetics 1: e53.
He L., Xu W., Jing Y., Wu M., Song S., Cao Y., Mei C. (2015). Yes-Associated Protein (Yap) Is Necessary for Ciliogenesis and Morphogenesis during Pronephros Development in Zebrafish ( Danio Rerio ) . International Journal of Biological Sciences 11: 935-947.
Heldin C.H., Lennartsson J. (2013). Structural and Functional Properties of Platelet-Derived Growth Factor and Stem Cell Factor Receptors. Cold Spring Harbor Perspectives in Biology 5: a009100-a009100.
Hu A., Song B.L. (2019). The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling. Current Opinion in Cell Biology 61: 31-38.
Huang W., August A. (2015). The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. Journal of Leukocyte Biology 97: 477-485.
Huang Z., Marsiglia W. M., Basu Roy U., Rahimi N., Ilghari D., Wang H., Chen H., Gai W., Blais S., Neubert T. A., Mansukhani A., Traaseth N. J., Li X., Mohammadi M. (2016). Two FGF Receptor Kinase Molecules Act in Concert to Recruit and Transphosphorylate Phospholipase Cγ. Molecular Cell 61: 98-110.
Hui C., Angers S. (2011). Gli Proteins in Development and Disease. Annual Review of Cell and Developmental Biology 27: 513-537.
Hurtado L., Caballero C., Gavilan M. P., Cardenas J., Bornens M., Rios R. M. (2011). Disconnecting the Golgi ribbon from the centrosome prevents directional cell migration and ciliogenesis. Journal of Cell Biology 193: 917-933.
Hynes M., Stone D. M., Dowd M., Pitts-Meek S., Goddard A., Gurney A., Rosenthal A. (1997). Control of Cell Pattern in the Neural Tube by the Zinc Finger Transcription Factor and Oncogene Gli-1. Neuron 19: 15-26.
Janda C. Y., Dang L. T., You C., Chang J., de Lau W., Zhong Z. A., Yan K. S., Marecic O., Siepe D., Li X., Moody J. D., Williams B. O., Clevers H., Piehler J., Baker D., Kuo C. J., Garcia K. C. (2017). Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature 545: 234-237.
Jonassen J. A., San Agustin J., Follit J. A., Pazour G. J. (2008). Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. Journal of Cell Biology 183: 377-384.
Jones M., Tussey L., Athanasou N., Jackson D. G. (2000). Heparan Sulfate Proteoglycan Isoforms of the CD44 Hyaluronan Receptor Induced in Human Inflammatory Macrophages Can Function as Paracrine Regulators of Fibroblast Growth Factor Action. Journal of Biological Chemistry 275: 7964-7974.
Kanie T., Jackson P. K. (2021). Connecting autoimmune disease to Bardet–Biedl syndrome and primary cilia. EMBO reports 22: e52180.
Kawata K., Narita K., Washio A., Kitamura C., Nishihara T., Kubota S., Takeda S. (2021). Odontoblast differentiation is regulated by an interplay between primary cilia and the canonical Wnt pathway. Bone 150: 116001.
Kazlauskas A., Cooper J. A. (1989). Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell 58: 1121-1133.
Kiesel P., Alvarez Viar G., Tsoy N., Maraspini R., Gorilak P., Varga V., Honigmann A., Pigino G. (2020). The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nature Structural & Molecular Biology 27: 1115-1124.
Kim S., Zaghloul N. A., Bubenshchikova E., Oh E. C., Rankin S., Katsanis N., Obara T., Tsiokas L. (2011). Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nature Cell Biology 13: 351-360.
Kitami M., Yamaguchi H., Ebina M., Kaku M., Chen D., Komatsu Y. (2019). IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochemical and Biophysical Research Communications 509: 222-226.
Kumar S., Das A., Barai A., Sen S. (2018). MMP Secretion Rate and Inter-invadopodia Spacing Collectively Govern Cancer Invasiveness. Biophysical Journal 114: 650-662.
Kunova Bosakova M., Varecha M., Hampl M., Duran I., Nita A., Buchtova M., Dosedelova H., Machat R., Xie Y., Ni Z., Martin J. H., Chen L., Jansen G., Krakow D., Krejci P. (2018). Regulation of ciliary function by fibroblast growth factor signaling identifies FGFR3-related disorders achondroplasia and thanatophoric dysplasia as ciliopathies. Human Molecular Genetics 27: 1093-1105.
Landgren E., Blume-Jensen P., Courtneidge S. A., Claesson-Welsh L., (1995). Fibroblast growth factor receptor-1 regulation of Src family kinases.. Oncogene 10: 2027-2035.
Leung M. R., Roelofs M. C., Ravi R. T., Maitan P., Henning H., Zhang M., Bromfield E. G., Howes S. C., Gadella B. M., Bloomfield‐Gadêlha H., Zeev‐Ben‐Mordehai T. (2021). The multi‐scale architecture of mammalian sperm flagella and implications for ciliary motility. The EMBO Journal 40: e107410.
Li Q., Sun Y., Jarugumilli G. K., Liu S., Dang K., Cotton J. L., Xiol J., Chan P. Y., DeRan M., Ma L., Li R., Zhu L. J., Li J. H., Leiter A. B., Ip Y. T., Camargo F. D., Luo X., Johnson R. L., Wu X., Mao J. (2020). Lats1/2 Sustain Intestinal Stem Cells and Wnt Activation through TEAD-Dependent and Independent Transcription. Cell Stem Cell 26: 675-692.e8.
Li W., Schlessinger J. (1991). Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Molecular and Cellular Biology 11: 3756-3761.
Łysyganicz P. K., Pooranachandran N., Liu X., Adamson K. I., Zielonka K., Elworthy S., van Eeden F. J., Grierson A. J., Malicki J. J. (2021). Loss of Deacetylation Enzymes Hdac6 and Sirt2 Promotes Acetylation of Cytoplasmic Tubulin, but Suppresses Axonemal Acetylation in Zebrafish Cilia. Frontiers in Cell and Developmental Biology 9: 676214.
Ma M., Tian X., Igarashi P., Pazour G. J., Somlo S. (2013). Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nature Genetics 45: 1004-1012.
Martin L., Kaci N., Estibals V., Goudin N., Garfa-Traore M., Benoist-Lasselin C., Dambroise E., Legeai-Mallet L. (2018). Constitutively-active FGFR3 disrupts primary cilium length and IFT20 trafficking in various chondrocyte models of achondroplasia. Human Molecular Genetics 27: 1-13.
McDermott K. M., Liu B. Y., Tlsty T. D., Pazour G. J. (2010). Primary Cilia Regulate Branching Morphogenesis during Mammary Gland Development. Current Biology 20: 731-737.
Menck K., Heinrichs S., Baden C., Bleckmann A. (2021). The WNT/ROR Pathway in Cancer: From Signaling to Therapeutic Intervention. Cells 10: 142.
Mia M. M., Cibi D. M., Abdul Ghani S. A. B., Song W., Tee N., Ghosh S., Mao J., Olson E. N., Singh M. K. (2020). YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLOS Biology 18: e3000941.
Mikels A. J., Nusse R. (2006). Purified Wnt5a Protein Activates or Inhibits β-Catenin–TCF Signaling Depending on Receptor Context. PLoS Biology 4: e115.
Nishita M., Park S.Y., Nishio T., Kamizaki K., Wang Z.C., Tamada K., Takumi T., Hashimoto R., Otani H., Pazour G. J., Hsu V. W., Minami Y. (2017). Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Scientific Reports 7: 1.
Noda K., Kitami M., Kitami K., Kaku M., Komatsu Y. (2016). Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proceedings of the National Academy of Sciences 113: E2589-E2597.
Noguchi T., Ishiba H., Honda K., Kondoh Y., Osada H., Ohno H., Fujii N., Oishi S. (2017). Synthesis of Grb2 SH2 Domain Proteins for Mirror-Image Screening Systems. Bioconjugate Chemistry 28: 609-619.
Nong J., Kang K., Shi Q., Zhu X., Tao Q., Chen Y.G. (2021). Phase separation of Axin organizes the β-catenin destruction complex. Journal of Cell Biology 220: 33651074.
Ocbina P. J. R., Tuson M., Anderson K. V. (2009). Primary Cilia Are Not Required for Normal Canonical Wnt Signaling in the Mouse Embryo. PLoS ONE 4: e6839.
Ong S. H., Guy G. R., Hadari Y. R., Laks S., Gotoh N., Schlessinger J., Lax I. (2000). FRS2 Proteins Recruit Intracellular Signaling Pathways by Binding to Diverse Targets on Fibroblast Growth Factor and Nerve Growth Factor Receptors. Molecular and Cellular Biology 20: 979-989.
Onnis A., Finetti F., Patrussi L., Gottardo M., Cassioli C., Spanò S., Baldari C. T. (2015). The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death & Differentiation 22: 1687-1699.
Pahara J., Shi H., Chen X., Wang Z. (2010). Dimerization drives PDGF receptor endocytosis through a C-terminal hydrophobic motif shared by EGF receptor. Experimental Cell Research 316: 2237-2250.
Pampliega O., Orhon I., Patel B., Sridhar S., Díaz-Carretero A., Beau I., Codogno P., Satir B. H., Satir P., Cuervo A. M. (2013). Functional interaction between autophagy and ciliogenesis. Nature 502: 194-200.
Peralta M., Ortiz Lopez L., Jerabkova K., Lucchesi T., Vitre B., Han D., Guillemot L., Dingare C., Sumara I., Mercader N., Lecaudey V., Delaval B., Meilhac S. M., Vermot J. (2020). Intraflagellar Transport Complex B Proteins Regulate the Hippo Effector Yap1 during Cardiogenesis. Cell Reports 32: 107932.
Piccolo S., Dupont S., Cordenonsi M. (2014). The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiological Reviews 94: 1287-1312.
Pigino G. (2021). Intraflagellar transport. Current Biology 31: R530-R536.
Rohatgi R., Milenkovic L., Scott M. P. (2007). Patched1 Regulates Hedgehog Signaling at the Primary Cilium. Science 317: 372-376.
Russell M. C., Cowan R. G., Harman R. M., Walker A. L., Quirk S. M. (2007). The Hedgehog Signaling Pathway in the Mouse Ovary1. Biology of Reproduction 77: 226-236.
Sacco A., Federico C., Giacomini A., Caprio C., Maccarinelli F., Todoerti K., Favasuli V., Anastasia A., Motta M., Russo D., Rossi G., Bozza N., Castelli R., Neri A., Ronca R., Cattaneo C., Tucci A., Mor M., Presta M., Roccaro A. M. (2021). Halting the FGF/FGFR axis leads to antitumor activity in Waldenström macroglobulinemia by silencing MYD88. Blood 137: 2495-2508.
Scheidel N., Blacque O. E. (2018). Intraflagellar Transport Complex A Genes Differentially Regulate Cilium Formation and Transition Zone Gating. Current Biology 28: 3279-3287.e2.
Schmid F. M., Schou K. B., Vilhelm M. J., Holm M. S., Breslin L., Farinelli P., Larsen L. A., Andersen J. S., Pedersen L. B., Christensen S. T. (2018). IFT20 modulates ciliary PDGFRα signaling by regulating the stability of Cbl E3 ubiquitin ligases. Journal of Cell Biology 217: 151-161.
Schneider L., Clement C. A., Teilmann S. C., Pazour G. J., Hoffmann E. K., Satir P., Christensen S. T. (2005). PDGFRαα Signaling Is Regulated through the Primary Cilium in Fibroblasts. Current Biology 15: 1861-1866.
Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K., Blum M. (2007). Cilia-Driven Leftward Flow Determines Laterality in Xenopus. Current Biology 17: 60-66.
Seixas C., Choi S. Y., Polgar N., Umberger N. L., East M. P., Zuo X., Moreiras H., Ghossoub R., Benmerah A., Kahn R. A., Fogelgren B., Caspary T., Lipschutz J. H., Barral D. C. (2016). Arl13b and the exocyst interact synergistically in ciliogenesis. Molecular Biology of the Cell 27: 308-320.
Sheldahl L. C., Park M., Malbon C. C., Moon R. T. (1999). Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in aG-protein-dependent manner. Current Biology 9: 695-S1.
Simons M., Gloy J., Ganner A., Bullerkotte A., Bashkurov M., Krönig C., Schermer B., Benzing T., Cabello O. A., Jenny A., Mlodzik M., Polok B., Driever W., Obara T., Walz G. (2005). Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics 37: 537-543.
Singh S., Adam M., Matkar P. N., Bugyei-Twum A., Desjardins J.F., Chen H. H., Nguyen H., Bazinet H., Michels D., Liu Z., Mebrahtu E., Esene L., Joseph J., Ehsan M., Qadura M., Connelly K. A., Leong-Poi H., Singh K. K. (2020). Endothelial-specific Loss of IFT88 Promotes Endothelial-to-Mesenchymal Transition and Exacerbates Bleomycin-induced Pulmonary Fibrosis. Scientific Reports 10: 4466.
Songyang Z., Cantley L. C. (1995). Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends in Biochemical Sciences 20: 470-475.
Stoetzel C., Bär S., De Craene J.O., Scheidecker S., Etard C., Chicher J., Reck J. R., Perrault I., Geoffroy V., Chennen K., Strähle U., Hammann P., Friant S., Dollfus H. (2016). A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nature Communications 7: 13586.
Strutt H., Langton P. F., Pearson N., McMillan K. J., Strutt D., Cullen P. J. (2019). Retromer Controls Planar Polarity Protein Levels and Asymmetric Localization at Intercellular Junctions. Current Biology 29: 484-491.e6.
Sung C.H., Leroux M. R. (2013). The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nature Cell Biology 15: 1387-1397.
Takeda S., Narita K. (2012). Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation 83: S4-S11.
Taschner M., Weber K., Mourão A., Vetter M., Awasthi M., Stiegler M., Bhogaraju S., Lorentzen E. (2016). Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin‐binding IFT ‐B2 complex . The EMBO Journal 35: 773-790.
Thouvenin O., Keiser L., Cantaut-Belarif Y., Carbo-Tano M., Verweij F., Jurisch-Yaksi N., Bardet P.L., van Niel G., Gallaire F., Wyart C. (2020). Origin and role of the cerebrospinal fluid bidirectional flow in the central canal. eLife 9: e47699.
Toropova K., Zalyte R., Mukhopadhyay A. G., Mladenov M., Carter A. P., Roberts A. J. (2019). Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nature Structural & Molecular Biology 26: 823-829.
Umberger N. L., Caspary T., Bettencourt-Dias M. (2015). Ciliary transport regulates PDGF-AA/αα signaling via elevated mammalian target of rapamycin signaling and diminished PP2A activity. Molecular Biology of the Cell 26: 350-358.
Vivar O. I., Masi G., Carpier J.M., Magalhaes J. G., Galgano D., Pazour G. J., Amigorena S., Hivroz C., Baldari C. T. (2016). IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proceedings of the National Academy of Sciences 113: 386-391.
Wang H., Malbon C. C. (2003). Wnt Signaling, Ca 2 + , and Cyclic GMP: Visualizing Frizzled Functions . Science 300: 1529-1530.
Wang M., Marco P., Capra V., Kibar Z., (2019). Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells 8: 1198.
Wardenburg J. B., Fu C., Jackman J. K., Flotow H., Wilkinson S. E., Williams D. H., Johnson R., Kong G., Chan A. C., Findell P. R. (1996). Phosphorylation of SLP-76 by the ZAP-70 Protein-tyrosine Kinase Is Required for T-cell Receptor Function. Journal of Biological Chemistry 271: 19641-19644.
Webb S., Mukhopadhyay A. G., Roberts A. J. (2020). Intraflagellar transport trains and motors: Insights from structure. Seminars in Cell & Developmental Biology 107: 82-90.
Wei Q., Zhang Y., Li Y., Zhang Q., Ling K., Hu J. (2012). The BBSome controls IFT assembly and turnaround in cilia. Nature Cell Biology 14: 950-957.
Wingfield J. L., Mekonnen B., Mengoni I., Liu P., Jordan M., Diener D., Pigino G., Lechtreck K. (2021). In vivo imaging shows continued association of several IFT-A, IFT-B and dynein complexes while IFT trains U-turn at the tip . Journal of Cell Science 134: jcs259010.
Xie Y., Su N., Yang J., Tan Q., Huang S., Jin M., Ni Z., Zhang B., Zhang D., Luo F., Chen H., Sun X., Feng J. Q., Qi H., Chen L. (2020). FGF/FGFR signaling in health and disease. Signal Transduction and Targeted Therapy 5: 181.
Yamagata K., Li X., Ikegaki S., Oneyama C., Okada M., Nishita M., Minami Y. (2012). Dissection of Wnt5a-Ror2 Signaling Leading to Matrix Metalloproteinase (MMP-13) Expression. Journal of Biological Chemistry 287: 1588-1599.
Yamaguchi H., Kitami M., Uchima Koecklin K. H., He L., Wang J., Lagor W. R., Perrien D. S., Komatsu Y. (2022). Temporospatial regulation of intraflagellar transport is required for the endochondral ossification in mice. Developmental Biology 482: 91-100.
Yang H., Zhang F., Long H., Lin Y., Liao J., Xia H., Huang K. (2021). IFT20 Mediates the Transport of Cell Migration Regulators From the Trans-Golgi Network to the Plasma Membrane in Breast Cancer Cells. Frontiers in Cell and Developmental Biology 9: 632198.
Yoshimura K., Kawate T., Takeda S. (2011). Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia 59: 333-344.
Zeng X., Zhang Y., Yang Q., Wang S., Zou B., Tan Y., Zou M., Liu S., Li X. (2020). Artesunate attenuates LPS-induced osteoclastogenesis by suppressing TLR4/TRAF6 and PLCγ1-Ca2+-NFATc1 signaling pathway. Acta Pharmacologica Sinica 41: 229-236.
Zhang W., Taylor S. P., Ennis H. A., Forlenza K. N., Duran I., Li B., Sanchez J. A. O., Nevarez L., Nickerson D. A., Bamshad M., Lachman R. S., Krakow D., Cohn D. H. (2018). Expanding the genetic architecture and phenotypic spectrum in the skeletal ciliopathies. Human Mutation 39: 152-166.
Zhang Y.C., Bai Y.F., Yuan J.F., Shen X.L., Xu Y.L., Jian X.X., Li S., Song Z.Q., Hu H.B., Li P.Y., Tu H.Q., Han Q.Y., Wang N., Li A.L., Zhang X.M., Wu M., Zhou T., Li H.Y. (2021). CEP55 promotes cilia disassembly through stabilizing Aurora A kinase. Journal of Cell Biology 220: e202003149.
Zhao B., Li L., Tumaneng K., Wang C.Y., Guan K.L. (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF β-TRCP . Genes & Development 24: 72-85.
Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C.Y., Chinnaiyan A. M., Lai Z.C., Guan K.L. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes & Development 22: 1962-1971.
Zhao L., Hou Y., McNeill N. A., Witman G. B. (2020). The unity and diversity of the ciliary central apparatus. Philosophical Transactions of the Royal Society B: Biological Sciences 375: 20190164.
Zhen H., Deng H., Song Q., Zheng M., Yuan Z., Cao Z., Pang Q., Zhao B. (2020). The Wnt/Ca 2+ signaling pathway is essential for the regeneration of GABAergic neurons in planarian Dugesia japonica . The FASEB Journal 34: 16567-16580.
Zhou M., Lin Y., Zhang Z. (2020). Intraflagellar transport 20: New target for the treatment of ciliopathies. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1867: 118641.
Zhu G., Ma B., Dong P., Shang J., Gu X., Zi Y. (2020). Melatonin promotes osteoblastic differentiation and regulates PDGF/AKT signaling pathway. Cell Biology International 44: 402-411.
Zhu X., Wang J., Li S., Lechtreck K., Pan J. (2021). IFT54 directly interacts with kinesin‐II and IFT dynein to regulate anterograde intraflagellar transport. The EMBO Journal 40: e105781.