Int. J. Dev. Biol. 66: 317 - 331 (2022)
The mesenchymal potential of trunk neural crest cells
Open Access | Review | Published: 8 July 2022
Abstract
It has long been held that the main difference between cranial and trunk neural crest (CNC and TNC, respectively) was the potential of CNC to originate mesenchymal cell types, especially skeletogenic. This is an age-old question that continues to challenge researchers, even today. Unfortunately, to date, no consensus has concluded the extent of TNC mesenchymal potential, nor has a systematic review been conducted to organize current knowledge about this fascinating question. However, the number of studies related to this question have expanded and deepened considerably in the last few years thanks to several new different species of vertebrates employed, the generation of transgenic animal strains, the combination of cell markers, and also the improvement of cell culture conditions through the use of different substrates and signaling molecules. Therefore, this review summarizes the literature showing that TNCCs can generate a broad range of mesenchymal cell types, including skeletogenic. This potential can be unveiled by certain favorable in vitro conditions, but it also seems to be expressed in some animal structures in vivo, to which TNCCs contribute. We also present several works that offer a contrary view and do not detect any mesenchymal/skeletogenic contribution of TNCCs in vivo. Perhaps, it is the controversy itself that makes this subject even more exciting.
Keywords
trunk neural crest, potential, mesenchymal, skeletogenic, vertebrates
Introduction
The Neural Crest (NC) is an embryonic structure that forms from the dorsolateral ridges of the neural primordium. The neural crest cells (NCCs) undergo an epithelial-to-mesenchymal transition, migrating extensively through defined routes along the vertebrate embryo’s body until they colonize and contribute to the formation of a variety of tissues and organs (Le Douarin and Kalcheim, 2011).
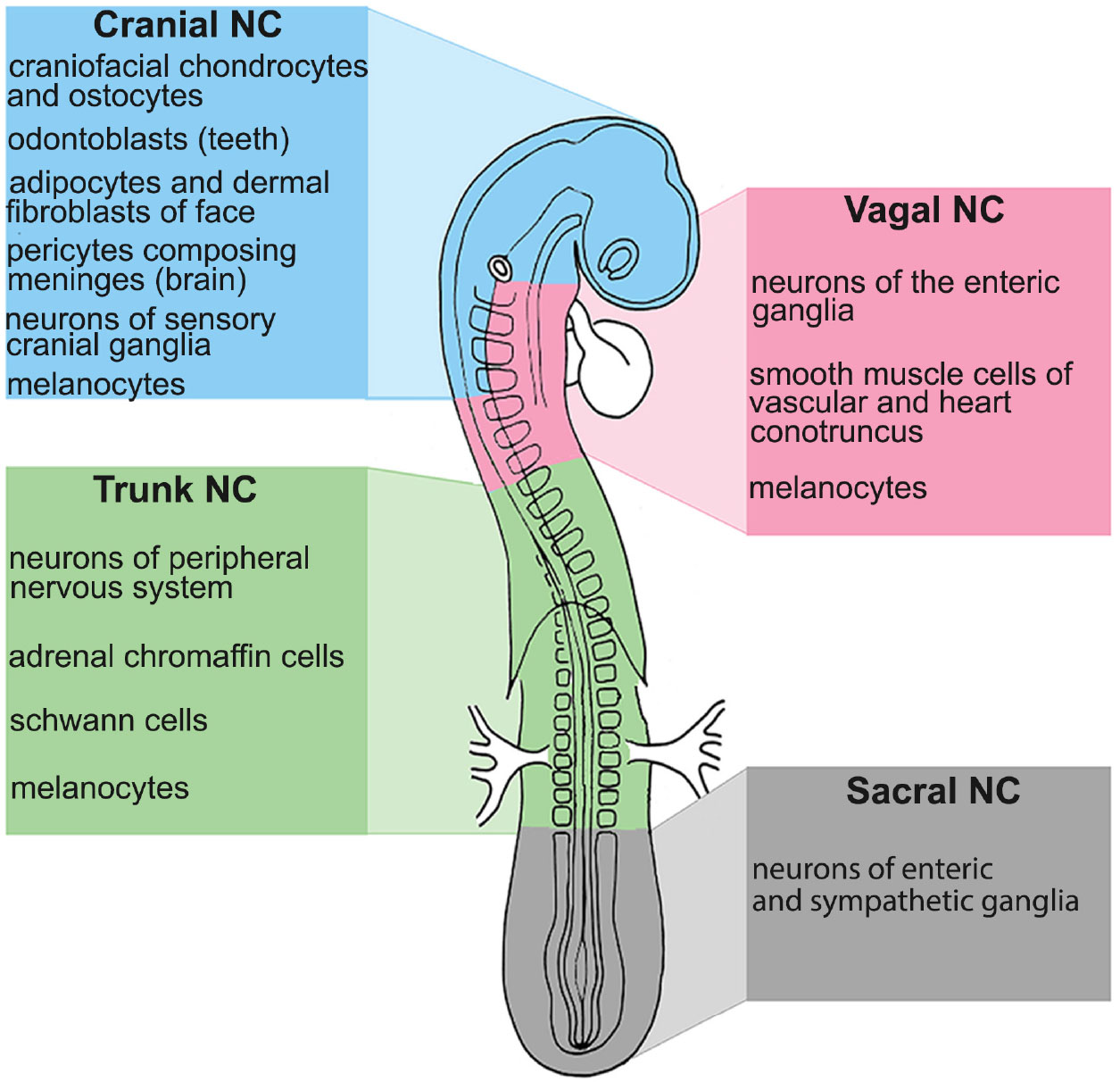
NCCs emerge from almost the entire axial body of the embryo, stretching from the posterior diencephalon to the lumbosacral region. According to the level from which they emerge, NCCs can be classified into four major subdivisions: cephalic, vagal, trunk, and sacral (Fig. 1) (Le Douarin and Kalcheim, 2011). These boundaries are very similar across vertebrates, but some variations occur. For example, recent studies speculate that a vagal NC population with the ability to form enteric neurons arose only in stem gnathostomes. Thus, agnathans might lack a “classic vagal” NC (Green et al., 2017).
Moreover, a “classic sacral” NC population may not exist in zebrafish since the enteric nervous system (ENS) of the zebrafish is derived entirely from the vagal NC. In amniotes, the ENS arises from a combination of vagal and sacral NCCs (Shepherd and Eisen, 2011). It is important to note that this classification is not merely anatomical since these subpopulations differ in their migratory patterns and behaviors, protein and gene expression profiles, and especially in a feature that represents the main concern of the present article: the differentiation potentials (see Hutchins et al., 2018; Rothstein et al., 2018 for excellent reviews).
We will focus on two significant NC populations: i) the Cephalic Neural Crest Cells (CNCCs), corresponding to cells originating from the forebrain to the 6th rhombomere (R6) of the hindbrain, and ii) the Trunk Neural Crest Cells (TNCC), including cells that migrate from the 8th somite to the 27th somite in avian embryos or the 24th somite in mouse (Fig. 1) (Le Douarin and Kalcheim, 2011).
Fig. 1. Neural crest derivatives along the anterior-posterior axis in avian embryos.
Neural crest subpopulations have been described in all vertebrate species based on their axial level of origin along the anterior-posterior axis. The scheme shows the main four major subdivisions: cephalic or cranial (blue), vagal (pink), trunk (green), and sacral (grey). These boundaries are very similar across vertebrates, but some variations occur. Colored boxes also show the main NC derivatives contributing to different tissues of the embryo.
The diversity of cell types generated by NC progenitors is astonishing in that they range from neural cells, such as glia, neurons, and chromaffin cells, to mesenchymal cells, including adipocytes, smooth muscle cells, fibroblasts, chondrocytes, osteoblasts, and odontoblasts. Currently, both in vivo and in vitro studies show that most of these NC progenitors are highly multipotent, meaning that one progenitor can originate a wide range of cell types (Baggiolini et al., 2015; Baroffio et al., 1988; Baroffio et al., 1991; Bronner-Fraser and Fraser, 1988; Calloni et al., 2007; Calloni et al., 2009; Da Costa et al., 2018; Dupin et al., 2018; Kaucka and Adameyko, 2019; Trentin et al., 2004).
However, it has long been assumed that only CNCCs are endowed with skeletogenic potential. Therefore, compared to CNCCs, the developmental potential of TNCCs is thought to be limited to neurons and glial cells of the peripheral nervous system (PNS), skin melanocytes, and endocrine (adrenomedullary) cells (Fig. 1) (Le Douarin and Kalcheim, 2011).
The notion that TNCCs lack skeletogenic potential was progressively built over the decades, mainly from results obtained by heterotopic transplantation studies performed with different vertebrates. Thus, exactly 20 years ago, the scientific community received, with some astonishment, an article with the provocative title “Trunk neural crest has skeletogenic potential” by Imelda McGonnell and Anthony Graham (McGonnell and Graham, 2002).This study showed, for the first time, that avian trunk neural crest cells (TNCCs) have chondrogenic and osteogenic potential when placed in a favorable environment, both in vivo and in vitro. As we will see in this review, this was not the beginning of the story because some studies had already proposed the contribution of TNCCs to skeletogenic structures in the normal development of fishes, amphibians, and reptiles. However, McGonnell and Graham’s article was the first to evoke the skeletogenic potential of TNCCs in amniotes. Many studies emerged from it, and, as we will see, it seems to have rekindled the interest of several research groups working with NCCs of fishes, reptiles, avians, and even mammals.
Moreover, even if TNCCs lack skeletogenic potential, as some authors argue, it does not mean it completely lacks mesenchymal potential. As we will see, classic graft experiments in avians and amphibians, as well as more modern experimental approaches in mammals, clearly show that.
Precisely, we will begin this review with a relatively less commented aspect of TNCCs: their ability to give rise to non-skeletogenic mesenchymal cells. Curiously, despite being a less-discussed aspect in the literature, it also seems less contentious, at least if compared to the skeletogenic potential of TNCCs. Therefore, it seems to be a good starting point for this review.
Fibroblastic and smooth muscle potential of TNCCs
In amphibians
Tadpoles have dorsal and ventral fins composed of an epidermis supported by a nucleus of mesenchyme. These mesenchymal cells are surrounded by great amounts of extracellular matrix, constituting the most significant part of the fins. Despite being mostly acellular, macrophages and fibroblasts are the dominant cell types in the dermal dorsal fin of the larval tail of anuran and urodelan (Takahama et al., 1992). A series of studies have demonstrated that TNCCs are, at least partially, at the origin of these mesenchymal (fibroblastic) cells composing the core of fins. Table 1 summarizes this literature. As far as we know, only one study (Taniguchi et al., 2015) has provided evidence that contradicts the contribution of TNCCs to fins (Table 1).
Table 1
Several works showing the contribution of TNCCs to the amphibian mesenchyme
| Reference | Descriptions/Conclusions of the works |
|---|---|
| Raven, 1931; Holtfreter, 1933; Holtfreter, 1935; DuShane, 1935; Twitty and Bodenstein, 1941; Woerdeman, 1946; Ford, 1949; Hörstadius, 1950; Bodenstein, 1952; Chibon, 1966 | These seminal works, which employed different amphibian species and methodologies, but generally intra- or interspecific transplants and ablations, put into evidence that the NC acts as the inductor of the amphibian fin. Unlike most embryonic induction phenomena, Bodenstein suggests that the inductor of the fin (in this case, NCCs) also participates in forming the organ it induces. Therefore, fin formation depends on the continued presence and real cooperation of NCCs. |
| Sadaghiani and Thiébaud, 1987 | The authors performed orthotopic grafts from different species of Xenopus stained with the fluorescent dye quinacrine, supplementing the analysis with scanning electron microscopy (SEM). The nuclei of Xenopus borealis show a number of bright fluorescent spots with quinacrine. On the other hand, the nuclei of Xenopus laevis stain homogeneously. Therefore, individual cells of each of the two species can be distinguished. The authors observed that some TNCCs derived from vagal-truncal grafts of Xenopus borealis had migrated and diffused into the mesenchymal tissue of the dorsal fin of Xenopus laevis hosts. |
| Krotoski et al., 1988 | They conducted a study similar to Sadaghiani and Thiébaud (1987) by using the same chimeric model and quinacrine to distinguish NCCs among distinct Xenopus species. Moreover, they employed the vital dyes lysinated fluorescein dextran (LFD) and lysinated rhodamine dextran (LRD). These dyes were injected into a single blastomere, thus acting as lineage tracers to detect the progeny of individual NCCs. The researchers observed TNCCs contributing to the mesenchyme of the dorsal fin in Xenopus laevis. It was not determined if the distribution of NCC cells in the fin resulted from passive translocation owing to the expansion of the dorsal fin or active migration through the matrix. |
| Collazo et al., 1993 | The use of vital fluorescent dyes Dil* and LRD at the clonal level allowed the researchers to observe TNCCs populating both the dorsal and ventral fins of Xenopus laevis and assuming a mesenchymal morphology. Moreover, two previously undescribed NCC migration routes were observed in the caudal trunk: the enteric pathway, in which TNCCs extend directly ventrad toward the anus and invade the ventral fin, and the tail tip pathway, in which TNCCs migrate along the dorsal surface of the neural tube or within the dorsal fin and then circumnavigate the tail tip and enter the ventral fin. The authors suggest that most NCCs might migrate actively to populate the fins. Two morphologically distinct types of NC-derived cells were observed populating the fins: (1) oval secretory cells containing vesicles and (2) abundant mesenchymal cells, presumably providing structural support to the fin. Importantly, this clonal study showed direct evidence for the multipotentiality of some NC progenitors in vivo. |
| Tucker and Slack, 2004 | Contrary to the results of Ford (1949), showing that NC controls both dorsal and ventral fin induction, the authors verified that ablation of the TNC in Xenopus laevis embryos only affects dorsal fin development, not ventral fin formation. Therefore, although the ventral fin core contains some TNC-derived mesenchyme, NC is not the only tissue contributing to ventral fin mesenchyme. The researchers concluded that the ventral fin is both induced by and populated with cells from the ventral mesoderm and is initially independent of the NC. |
| Sobkow et al., 2006 | They employed transgenic axolotl (Ambystoma mexicanum) ubiquitously expressing GFP. The neural folds of GFP+ embryos were grafted into wild-type embryos at stage 16, which were allowed to develop until stages 40-41. In all embryos, GFP+ cells were found in the apical portions of the dorsal fin epidermis, but, surprisingly, only half of the internal mesenchyme of the dorsal fin was labeled. According to Bodenstein (1952) and Tucker and Slack (2004), NC was the sole contributor of mesenchymal cells of the dorsal fin. These results led researchers to speculate on the existence of another source of mesenchymal cells for the dorsal fin. Therefore, they grafted somites from GFP+ donors into wild-type hosts at stage 23 and allowed them to develop until stage 42. After this period, GFP+ cells were localized within the dorsal fin mesenchyme. These results allowed the researchers to re-interpret the previous transplantation results as consistent with a dual neural crest and somite origin of dorsal fin mesenchyme. |
| Garriock and Krieg, 2007 | They used the vital dye Dil to track cells that contribute to the dorsal fin mesenchyme of the frog Xenopus laevis. Dil injections were performed in somites or in the neural tube in the trunk region of stage 19 embryos (the stage in which TNCCs have not started migrating), which were allowed to develop for 36h. When Dil was injected into somites, 86% of the embryos presented Dil+ cells in the dorsal fin. In comparison, Dil injections in the neural tube resulted in 63% of the analyzed embryos presenting Dil+ cells in the dorsal fin. Therefore, these results showed a dual contribution (somites and TNCCs) to the dorsal fin in Xenopus laevis. The researchers also observed that NC and somite cell populations that migrate into the fin matrix expressed the frog orthologue of Wnt11 (Wnt11-R). Wnt11-R is expressed prior to migration and persists in mesenchymal cells once they have distributed throughout the fin. |
| Epperlein et al., 2007 | They confirmed a contribution of TNCCs to the dorsal fin mesenchyme of axolotl (Ambystoma mexicanum), as already shown by Sobkow et al., (2006), but dermomyotome makes a significant contribution. These data were obtained through Dil injections into dorsal mid trunks of axolotl tailbuds at stages 25 and 33.After two days (stages 36–37), only a few labeled cells were detected in the developing dorsal fin. However, when DiI was injected into larvae at stage 35, the dorsal fin mesenchyme was abundantly labeled after four days.In conclusion, at early larval stages, TNCCs appear to make only a limited contribution to the developing axolotl dorsal fin mesenchyme; however, their contribution increases later. |
| Taniguchi et al., 2015 | Contradicting all the studies mentioned above, this work proposes that the fin mesenchyme of axolotl (Ambystoma mexicanum) and Xenopus laevis is derived exclusively from the mesoderm. Experiments of homotopic transplantation were done, grafting three distinct GFP+ neural plate regions to a white host (stage 15). Derivatives of the graft-derived GFP+ cells were visualized at stage 41. The transplants revealed that the posterior neural plate and neural folds (named by authors as region 3) are the primary sources of tail fin mesenchyme and tail muscle. The absence of expression of tfap2a, an NC gene marker from central region 3, suggests that little, if any, NC arises from this domain. Therefore, according to the authors, an NC contribution to fin mesenchyme can almost be ruled out. Interestingly, when authors replaced the region 3 neural fold of a white axolotl host with a cranial neural fold fragment of a GFP+ axolotl donor, they observed GFP+ mesenchyme in both the dorsal and ventral tailfins of donor larvae, suggesting that cephalic NC can form fin mesenchyme. These data suggest that amphibian TNCCs do not display mesenchymal potential. However, the median fin environment can support NC migration and differentiation into ectomesenchyme. |
In birds and mammals
The work of Nakamura and Le Lièvre (1982) was the first to describe in birds that TNCCs transplanted heterotopically to the mesencephalic NC level produced a few fibroblast-type cells contributing to connective tissues and blood vessel pericytes (Nakamura and Ayer-le Lievre, 1982). However, cells of chondro-osteogenic lineages were not detected, corroborating the observations made by Nicole Le Douarin’s group in the 1970s that avian TNCCs lack skeletogenic potential (Le Douarin et al., 1977; Le Douarin and Teillet, 1974).
Later, in 1996, David Anderson’s research group identified mesenchymal cells in clonal cultures derived from rat TNC (Shah et al., 1996). The authors noted that 93% of these cells were non-neural and expressed smooth muscle actin (SMA) in addition to calponin, also a muscle cell marker. Treating the clones with the molecule Tgfß1, the authors verified that all colonies differentiated into smooth muscle cells. Around 86% of the colonies consisted exclusively of SMA+ or calponin+ or both (Shah et al., 1996). The authors explain their results in light of the Nakamura and Le Lièvre (1982) data, showing that TNC can originate mesenchymal derivatives when transplanted to a favorable, or at least permissive, environment.
In 1999, the same research group isolated and cloned rat sciatic nerve cells and detected the existence of colonies containing glia + fibroblasts (GF), others containing only glia (G), and also colonies containing only fibroblasts (F) (Morrison et al., 1999). This result showed clearly that nerves of adult rats contained undifferentiated TNCC progenitors and that some of them were endowed with mesenchymal potential (Morrison et al., 1999).
The most skeptical can argue that these results observed in vitro may be a consequence of the artificialities intrinsic to cell culture methods. However, an important study, also from Anderson’s lab, shed new light on this issue. Joseph and colleagues, through the Cre-recombinase system, identified that a population of TNCCs forms the endoneurium of the peripheral nervous system of rats. The endoneurium consists of loose connective tissue and fibroblastic cells that surround each nerve fiber of peripheral nerves. Therefore, TNCCs are the source of the endoneurial fibroblasts that contribute to the constitution of nerve fibers in vivo (Joseph et al., 2004).
The authors also showed that both Schwann cells and endoneurial fibroblasts arise from a common progenitor population, expressing desert-hedgehog (Dhh) in the nerve environment. The nerve NC stem cells expressed Dhh strongly, suggesting that these stem cells are the origin of both Schwann cells and endoneurial fibroblasts. Moreover, the combination of neuregulin, Delta, and Bmp4 promotes the generation of Schwann cells and fibroblasts but not neurons, from NC stem cells obtained from sciatic nerve cultures (Joseph et al., 2004). Curiously, the marker used to identify fibroblasts in culture, SMA, was not expressed by endoneurial fibroblasts in vivo.
Still, in 2004, Trentin and colleagues corroborated in the avian model the observations of Anderson’s group in mammals that some TNC progenitors are capable of self-renewal, which is a fundamental characteristic when considering a cell as an actual stem cell. Importantly, Trentin detected the existence of four types of oligopotent progenitors containing smooth muscle cells: GF (glia/smooth muscle cells), GNF (glia/neurons/smooth muscle cells), GMF (glia/melanocytes/smooth muscle cells), and GNMF (glia/neurons/melanocytes and smooth muscle cells). Subcloning experiments, performed under plastic plates covered with collagen type I, showed that GF progenitors are able to self-renew for two generations (Trentin et al., 2004). Some years later, our research group observed that Fgf2 can prolong up to four generations the appearance of GF progenitors (Bittencourt et al., 2013).
It would be interesting to know if TNCCs also contribute in vivo to endoneurial fibroblast populations in the avian model, as seen in mammals. The fact that Trentin and Morrison found very similar progenitors endowed with mesenchymal potential in cell cultures suggests that this could be the case. Moreover, the findings that Schwann cells and endoneurial fibroblasts arise from a common progenitor suggest that these progenitors may constitute an actual stem cell population. The fact that they have been observed in sciatic nerves also indicates that this population may constitute a possible source of stem cells in adult tissues, fostering studies related to therapeutic applications.
As mentioned above, these clonal and subclonal cultures were performed on culture plates coated with collagen type I. Perhaps; these results could have been aggrandized if the experiments had been carried out on other substrates. For example, clonal assays demonstrated that the extracellular matrix molecule fibronectin can significantly increase the proportion of clones containing only smooth muscle cells (F) and glial-fibroblastic progenitors (GF clones) when compared to collagen type IV (Costa-Silva et al., 2009). More recently, we demonstrated that a simple peptide sequence of 16 amino acids commercially sold under the name of PuraMatrix™ can produce higher quantities of smooth muscle cells from TNC mass cultures (400 cells/well) (Taufer et al., 2020) (see Fig. 3I). This highlights the role that a microenvironment composed of different substrates, such as fibronectin, PuraMatrix™, and growth factors, such as Fgf2, can play in multipotentiality, self-renewal, and mesenchymal differentiation of TNCCs. Therefore, depending on the culture conditions, the mesenchymal (fibroblastic) potential of TNCCs can be strongly evidenced.
Adipogenic and chondro-osteogenic potential of TNCCs: emphasis on in vitro experiments
The results of some in vivo studies performed over several decades of the last century did not encourage the search for skeletogenic derivatives originating from TNCCs.
For example, TNCCs grafted into the head region of axolotl neurulae did not exhibit signs of cell migration and cartilage formation (Horstadius and Sellman, 1946; Raven, 1931). Years later, Chibon (1966) observed that TNCCs give rise to the dorsal fin mesenchyme in the amphibian Pleurodeles; however, it never produces cartilage or bone, even when transplanted to the cephalic region (Chibon, 1966). Around twenty years later, Graveson and Armstrong (1987) tested the chondrogenic potential of TNCCs in explant cultures of the axolotl (Ambystoma mexicanum). Cartilage was observed in 90% of cultures in which CNC was put in intimate contact with inductive endoderm. On the other hand, explants containing TNC never formed cartilage (Graveson and Armstrong, 1987). Despite the absence of chondrogenic potential, it is noteworthy that teeth formed in combinations of mandibular arch epithelium with NC explanted from the trunk level. Therefore, an odontogenic and osteogenic potential, even if limited, was observed by the most rostral TNC in both axolotl (Graveson, 1993) and mice (Lumsden, 1988).
Later, it was observed that transplanted TNC fails to migrate from the axolotl cranial neural tube (Graveson and Hall, 1995). Therefore, this observation opened the possibility that TNCCs were, in fact, not devoid of skeletogenic potential but instead could not migrate properly after transplantation in the new site to differentiate.
Experiments performed in birds by Nicole Le Douarin’s group in the 1970s also argued against the skeletogenic potential of TNCCs. Bilateral grafts of the trunk neural primordium to cephalic level resulted in severe malformations similar to those observed after CNC excision due to the absence of facial and/or pharyngeal mesectoderm. On the other hand, cranial NC transplanted to the level of the trunk could generate some cartilage and connective tissue (Le Douarin et al., 1977; Le Douarin and Teillet, 1974). As already mentioned, the study of Nakamura and Le Lièvre (1982) showed that TNCCs can migrate and differentiate into fibroblastic cells when transplanted heterotopically to cranial levels. Despite their inability to originate skeletogenic derivatives, this indicates that TNCCs are endowed with mesenchymal potential, but it is not expressed in vivo owing to the presence of inhibitory cues or the lack of permissive factors in the trunk environment.
In addition, a seminal study performed by Smith and colleagues in 1994 showed, for the first time, that TNCCs contribute to the medial and caudal fin ray mesenchyme of zebrafish (Smith et al., 1994). However, it was uncertain whether NC cells actually formed skeletogenic tissue or remained mesenchymal, even though they were associated with osteogenic fin rays. We will present this work in more detail below when discussing specifically the skeletogenic potential of TNCCs in fishes.
Then, in 2000, Epperlein and colleagues showed evidence that cartilage can actually develop from TNCCs in amphibians (axolotl). Heterotopic transplantations showed that TNCCs do not migrate along normal cranial NC pathways but instead move in a disoriented manner. However, they form cartilage, contributing to elements of the visceral skeleton (Epperlein et al., 2000).
Therefore, until the 2000s, a few studies demonstrated in vivo a possible chondro-osteogenic potential of TNCCs. This observation was restricted to fish and amphibians and, as we have seen, was far from unanimous in the scientific community.
Then, a paper published in 2002 seemed to represent a milestone that sparked the interest of many groups in the NC field. The article entitled "Trunk Neural has skeletogenic potential" by Imelda McGonnell, and Anthony Graham described, for the first time, that avian TNCCs could differentiate into bone and cartilage when placed in a suitable environment (McGonnell and Graham, 2002).
Importantly, they demonstrated the skeletogenic potential of TNCCs in both in vitro and in vivo experiments. For in vitro assays, TNCCs were obtained from neural tubes at the region of the last three somites formed; embryos were between stages 10 and 15HH. After 24 hours, neural tubes were removed, and NCCs were treated with dexamethasone, ascorbic acid, and β-glycerophosphate. After 2-3 weeks of culture, 50% of the total cells were marked with the chondrogenic marker collagen type II. The morphology of these cells matched that of chondrocytes. Furthermore, after 3-4 weeks of culture, cells with osteoblastic morphology were also detected. Around 20% to 30% of the total cells in each culture were marked with the osteoblastic marker collagen I.
For the in vivo experiments, the researchers inserted compact aggregates of quail TNCCs in the region of the developing maxilla and mandible of chicken embryos in stage 14HH, letting them develop until stage 36HH. After this period, quail cells were identified through the antibody QCPN (Quail Cell Marker Antibody – specific for identifying cells from this species). Quail TNCCs were detected spreading over the entire region of the chicken face, contributing to facial structures, such as Meckel’s cartilage and the scleral cartilage that surrounds the embryo’s eye. However, whether these cells expressed skeleton-specific markers remained elusive.
In the following year, Abzhanov and colleagues endorsed the findings of McGonnell and Graham regarding the skeletogenic potential of the avian TNC in vitro (Abzhanov et al., 2003). Here, TNCCs were obtained from the sacral region of chick embryos and cultivated on fibronectin in a medium free from any stimulator of skeletogenic differentiation and kept in culture for a long time (2-4 weeks). On the 14th day of culture, researchers detected chondrogenic cells expressing collagen type II. The scientists proposed that TNCCs in long-term cultures become similar to CNC since real-time PCR identified a high expression of two characteristic genes of the CNC: Id2 and Noelin1. Besides, the researchers analyzed the expression of Hoxb4, which was down-regulated in the second week of TNCCs culture. With this result, the authors proposed that chondrogenesis is possible, owing to the downregulation of Hox genes during long-term cultures (Abzhanov et al., 2003).
These initial observations that avian TNCCs can form cartilage under appropriate conditions prompted Marianne Bronner’s group in 2004 to revisit the classic grafting experiments of Le Douarin’s lab, now using more sophisticated approaches and molecular markers of cell labeling and differentiation. Grafts of quail trunk NCCs to the midbrain of chick host embryos demonstrated little ability to populate the branchial arches and no ability to form cartilage. Moreover, grafts of quail trunk dorsal neural tubes directly into the first branchial arch showed that quail NC cells formed aggregates adjacent to the chick cartilage-forming region. However, these quail cells never incorporated into the cartilage-forming region or expressed collagen II (Lwigale et al., 2004). These results align with classic grafting experiments showing that avian TNCCs do not form cartilage, even when grafted directly in a conducive environment in vivo (Le Douarin et al., 1977; Le Douarin and Teillet, 1974; Nakamura and Ayer-le Lievre, 1982).
Then, in 2006, Ido and Ito demonstrated the potential of TNCCs to form cartilage, but now in mice and exclusively by in vitro assays. However, in contrast to avian TNC cultures, chondrogenic differentiation was not observed in long-term experiments and was found to occur only in cultures treated with fibroblast growth factor 2 (Fgf2) (Ido and Ito, 2006). In order to obtain TNCCs, the neural tubes were excised from the region corresponding to the last six somites of 21-29 somite mice embryos. The neural tubes were cultivated on collagen gels and treated with Fgf2. The Fgf2 was so effective that on the fifth day of culture, it was already possible to verify chondrocytes expressing collagen type II and stained by alcian blue.
Moreover, a remarkably rapid decrease of Hox9 expression and upregulation of Id2 was identified in Fgf2-treated cultures on culture day 5. However, on the 10th day of culture, the two genes showed similar expression, both in the presence and the absence of Fgf2. The authors explained this result in two ways. First, the down-regulation of Hox genes and the upregulation of Id2 are essential for chondrogenesis at the beginning of the culture. Second, TNC chondrogenic potential is independent of Hox down-regulation and Id2 upregulation but rather depends on other signaling effects promoted by Fgf2 (Ido and Ito, 2006). Therefore, mandatory down-regulation of Hox genes for expressing the chondro-osteogenic potential of TNCCs remains an open question.
Finally, in 2007, the work of Nathalie Billon and colleagues demonstrated, for the first time, another mesenchymal potential displayed by TNCCs: adipogenic differentiation (Billon et al., 2007). The researchers used quail embryos with 18-25 somites, removing the region of the last ten somites of the neural tube. TNCCs migrated during 48 hours of primary cultures, and then secondary cultures were performed. After four days of secondary cultures, adipogenic differentiation was stimulated through two different culture media types: DIF1, containing insulin, triiodothyronine, and rosiglitazone, or DIF2, containing insulin, rosiglitazone, dexamethasone, and IBMX. The cultures were kept in contact with these differentiation media for two days, and adipocytes were found in greater quantity (40% of the cultures) under the stimulus of the DIF2 medium. To the best of our knowledge, it should be noted that the adipogenic potential of TNCCs has not been observed in vivo so far.
Based on these data, a series of questions arise. First, what would a segregation model of these TNC progenitors look like? Second, would TNC progenitors be capable of simultaneously originating neural and skeletogenic/adipogenic derivatives? Third, in the alternative, would the TNC population be composed of a heterogeneous cell population with some progenitors responsible for the origin of neural lineages and others responsible for giving rise exclusively to skeletogenic and/or adipogenic derivatives?
In order to begin to address these questions, clonal studies were performed, revealing for the first time, the existence of a TNC progenitor exhibiting glial, fibroblastic, and chondrogenic potential (clone GFC) at the same time (Calloni et al., 2007). In this study, the TNC was obtained from the region of the last 10 somites of quail embryos with 20-25 somites. The TNCCs were clonally seeded on a feeding monolayer of mouse embryonic fibroblasts (3T3), which is very efficient in allowing the survival and differentiation of both neural and mesenchymal derivatives (Baroffio et al., 1988; Baroffio et al., 1991). The GFC progenitor turned out to be extremely rare, as it was the only one obtained from around 500 clones analyzed. It is important to note that this progenitor was observed in cultures treated with the morphogen Sonic hedgehog (Shh). Shh was shown to be extremely important for increasing the percentage of progenitors with chondrogenic potential in CNC cultures (Calloni et al., 2007; Da Costa et al., 2018). The fact that Shh stimulated the appearance of cartilage nodules in TNC mass and clonal cultures highlights even more how the mesenchymal potential of TNCCs needs special culture conditions to be unveiled (Calloni et al., 2007).
These results prompted Coelho-Aguiar et al. (2013) to search for TNCC progenitors endowed with osteogenic and adipogenic potential. TNC primary cultures were performed with the last 10 formed somites of quail embryos with 18-25 somites. Meanwhile, the cloning experiments (secondary cultures) were performed in different substrates due to methodological difficulties. In order to analyze adipogenesis, the cloning assays were carried out in culture plates coated with collagen type I. At the same time, to analyze osteogenesis, the TNCCs were cultured on 3T3 monolayers. Then, to stimulate adipogenesis, clonal cultures received insulin, triiodothyronine, and rosiglitazone from the 7th day onward. On the other hand, dexamethasone, ascorbic acid, and β-glycerophosphate were added to clonal TNCC cultures to promote osteogenic differentiation (Coelho-Aguiar et al., 2013).
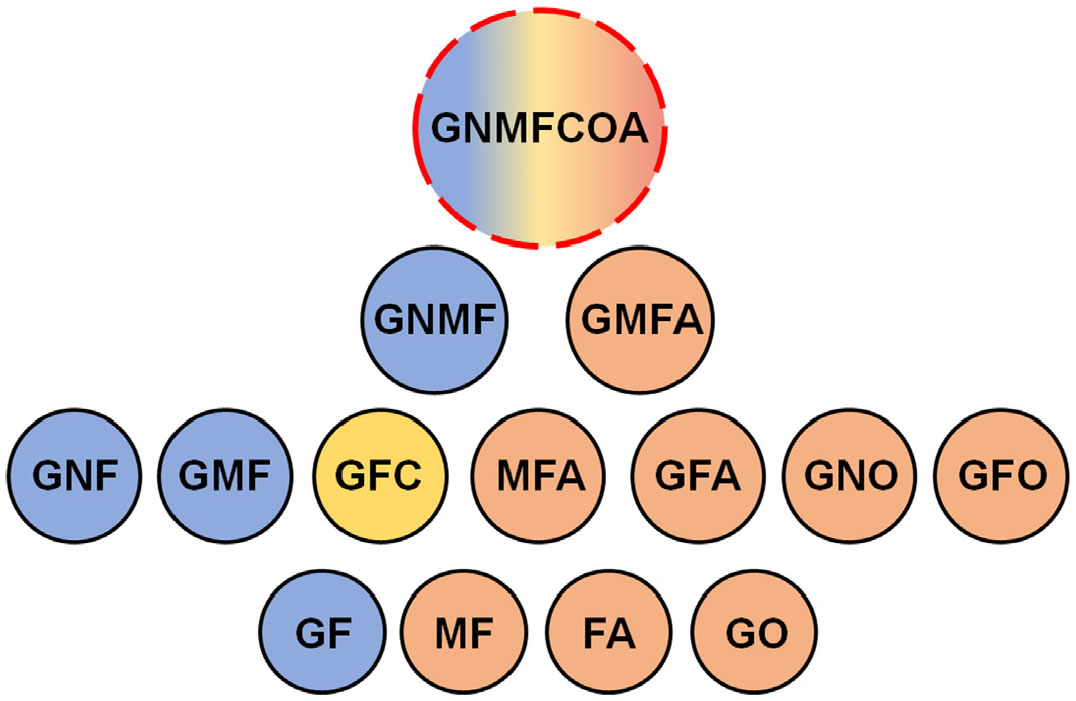
In cultures treated with the osteogenic cocktail, results showed that around 80% of the clones contained cells expressing Runx2. Moreover, besides osteoblasts, almost all these clones contained glial derivatives (GO progenitors) or glial cells and neurons (GNO progenitors). Adipocytes were found in around 40% of the clones treated with the adipogenic differentiation medium. However, no culture presented adipocytes without the treatment, evidencing that adipogenic potential is more difficult to achieve “naturally” in TNC cultures (Coelho-Aguiar et al., 2013). This work leaves no doubt that the TNC has progenitors with skeletogenic-neural potential (clones GNO, GFO, GO) and adipogenic-neural potential (GMFA, GFA) (Fig. 2). Nevertheless, given that the clonal cultures were carried out in different substrates, this study could not demonstrate the existence of a common progenitor for osteogenic and adipogenic derivatives. Moreover, the authors did not identify any clones with chondrogenic potential in their cultures.
Fig. 2. TNC progenitors exhibiting mesenchymal potentialities.
Scheme of the multipotent progenitors identified in the works of Trentin et al. (2004 (blue circles); Calloni et al. (2007 (yellow circle) and Coelho-Aguiar et al. (2013 (salmon circles). The majority of progenitors display neural-mesenchymal potentials. The existence of a highly multipotent progenitor able to give rise to seven distinct cell types is merely hypothetic. G-glia, N-neuron, M-melanocyte, F-fibroblast, A-adipocyte, C-chondrocyte, O-osteoblast.
So far, it can be seen that the TNC has dormant adipogenic and skeletogenic potential that can be unveiled if the cells are cultivated in an environment favorable to the expression of such potential, e.g., substrate, hormones, growth factors, or time of culture. Next, we asked if the multipotent progenitors found so far would behave as true stem cells; that is, would they be capable of self-renewal, as observed in vitro for GF (neural-fibroblastic) progenitors?
The core issue here is related to methodology. This means that in order to verify whether a given progenitor is capable of self-renewal, it is necessary to perform serial clonal assays (subcloning). In these assays, a progenitor is removed from a given generation and replated in order to analyze its progeny. Following this, we observe if it can maintain itself for several passages or self-renew by so-called asymmetric cell division.
Unfortunately, the substrates used in the studies described so far have some unsuitable characteristics. For example, plastic plates coated with isolated extracellular matrix molecules, such as fibronectin, laminin, or collagens, are unfavorable for the differentiation of chondrocytes and osteoblasts. Furthermore, these experiments were carried out in two-dimensional (2D) environments, which we now know to be poorly permissive and even inhibitory, to differentiate chondroblasts and osteoblasts.
NC clonal experiments using mitomycin-arrested 3T3 monolayers vastly expanded our knowledge about CNC and TNC potentials (Baroffio et al., 1988; Baroffio et al., 1991; Calloni et al., 2007; Calloni et al., 2009; Coelho-Aguiar et al., 2013; da Costa et al., 2018; Trentin et al., 2004). However, 3T3 brings other problems in its use, including the impossibility of separating NC cells from mouse fibroblastic cells for subcloning experiments. In addition, various growth factors secreted by 3T3 cells are unknown, as well as their concentrations, which may directly and/or indirectly influence the differentiation of NCCs. Thus, over the last years, the priority of our research group has become that of finding new substrates that could allow the maximum expression of NC potential and, at the same time, be malleable enough to allow the removal of cells to perform subcloning assays.
In 2013, we analyzed the differentiation of TNCCs on Matrigel™ (Ramos-Hryb et al., 2013). Matrigel™ corresponds to the extracellular matrix of the basement membrane of rat sarcoma. In this matrix, several proteins are adsorbed, including a range of growth factors in different concentrations. It is a completely biodegradable and non-toxic material (Kleinman and Martin, 2005). In addition, Matrigel™ allows the constitution of two-dimensional (2D) and three-dimensional (3D) environments that provide a framework for cells cultured on a matrix. This material is widely used in several cell types’ differentiation, growth, adhesion, and morphogenesis studies (Kleinman and Martin, 2005). Our research group was the first to evaluate the potential of embryonic TNCCs on this material. We showed that Matrigel™ supported the differentiation of neurons, glial cells, melanocytes, smooth muscle cells, and chondrocytes, even without adding specific molecules that stimulate mesenchymal differentiation. The high frequency of wells containing cartilage nodules (more than 80% of wells) was noteworthy. Most of these nodules were present in peripheral regions of the wells where Matrigel™ forms a 3D environment (Ramos-Hryb et al., 2013). Significantly, this matrix can be dissociated, allowing for the removal of cells for replating, thus making it possible to perform serial cloning assays (subcloning) to study the self-renewal of NC cells.
Unfortunately, the number of cartilage nodules observed under Matrigel™ was insufficient to encourage us to carry out cloning experiments. Moreover, some characteristics of Matrigel™ must be considered, such as the heterogeneity of extracellular matrix molecules and their growth factors. These molecules can directly or indirectly influence the differentiation of NCCs, thus complicating an understanding of the phenomena under analysis. This heterogeneity is also present in different batches of Matrigel™, and this characteristic may interfere with the replicability of the experiments. It has been shown that this heterogeneity can be circumvented by mixing different batches, but it ends up making the experiment very expensive (Ramos-Hryb et al., 2013).
These perceptions prompted us to search for materials with more pure composition, yet as efficient as Matrigel™, in allowing a wide range of differentiation of TNC phenotypes and permitting the removal of NCCs for subcloning assays. Consequently, we tested a hydrogel named PuraMatrix™. This hydrogel was developed by Dr. Zhang and colleagues (2005) and is composed of 99% water and 1% of a repetitive sequence of just 16 amino acids, making it highly pure (Zhang et al., 2005). When in contact with saline solutions, these amino acids can self-assemble as fibers, adapting to gel consistency. This hydrogel can be diluted and, thus, have its structure (porosity) regulated to perform 2D and/or 3D cultures. PuraMatrix™ had never been used as a substrate for the culture of embryonic NCCs, and we were the first research group to perform this test (Taufer et al., 2020).
In short, TNCCs were removed from the region of the last 10 somites of quail embryos at the stage of 18-24 somites. For 15 hours, these cells migrated in plastic plates and then were removed and seeded in a 96-well plate pre-prepared with different concentrations of PuraMatrix™ (0.15%, 0.25%, and 0.5%). The cultures were maintained for seven days in a control culture medium, and then some were treated with the same cocktail of osteogenic and adipogenic inductors as those used by Coelho-Aguiar et al.,2013). The cultures were maintained until the 14th or 21st day.
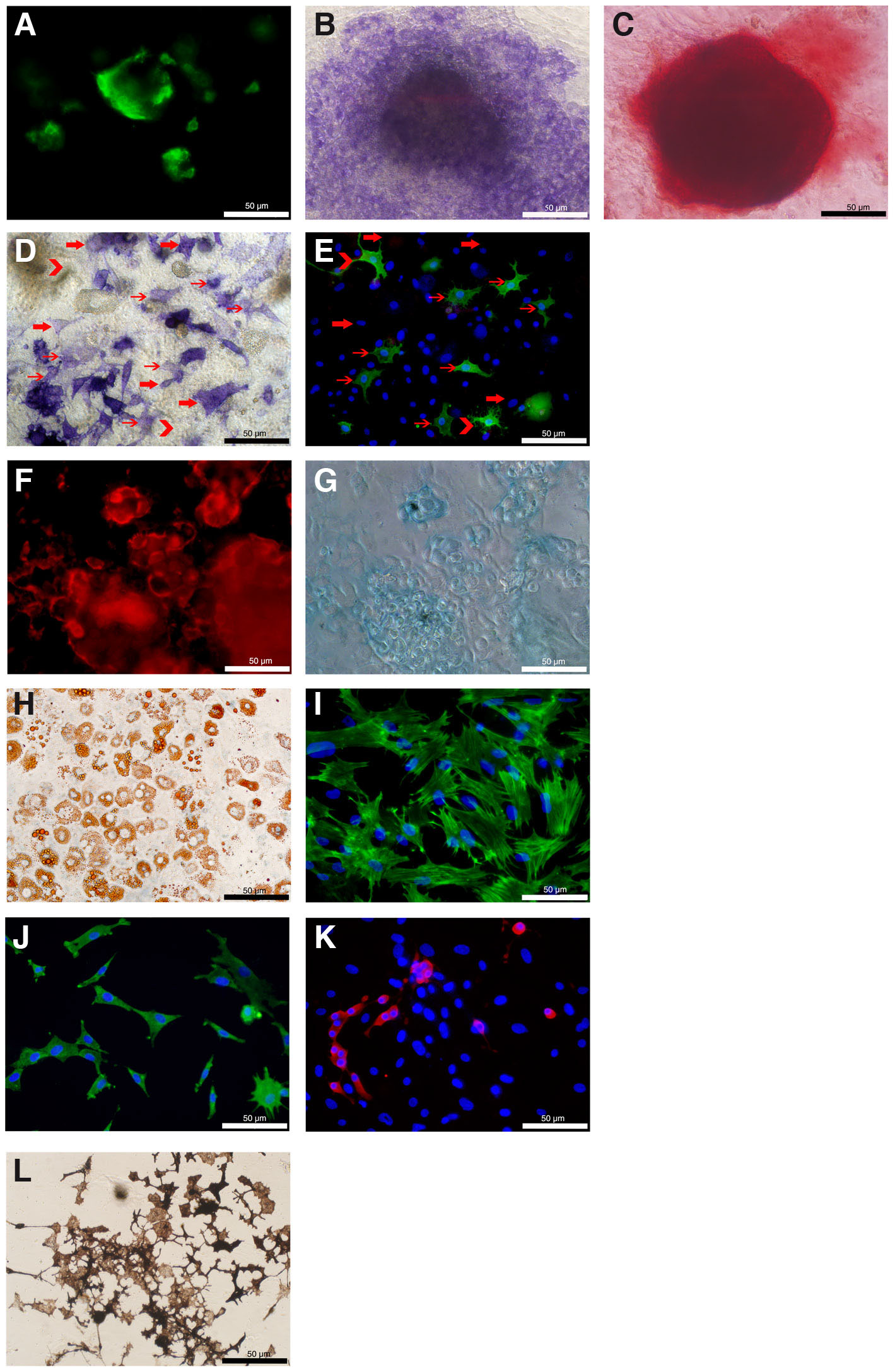
We observed that PuraMatrix™ supports the differentiation of osteoblasts/osteocytes, adipocytes, and chondrocytes, even without adding substances that induce the differentiation of these cell lineages (Fig. 3).
For detection of cell types from the bone lineage, the following markers were used: a) the antibody SB5, developed by Arnold Caplan in 1989, which recognizes osteocytes very specifically in birds; b) alkaline phosphatase (ALP), which marks osteoblasts in different animal species; and c) alizarin red dye (AR), widely used for the detection of mineralized osteoblasts. Cells of bone lineage were visualized as early as day 14th of culture through marking with SB5 (Fig. 3A). On the 21st day of culture, bone nodules and mineralized matrix were strongly marked by ALP and AR (Figs. 3B and 3C, respectively). In these same cultures, isolated cells marked with ALP and SB5 were also found, interestingly presenting three subpopulation types: 1) ALP+ and SB5- cells; 2) ALP- and SB5+ cells, and 3) ALP+ and SB5+ cells (Figs. 3 D-E). According to Bruder and Caplan (Bruder and Caplan, 1989), these subpopulations can be explained as distinct osteogenic differentiation stages. This observation shows that PuraMatrix™ allows an extensive development of osteoblastic cells at different stages of maturation or differentiation.
For the chondrogenic lineage, we employed two markers considered very specific for detecting cartilage and chondrocyte nodules: chondroitin-sulfate (CS) antibody and alcian blue dye (Figs. 3F and G, respectively). Furthermore, the chondrocytes were easily recognized by their morphology and organization into nodules by bright-field microscopy. Noteworthy, while Matrigel™ presented few cartilage nodules per well (not more than five), PuraMatrix™ easily presented more than 50 nodules per well in 90% of the wells (Taufer et al., 2020).
PuraMatrix™ also allowed the spontaneous development of adipocytes. At 0.15% and 0.25% PuraMatrix™ concentrations, adipocytes were observed in 28% and 40% of the wells, respectively. More importantly, TNCCs differentiated into adipocytes without adding any specific inducers for this cell type. However, the addition of mesenchymal differentiation factors increased the number and increased adipocyte frequency to 100% of the culture wells (Taufer et al., 2020) (Fig. 3H).
Finally, it is essential to note that the cell type detected in higher quantity in all PuraMatrix™ concentrations tested was a mesenchymal, the smooth muscle cells (Fig. 3I). In addition, the hydrogel allowed the differentiation of glial cells, adrenergic neurons, and melanocytes (Figs. 3J, L, and M, respectively).
Therefore, the use of this matrix constituted of a single amino acid sequence and extreme purity had the capacity to allow the full expression of the neural and mesenchymal potentials of TNC. This differentiation occurs even without the addition of growth factors and hormones commonly employed to stimulate mesenchymal differentiation. Together, these data reinforce the conclusion that TNC mesenchymal potential can be revealed through specific permissive culture conditions and sometimes inductors of this expression (Taufer et al., 2020). Currently, our research group is performing clonal cultures using PuraMatrix™ to address the questions related to the multipotentiality and self-renewal of these TNCC progenitors. These studies should shed light on the extended potential of TNCCs compared to CNCCs.
Fig. 3. PuraMatrix™ (PM) supports differentiation of neural-mesenchymal phenotypes in trunk neural crest cell (TNCC) cultures.
Several mesenchymal/skeletogenic cell types were detected in d14 and d21 TNCCs cultures grown at PM. (A) Osteoblasts were assessed through immunoreactivity to SB-5 (green) in d14. (B) Microscopic views of bone cells stained by Alkaline Phosphatase and (C) Alizarin Red at d21. (D) Phase- contrast microscopy showing alkaline phosphatase (ALP) staining and (E) epifluorescence microscopy of SB-5 immunocytochemistry. Thin arrows indicate cells with both ALP and SB-5 staining, thick arrows indicate cells stained only with ALP, and chevron arrows indicate cells marked only with SB-5. (F) Chondrocytes were assessed through immunofluorescence to Chondroitin Sulfate and (G) the same cartilage nodules were stained by Alcian Blue. (H) Representative pictures of phase-contrast microscopy displaying adipocytes stained by Oil Red and (I) immunofluorescence to smooth muscle actin (SMA) for detection of smooth muscle cells (green). (J) Representative images of immunofluorescence to HNK-1 for detection of glial cells (green) and (L) immunofluorescence to Tyrosine Hydroxylase (TH) to detect adrenergic cells (red). (M) The presence of melanin pigment identified Melanocytes through phase contrast microscopy. Cell nuclei in (E, I, J and K) were detected by staining with DAPI (in blue). (Magnification of all pictures: 400x).
Evidence of TNCC skeletogenic potential in vivo
In teleost fish
For a long time, it has been assumed that the NC contributes to superficial dermal calcified tissues in some fishes. In extinct animals, like ostracoderms, these primitive exoskeletal armor-bearing, tooth-like structures cover the animal’s body in some species from head to trunk. This assumption was based mainly on the fact that these exoskeletal structures are often covered by enamel and dentin layers, proteins considered to be produced exclusively by NC cells (Géraudie, 1988; Smith, 1991; Smith and Hall, 1990; Smith and Hall, 1993). In extant animals, the exoskeleton is retained in the form of dermal dentine scales in cartilaginous fish, dermal scales, and fin rays in bony fish. However, the presence of dentin does not necessarily imply that NC cells in the trunk region form these exoskeletal structures; instead, it could result from a massive caudal migration of CNCCs.
A pioneering study addressed this question in the 1990s (Smith et al., 1994). The researchers injected Dil in 15 somite stage zebrafish (Danio rerio) embryos at two different sites: the caudal neural keel (before the onset of NC migration) and the somites. The embryos were allowed to develop for nine days and then analyzed through fluorescence microscopy. When Dil was injected at the neural keel, TNC labeled cells were observed in the caudal fin mesenchyme, assuming a mesenchymal morphology. Dil injection at the somite resulted in labeled cells at the myotomes and the epidermis. Therefore, the authors showed, for the first time, the contribution of TNCCs to the caudal and medial fin ray mesenchyme of zebrafish. However, owing to technical difficulties in long-term cell-lineage analysis, embryos were not followed for long enough to determine whether these cells actually give rise to skeletogenic lepidotrichia (the final portion of the fin rays) or remain as mesenchymal cells associated with osteogenic fin rays.
Currently, the possible contribution of TNCCs to skeletogenic tissues in fish is still far from reaching consensus among the different research groups. While some studies align with Smith's assumptions, others go in the opposite direction and do not detect any contribution of TNCCs to skeletogenic/mesenchymal structures in fish. These studies are summarized in Tables 2A and 2B.
Table 2A
Works showing the mesenchymal or skeletogenic potential of TNCCs in fish
| Reference | Brief description of the work |
| They performed labeling with HNK-1 antibody, a marker for migrating NC cells, in the larval flounder Paralichthys olivaceus at 72 hpf (hours post-fertilization). An accumulation of HNK-1+ cells was observed at the proximal part of the embryonic dorsal fin fold. This part of the fin fold is composed of mesenchymal cells, some of which will differentiate into prescleroblasts, precursors of scleroblasts (skeletal cells), forming the lepidotrichia at later stages. The same was observed in pufferfish embryos, but here it was discovered through in situ hybridization against Slug and Msxb mRNA, genes known to serve as NC markers. The authors also propose that NCCs could give rise to radial cartilage precursors in fin folds. However, it was impossible to determine the fin components into which the TNCCs differentiate since the fate of the labeled cells had not been traced up to actual skeletogenesis. | |
| They developed transgenic zebrafish embryos (Sox10:cre) through the CreLoxP technique. This allows performing lineage tracing experiments to follow NC cells through the expression of Sox10 gene, a widely known NC marker. Between 2 and 8 dpf, Sox10+ cells were observed clustering around the tip of the notochord, and a few were identified entering the fin fold. At 16 dpf, the number of Sox10+ cells invading the caudal fin increased substantially. Finally, at 21 dpf, Sox10+ cells were associated with a well-formed lepidotrichia, presenting osteoblastic morphology. These Sox10+ cells also expressed the osteoblast marker Runx2. Labeled cells similarly positioned in the dorsal fin were also observed. Therefore, sophisticated and accurate lineage tracing experiments confirmed the speculation that TNCCs might contribute to bony lepidotrichia. | |
| Chen et al., 2017 | They studied flounder embryos (Paralichthys olivaceus), as did Suzuki in 2003; however, they followed the development from larval to adult. They investigated the cellular origin of the dorsal fin bud. Fin buds are structures formed inside the embryonic fin fold during teleost metamorphosis, transforming into the adult median fins as the fish develops. By in situ hybridization, the authors observed that Slug, Hnk-1, and Msx2 started to be expressed in the developing dorsal fin bud at 3 days post-hatch (dph) and persisted during all the process of fin bud formation and later skeletogenesis. Moreover, all marker genes were expressed in the pectoral fin three days post-hatch, indicating that NCCs are also involved developing the pectoral fin. Col10a1, a marker of scleroblasts, according to the authors, was expressed at three days post-hatch and maintained at a high level during the whole process of fin development. According to the authors, both paraxial mesoderm and TNCCs contribute to the first steps of fin bud formation. However, strong expression of Slug and Col10a1 during dorsal fin skeletogenesis indicates that NCCs and scleroblasts participate mainly in adult dorsal fin ray development. |
Table 2B
Works that do not observe the mesenchymal or skeletogenic potential of TNCCs in fish
| Reference | Brief description of the work |
| Lee et al. 2013b | The authors analyzed the origin of fin rays and scales in zebrafish embryos through CreLoxP, using the transgenic lines Sox10:cre and Tbx6:cre to track NCCs and paraxial mesodermal cells, respectively. The researchers observed NCCs running the length of the lepidotrichia at 90 dpf, but none were identified as osteoblasts since immunostaining against zns-5, an osteoblast marker, failed to label these cells. In contrast, cells expressing Tbx6 were strongly present in the lepidotrichia at 90 dpf, and some reacted positively to zns-5 antibody, suggesting a paraxial mesodermal origin to the fin rays. Other structures thought to be derived from the NC are the elasmoid scales, which are composed of a superficial layer of osteoblasts and an inner layer rich in collagen. The authors showed that only the embryos from the Tbx6:cre line presented marked cells co-labeled with zns-5 antibody in the scales, also indicating a mesodermal origin to these structures. Therefore, contrary to Kague et al., (2012), who also employed sophisticated lineage tracing experiments, here, TNCCs of anamniotes appear not to be skeleto-odontogenic in situ. |
| They employed Cre-ERT2LoxP to test the contribution of NC to the elasmoid scales in zebrafish embryos, tracking these cells through Sox10 expression. Intense marking was observed in cranial skeletogenic tissue, but no substantial labeling was detected in the trunk skeletal elements, except for a small number of scales. The authors discarded an NC origin to the Sox10+ osteoblasts inside the scales because 84% of these cells were spatially associated with labeled skeletal muscle cells. However, the authors are cautious and claim that their analysis does not exclude the possibility that more ancestral types of scales with a structure closer to that of teeth, such as placoid scales of cartilaginous fish, may include NC-derived tissues. | |
| Shimada et al., 2013 | They showed evidence of the mesodermal origin of the elasmoid scales and fin rays in medaka fish. The authors produced a transgenic line expressing two different markers: DsRed under the control of the ubiquitous β-actin promoter and GFP controlled by the Osterix promoter of the Osterix gene, which encodes a protein found in bone tissues. Before NC emigration, the neural tubes of these transgenic fishes were transplanted into wild-type embryos, allowing lineage tracing of NC derivatives. The embryos were allowed to develop until 2-3 weeks of age; at this time, NC was observed colonizing the dorsal, anal, and caudal fins and the dermal scales. However, these NC cells never expressed Osterix, indicating that they did not differentiate into osteoblasts. In contrast, when the somites were transplanted, instead of the neural tubes, DsRed+/Osterix+ cells were seen in both the scales and median fin rays, showing that these structures are derived from mesoderm and not NC. The authors confirmed their results through another long-term labeling technique known as the infrared laser-evoked gene operator (IR-LEGO) system. |
| The authors employed the CreLoxP system in zebrafish in order to analyze the origin of the larval caudal fin mesenchyme, a tissue that forms earlier than the lepidotrichia. NC cells were identified by using a transgenic line expressing the mCherry marker under the control of the promoter of Sox10. Between 24 to 72 hpf, a limited number of NC cells were observed entering the caudal fin, but they did not assume a mesenchymal identity. Furthermore, when the NC was genetically ablated (FoxD3 and tfap2a deficiency), the embryos still retained fully formed median fins. On the other hand, when the researchers used the tbx6 promoter, extensive labeling of the fin mesenchyme was seen, indicating a mesodermal origin. To determine precisely the mesodermal tissue from which this mesenchyme was derived, the authors developed another transgenic line that marks cells expressing Pax3a, a gene broadly expressed in the paraxial mesoderm, mainly in the dermomyotome. This experiment led to intense labeling of the entire fin extension. Together, these results suggest that the fin mesenchyme is entirely derived from the mesoderm, or, more specifically, from the dermomyotome, and thus TNCCs do not contribute to any mesenchymal derivative in zebrafish. |
In non-teleost fish
Some studies also show that TNCCs contribute to the fin mesenchyme of non-teleost fishes. Newth (1956) performed a series of extirpation experiments on different populations of NC cells in lamprey embryos. The complete extirpation of part of the TNC resulted in a reduction in the size of the embryonic dorsal fin fold in most of the trunk, suggesting that TNCCs contribute, at least partially, to the formation of this structure (Newth, 1956). Several years later, Hirata, Ito, and Tsuneki (1997), also working with lamprey embryos, identified TNCCs colonizing the embryonic dorsal fin fold at stages 24-25 through labeling with HNK-1 antibody (Hirata et al., 1997). McCauley and Bronner-Fraser (2003) injected Dil into the rostral trunk neural tube of stage 22 lamprey embryos. At stage 25, marked cells were observed inside the embryonic dorsal fin fold, some of which were between the fin mesenchyme, possibly contributing to its formation (McCauley and Bronner-Fraser, 2003). Freitas, Zhang, and Cohn (2006) showed a small NC contribution to the mesenchyme of the embryonic dorsal fin fold of lampreys and catshark through immunostaining with the antibody Zn12, which recognizes the epitope of HNK-1 (Freitas et al., 2006). Finally, Häming et al., (2011) showed that Dil-labeled TNCCs were seen colonizing the fin mesenchyme of 34-day-old lampreys (Häming et al., 2011).
It is tempting to speculate whether the cells observed by Cattel et al., (2011) in lamprey dorsal fin mesenchyme and described as expressing transcripts of RunxB, Col2a1a, and Alx would correspond to a population of TNCCs. According to the authors, the coexpression of Runx and Alx is not seen in any skeletal tissue in the head, suggesting that lamprey dorsal fin mesenchyme may represent a mucocartilage-like tissue unique to the trunk (Cattell et al., 2011).
More recently (2017), Gillis and colleagues showed that TNCCs are at the origin of odontoblasts of the dermal denticles of the little skate Leucoraja erinacea. The researchers injected Dil into the dorsal neural tube of embryos at stage 18 (18-22 dpf), allowing them to develop for 4-5 months (stage 33). After this period, Dil+ odontoblasts were found in the dermal denticles. No labeled odontoblasts were observed when Dil injections were performed in the paraxial mesoderm, confirming the TNC origin of the odontoblasts (Gillis et al., 2017).
In reptiles
Studies show the skeletogenic potential of TNCCs in vivo regarding the formation of the plastron and the nuchal bone of turtles. The Scott Gilbert group performed a series of studies to address this question. We first present some features of the turtle’s shell to understand the experimental approaches better.
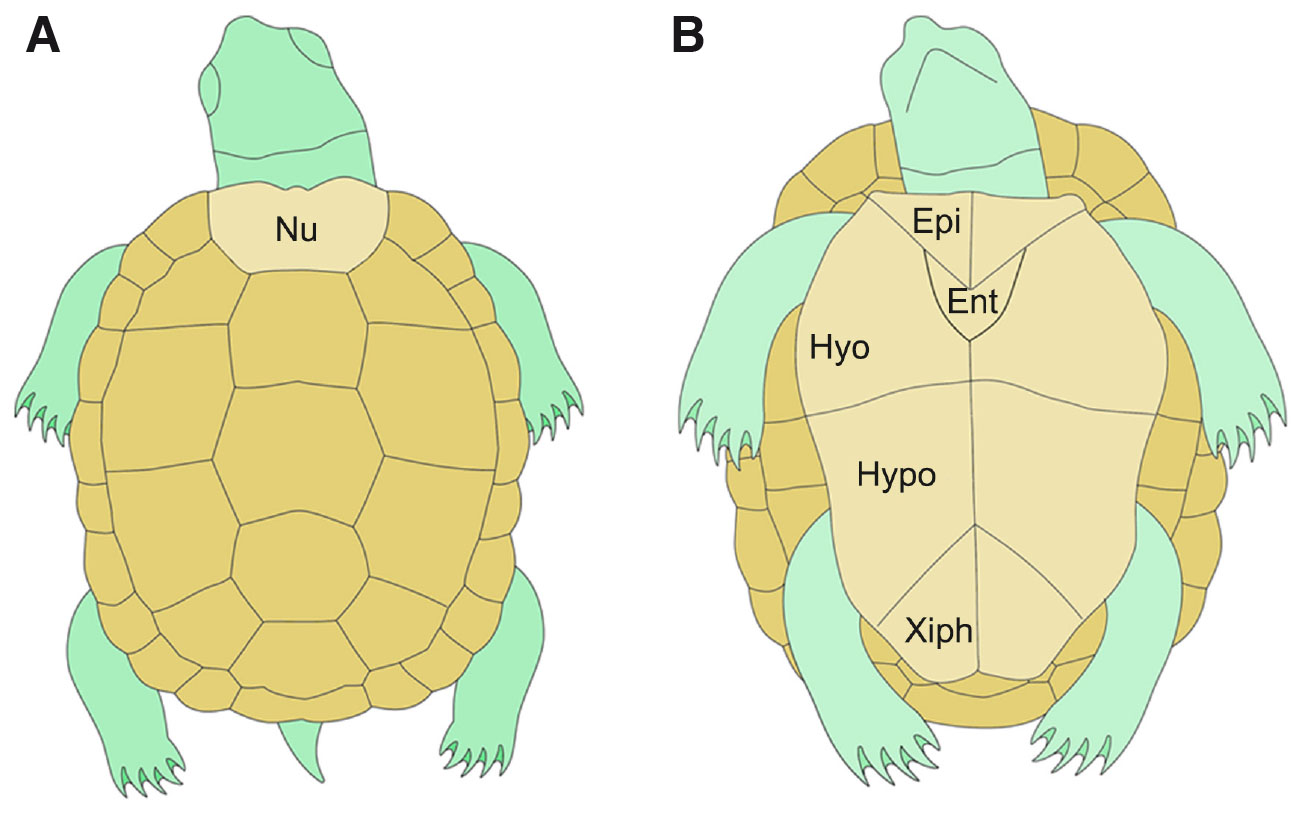
The turtle’s shell is composed of two principal regions: the ventral plastron and the dorsal carapace, which are connected along the mid flanks by lateral bridges. The carapace (Fig. 4A) contains ribs and vertebrae. Dermal bones surround the skeletons marginally. For example, the nuchal bone (light brown in Fig. 4A) occupies the rostral margin of the carapace, with peripheral plates aligned along the lateral margin. The suprapygal plates and a pygal plate form the caudal margin of the carapace but are often absent. In some species, these bony structures are covered with keratinous tissue, whereas in others, such as the soft-shelled turtles, the epidermis of the shell is not keratinized (Cebra-Thomas et al., 2007; Lyson et al., 2013; Nagashima et al., 2011; Nagashima et al., 2014).
The plastron (light brown in Fig. 4B) is usually composed of nine bones formed by intramembranous ossification of condensing mesenchymal cells (Gilbert et al., 2001). The anterior bones, the entoplastron, and the paired epiplastron are thought to be homologous to the interclavicles and the clavicle, respectively. In contrast, the posterior bones are considered homologous to the gastralia, a series of dermal bones currently restricted to some reptiles, like tuatara and crocodilians, but widely present in extinct tetrapods (Cebra-Thomas et al., 2007; Lyson et al., 2013; Nagashima et al., 2011; Nagashima et al., 2014).
Fig. 4. The turtle’s shell.
The turtle’s shell is composed of the dorsal carapace (A) and the ventral plastron (B). More than 50 bones compose the dorsal carapace, the most rostral is the nuchal bone (Nu; represented in light brown) and claimed to be of TNCCs origin by Scott Gilbert's group. The group also claims that the nine bones forming the plastron (B) are of NC origin. Epi, epiplastron; Ent, entoplastron; Hyo, hyoplastron; Hypo, hypoplastron; Xiph, xiphiplastron.
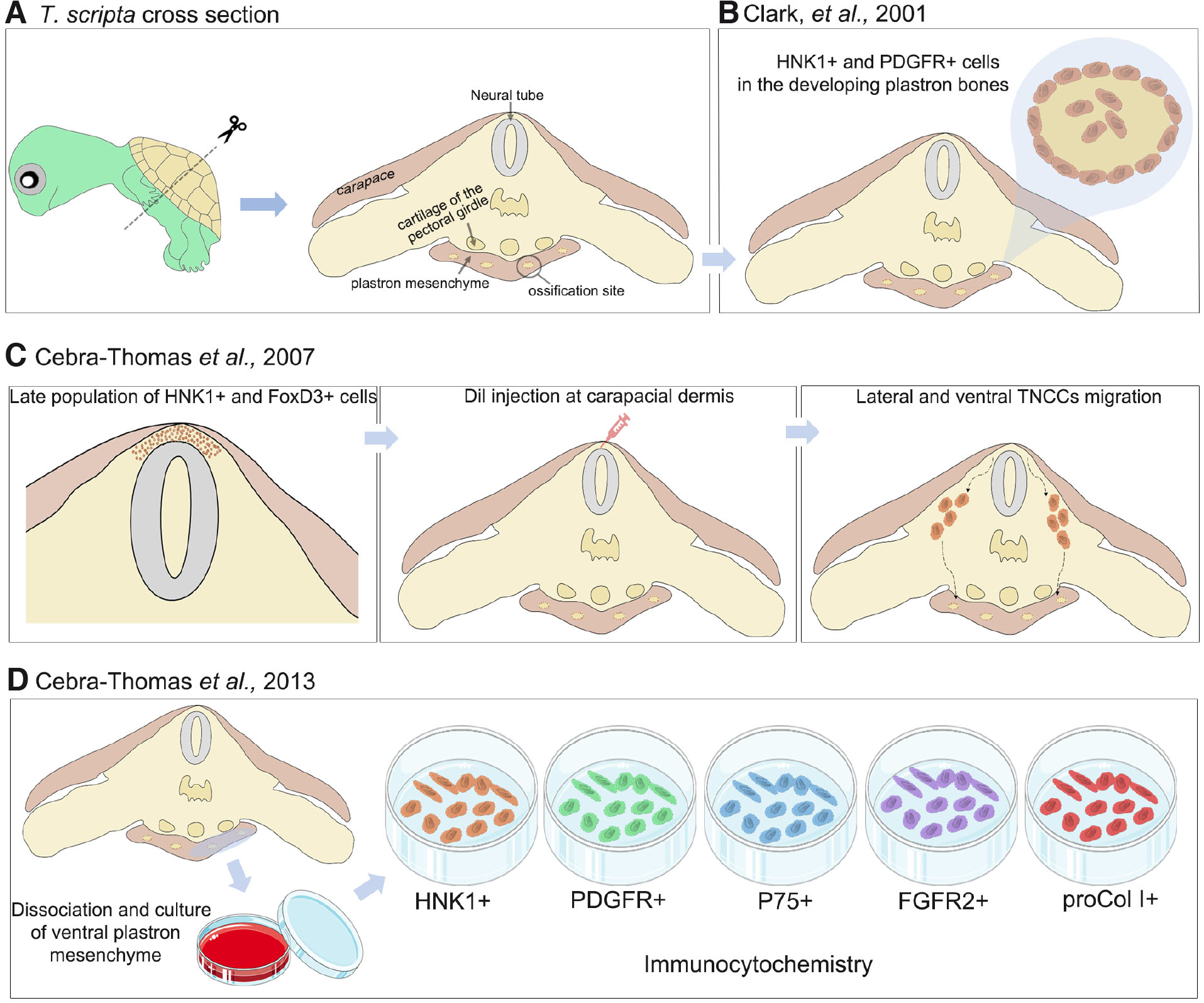
Scott Gilbert’s research group obtained the first evidence of an NC contribution to turtle plastron through immunostaining experiments performed on transverse and sagittal sections of 50-day-old T. scripta embryos (Fig. 5A) (Clark et al., 2001). HNK-1 staining was detected in all expected NC-related places: spinal ganglia, neural tube, and presumptive melanoblasts. Moreover, labeling for HNK-1 was also strong in the cells surrounding the region of differentiating membranous bone and often within the differentiating bone tissue of the nine bones of the turtle plastron. The antigen diminished the expression as the differentiating cells secrete their matrices (Clark et al., 2001). Another marker was used, the antibody against the alpha sub-unit of PDGFR. This marker is used to detect skeletogenic and odontogenic NCCs in mice and frogs (Ho et al., 1994; Schatteman et al., 1992; Takakura et al., 1997). PDGFRα staining was prominently observed in the cells within and around each of the developing plastron bones (Fig. 5B). It is essential to note that the authors point out the need for more studies to determine which region of the NC was forming these bones, not completely excluding, even if less plausible, the possibility of an extensive migration of CNCCs to form the plastron bones (Clark et al., 2001).
Then, some years later, the group identified, again through HNK-1 labeling, a late population of TNCCs emerging from the T. scripta neural tube embryos at stage G17 (22 days of incubation) (Cebra-Thomas et al., 2007). This population resides temporally within the carapacial dermis while expanding, and at stage G18 (25 days of incubation), these cells form a thick band directly above the neural tube. The NC identity of these HNK-1+ emerging cells was confirmed by colocalized expression of the well-known NC markers FoxD3 and p75 (Fig. 5C, left panel). Then the researchers performed Dil injections at the carapacial dermis in the exact position of the HNK-1+/FoxD3+/p75+ cells (Fig. 5C middle panel). After 36-48 hours, Dil-labeled cells were observed migrating ventrally and laterally across the midline. At stage G18, HNK-1+/p75+ cells were observed aggregating in the ventral mesenchyme, forming compact nodules where the plastron bones are formed (Fig. 5C, right panel). At stage G19 (28 days of incubation), the center of these aggregates was stained for bone tissue, while the periphery remained HNK-1+ (Cebra-Thomas et al., 2007). In order to confirm if this late-migrating TNC population contributes to the formation of the plastron, Cebra-Thomas et al., (2013) characterized in vitro the cells composing the ventral mesenchyme of T. scripta embryos at stage G19. To accomplish this, cells dissociated from the tissue were cultured over fibronectin and then analyzed through immunocytochemistry. Almost all cells were positive for HNK-1, p75, FGFR2, and PDGFRα, showing that this mesenchyme contained NC cells (Fig. 5D). In addition, they observed a high expression of Id-2 and a lower, but detectable, expression of Noelin-1, genes characteristic of the CNC. When treated with ascorbic acid, β-glycerophosphate, and dexamethasone, the ventral mesenchyme cells showed a high osteogenic potential, as observed through the high expression of procollagen type 1 and alkaline phosphatase, while maintaining the expression of HNK-1, p75, FGFR2 and PDGFRα (Cebra-Thomas et al., 2013).
Fig. 5. Experiments of Scott Gilbert's group demonstrating TNCCs at the origin of some turtle’s bones.
(A) Transverse sections of 50-day-old T. scripta embryos show the different anatomical structures under analysis. (B) Clark, et al., 2001, showed HNK-1+ cells surrounding and within bone tissue of the plastron (C) Cebra-Thomas et al., 2007 detected that is a late population of TNCCs that migrate and colonizes the developing plastron bones. (D) Cebra-Thomas, et al., 2013, confirmed all these results, go further and detect the expression of several known neural crest markers in cells obtained from ventral plastron mesenchyme.
It is important to note that Cebra-Thomas et al., (2007) also observed significant staining of HNK-1 in the ribs and vertebral cartilage at stage G17 and older T. scripta embryos, suggesting that the TNC may also contribute to these structures in turtles (Cebra-Thomas et al., 2007). In another study in the same year, Gilbert et al., (2007) observed that the nuchal bone, the most anterior bone of the carapace (see Fig. 4A), of 118-day-old T. scripta embryos, reacted positively to HNK-1 and PDGFRα antibodies, indicating that this structure may also be derived from the NC. In the same article, they found HNK-1 markings surrounding alligator gastralia. This structure it is belived to be homologous to the three posterior sets of paired plastron bones. Originally, Voeltzkow and Döderlein (1901) stated that the gastralia is a bone tissue. Here, however, the authors show that, in fact, gastralia is a cartilaginous tissue (Gilbert et al., 2007).
More recently, the late-emerging TNCCs population described by Scott Gilbert’s research group was also observed by Goldberg et al., 2020, while studying the migration patterns of the TNCCs in T. scripta embryos. In addition, the authors propose that earlier waves of TNCCs migration also contribute to the formation of the plastron and the carapace. Using Dil injections and immunoreactivity against HNK-1 and Sox10, they observed TNCCs migrating in the direction of the plastron and the carapace through the lateral mesoderm and the dorsal root ganglia in earlier developmental stages of turtle embryos (Goldberg et al., 2020).
Some authors pointed out some problems in the studies mentioned above. They first noted the use of a single marker to study a determined cell population, like HNK-1, which is usually called a “neural crest marker” but expressed in other cell types, thus making the marking imprecise (Hall, 2015; Nagashima et al., 2011). However, it is important to be cautious with this criticism because almost all experiments employed HNK-1 with the combination of at least two other different well-known NC markers, such as FoxD3 and p75. They also noted that the results obtained with Dil must not be taken as certain since lipophilic dye can contaminate neighboring cells (Nagashima et al., 2014). Finally, they commented on the use of different fixatives that may alter the results. For example, if embryos of the soft-shelled turtle Pelodiscus sinensis were fixed with Bouin’s solution, HNK-1 labeling would not be detected on early anlagen of plastral bones but only on the dorsal root ganglia (Nagashima et al., 2011). On the other hand, all the studies performed by Scott Gilbert’s group used paraformaldehyde as a fixative (Cebra-Thomas et al., 2007; Clark et al., 2001; Gilbert et al., 2007).
Trying to resolve this last point, Nagashima et al., (2014) decided to employ a fixative solution developed by Serra (1946) in hard-shelled (T. scripta) and soft-shelled (P. sinensis) turtles. It contains acetic acid to stabilize the HNK-1 epitope (Nagashima et al., 2011; Nagashima et al., 2014; Serra, 1946). They observed that the plastral bones develop from HNK-1 negative primordia in both turtle species, but T. scripta have unique expression patterns for the HNK-1 epitope in that the antibody recognizes osteoblasts as well as the dorsal mesenchyme in the particular developmental time window (Nagashima et al., 2014).
Finally, a very recent study (in the preprint version) used the elapid snake Naja haje haje, in which the authors may have detected another possible example of the mesenchymal potential of TNCCs in reptiles. Khannoon, Alvarado, and Bellard (2021) studied the migration of TNCCs through immunostaining against HNK-1 in snake embryos from early-stage to 14 days postoviposition. The authors observed a large population of HNK-1+ cells migrating at stages 1-3, possibly contributing to the cobra’s scale precursors (Khannoon et al., 2021). The researchers compared this TNCCs population to that migrating between DRGs and through mesodermal regions that ultimately contribute to forming the turtle’s plastron, as described by Goldberg et al., 2020.
In conclusion, the contribution of TNCCs to skeletogenic tissues forming the turtle’s carapace is also a controversial topic and deserves attention. The generation of transgenic turtle lineages allowing the observation of long-term cell labeling is awaited and must shed light on this controversy. However, as we have observed in fish studies, even the use of sophisticated cell lineage techniques does not guarantee that the dispute will easily end.
In mammals
Dasypodids, known as armadillos, are the only mammals with osteoderms, characterized by dermal bone plates covering the entire dorsal region (up to the tail) of the animal’s epidermis (Krmpotic et al., 2021).
Recently, Krmpotic and colleagues, 2021 used two armadillo fetuses of the species Dasypus hybridus to analyze the origin of osteoderms. Skin samples from the dorsal, ventral and caudal regions were immunolabeled with HNK-1 and PDGFR (Krmpotic et al., 2021). HNK-1 markings were identified in the subepidermal dorsal mesenchyme of the entire dorsal region of the analyzed fetuses. Another sample identified HNK-1+ osteoblasts surrounding the developing osteoderms. These two regions were also positive for PDGFR, colocalizing with HNK-1 labeling. In samples of the ventral region, no positive markings for HNK-1 were found since this region does not have osteoderms. Osteoderms can be found all around the tail, and, confirming this observation, positive markings for HNK-1 were identified in dorsal, ventral, and lateral regions of the tail of these animals (Krmpotic et al., 2021).
Conclusions
This review presents several studies that point to TNCCs as the origin of some mesenchymal and skeletogenic tissues in vertebrates. We also describe several works that show a contrary view to this contribution. So far, no evidence suggests that TNCCs give rise to cartilage and/or bone during normal development in either mice or chicks. However, this does not mean that skeletogenic potential cannot be expressed in amniotes since a recent study shows that TNCCs are at the origin of the dermal bone of armadillos. These observations suggest that the mesenchymal/skeletogenic potential of TNC is latent and can be unveiled under specific circumstances and then manifested in quite specific places in the body of some vertebrate species. The results of in vitro experiments made with avians and mammals strengthen these observations. Thus, both environmental cues and intrinsic properties may participate in determining whether or not TNCCs undergo skeletogenesis.
In 2015, Simões-Costa and Bronner identified a cranial-specific gene regulatory network that endows NC to differentiate into the craniofacial skeleton. The trunk neural tube of avian embryos electroporated with three key genes of this circuit (Sox8, Tfap2b, and Ets1) adopt a cranial-like expression profile, indicating a shift from trunk to cranial identity. When transplanted to the cranial regions of wild-type chick embryos, the reprogrammed TNCCs acquired chondrogenic potential and formed ectopic cartilage nodules. Therefore, introducing components of a cranial-specific transcriptional circuit is sufficient to reprogram TNCCs and drive them to a chondrogenic differentiation pathway (Simoes-Costa and Bronner, 2016).
These data suggest that all, or at least some, TNCC progenitors are intrinsically constrained by the lack of a genetic regulatory network (GRN) that can guide (or allow) NCCs through a mesenchymal/skeletogenic differentiation pathway. It is tempting to speculate if some flexibility of GRN would allow a “natural” rewiring to alter progenitor cell identity and fate during the embryonic development of some vertebrates. This hypothesis is supported by the results of some experiments indicating that a shift from trunk to cranial identity occurs under certain circumstances in vitro. For example, in 2003, Abzhanov and colleagues obtained chondrocytes in long-term cultures of avian TNCCs. According to the authors, a long time was necessary to allow the downregulation of the gene Hoxb4 in the second week of culture. Importantly, TNC started to express high levels of two characteristic genes of the CNC: Id2 and Noelin1 (Abzhanov et al., 2003). In mice, similar results were obtained, but in this case, adding the growth factor Fgf2 was mandatory to obtain chondrogenesis from TNC. It was observed that Fgf2 promotes downregulation of Hox9 and upregulation of Id2 in TNCCs. Interestingly, on the 10th day of culture, the two genes showed a similar expression, both in the presence and absence of Fgf2, indicating the high plasticity of the system, as long as some essential windows of time are respected (Ido and Ito, 2006). Finally, the late-migrating TNC population, which contributes to the formation of the turtle’s plastron, is characterized in vitro by cells exhibiting high expression of Id-2 and a lower but detectable, expression of Noelin-1 (Cebra-Thomas et al., 2013).
Thus, assuming reduced or even total blockage, of some constraints imposed by the environment or the emergence of some promoting elements, a rewiring of the GRN may occur, promoting a spatial-localized shift from trunk to cranial identity and finally allowing TNCC progenitors to express their full potentials. Epigenetic modifications promoted by specific local signaling molecules are one possible mechanism to be considered for such rewiring in vivo. The epigenetic program can be conserved over generations and, simultaneously, be sufficiently flexible to allow some tissue-localized modifications.
Nevertheless, how flexible could this system be in vivo? Recent approaches using sophisticated genetic lineage tracing experiments confirm that most cranial (Tang et al., 2021) and trunk (Baggiolini et al., 2015) NC precursors are multipotent in vivo. Therefore, considering that migratory TNCCs are not fate-restricted before emerging from the neural primordium and that environmental influences, rather than intrinsic information, govern cell fate choice of multipotent NCCs, it is very suggestive of proposing that the expression of TNCCs’ mesenchymal potential is under the control of localized tissue-specific environmental influences. Moreover, a very recent study shows that the pluripotency factor Oct4 is transiently reactivated in mice CNCCs and is required for the subsequent formation of ectomesenchyme. The migratory CNCC loses expression of neuroepithelial positional genes and adopts a more uniform transcriptional signature. Thus, during CNCCs delamination, a positional identity is erased, and the authors speculate that this erasure generates a functionally equivalent CNCC population, which can readily adapt to future migratory and post-migratory locations. The authors proposed that CNCCs expand their developmental potential via a transient reacquisition of molecular signatures of pluripotency (Zalc et al., 2021). It is tempting to speculate that erasure of this positional identity and reacquisition of molecular signatures of pluripotency can also occur during TNCC migration. As a whole, these results indicate that the system is much more flexible than previously thought and that the window of time necessary to rewire a GRN should be wider.
In line with this, the work of Coelho-Aguiar, 2013 shows by in vitro clonal experiments that most TNCCs are highly multipotent and composed of progenitors endowed with both neural and mesenchymal potential, as already demonstrated by CNCCs (Calloni et al., 2007; Calloni et al., 2009; da Costa et al., 2018). Thus, the potentials of TNCCs seem to be much more similar to those of CNCCs. Thus, one can put forward the hypothesis that most, if not all, NCCs were originally of the mesenchymal-neural type. The extent of this in vivo mesenchymal/skeletogenic TNCC contribution to the vertebrate’s body continues and will probably continue to be a subject of intense debate in the scientific community.
Abbreviations
A, Adipocytes ; α-SMA, α-smooth muscle actin ; 2D, Bi-dimensional ; 3D, Tri-dimensional ; ALP, Alkaline Phosphatase ; AR, Alizarin Red ; C, Chondrocytes ; CNCCs, Cephalic Neural Crest Cells ; CS, Condroitin Sulfate ; DAPI, 4’-6-diamidino-2-fenilindol;Dil,1,1’Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate ; dpf, days post fertilization ; F, Fibroblasts ; Fgf2, Fibroblast growth factor 2 ; G, Glia ; GFP, Green Fluorescent Protein ; GRN, genetic regulatory network ; HH, Hamburger-Hamilton ; HNK-1, Human Natural Killer 1 ; N, Neurons ; M, Melanocytes ; NC, Neural Crest ; NCCs, Neural Crest Cells ; O, Osteoblasts ; PM, PuraMatrix ; ss, somite stage ; TH, Tyrosine Hydroxylase ; Tgfß1, Transforming Growth Factor beta-1 ; TNCCs, Trunk Neural Crest Cells ;References
Abzhanov A., Tzahor E., Lassar A. B., Tabin C. J. (2003). Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 130: 4567-4579.
Baggiolini A., Varum S., Mateos J. M., Bettosini D., John N., Bonalli M., Ziegler U., Dimou L., Clevers H., Furrer R., Sommer L. (2015). Premigratory and Migratory Neural Crest Cells Are Multipotent In Vivo. Cell Stem Cell 16: 314-322.
Baroffio A., Dupin E., Le Douarin N. M. (1988). Clone-forming ability and differentiation potential of migratory neural crest cells. Proceedings of the National Academy of Sciences 85: 5325-5329.
Baroffio A., Dupin E., Le Douarin N.M. (1991). Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development 112: 301-305.
Billon N., Iannarelli P., Monteiro M. C., Glavieux-Pardanaud C., Richardson W. D., Kessaris N., Dani C., Dupin E. (2007). The generation of adipocytes by the neural crest. Development 134: 2283-2292.
Bittencourt D. A., da Costa M. C., Calloni G. W., Alvarez-Silva M., Trentin A. G. (2013). Fibroblast Growth Factor 2 Promotes the Self-Renewal of Bipotent Glial Smooth Muscle Neural Crest Progenitors. Stem Cells and Development 22: 1241-1251.
Bodenstein D. (1952). Studies on the development of the dorsal fin in amphibians. Journal of Experimental Zoology 120: 213-245.
Bronner-Fraser M., Fraser S. E. (1988). Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 335: 161-164.
Bruder S. P., Caplan A. I. (1989). Discrete Stages Within the Osteogenic Lineage are Revealed by Alterations in the Cell Surface Architecture of Embryonic Bone Cells. Connective Tissue Research 20: 73-79.
Calloni G. W., Le Douarin N. M., Dupin E. (2009). High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proceedings of the National Academy of Sciences 106: 8947-8952.
Calloni G. W., Glavieux-Pardanaud C., Le Douarin N. M., Dupin E. (2007). Sonic Hedgehog promotes the development of multipotent neural crest progenitors endowed with both mesenchymal and neural potentials. Proceedings of the National Academy of Sciences 104: 19879-19884.
Cattell M., Lai S., Cerny R., Medeiros D. M. (2011). A New Mechanistic Scenario for the Origin and Evolution of Vertebrate Cartilage. PLoS ONE 6: e22474.
Cebra-Thomas J. A., Betters E., Yin M., Plafkin C., McDow K., Gilbert S. F. (2007). Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evolution & Development 9: 267-277.
Cebra-Thomas J. A., Terrell A., Branyan K., Shah S., Rice R., Gyi L., Yin M., Hu Y., Mangat G., Simonet J., Betters E., Gilbert S. F. (2013). Late-emigrating trunk neural crest cells in turtle embryos generate an osteogenic ectomesenchyme in the plastron. Developmental Dynamics 242: 1223-1235.
Chen J., Liu X., Yao X., Gao F., Bao B. (2017). Dorsal fin development in flounder, Paralichthys olivaceus: Bud formation and its cellular origin. Gene Expression Patterns 25-26: 22-28.
Chibon P. (1966). Analyse experimentale de la regionalisation et des capacites morphogenetiques de la crete neurale chez l'amphibien urode' le Pleurodeles waltlii Michah. Me´m Soc Zool Fr 36: 1-107.
Clark K., Bender G., Murray B. P., Panfilio K., Cook S., Davis R., Murnen K., Tuan R. S., Gilbert S. F. (2001). Evidence for the neural crest origin of turtle plastron bones. Genesis 31: 111-117.
Coelho-Aguiar J. M., Le Douarin N. M., Dupin E. (2013). Environmental factors unveil dormant developmental capacities in multipotent progenitors of the trunk neural crest. Developmental Biology 384: 13-25.
Collazo A., Bronner-Fraser M., Fraser S.E. (1993). Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development 118: 363-376.
Costa-Silva B., da Costa M. C., Melo F. R., Neves C. M., Alvarez-Silva M., Calloni G. W., Trentin A. G. (2009). Fibronectin promotes differentiation of neural crest progenitors endowed with smooth muscle cell potential. Experimental Cell Research 315: 955-967.
da Costa M. C., Trentin A. G., Calloni G. W. (2018). FGF8 and Shh promote the survival and maintenance of multipotent neural crest progenitors. Mechanisms of Development 154: 251-258.
Dupin E., Calloni G. W., Coelho-Aguiar J. M., Le Douarin N. M. (2018). The issue of the multipotency of the neural crest cells. Developmental Biology 444: S47-S59.
DuShane G. P. (1935). An experimental study of the origin of pigment cells in Amphibia. Journal of Experimental Zoology 72: 1-31.
Epperlein H., Meulemans D., Bronner-Fraser M., Steinbeisser H., Selleck M.A. (2000). Analysis of cranial neural crest migratory pathways in axolotl using cell markers and transplantation. Development 127: 2751-2761.
Epperlein H.H., Selleck M. A.J., Meulemans D., Mchedlishvili L., Cerny R., Sobkow L., Bronner-Fraser M. (2007). Migratory patterns and developmental potential of trunk neural crest cells in the axolotl embryo. Developmental Dynamics 236: 389-403.
Ford P. (1949). The origin of the segmental musculature of the tail of the Axolotl (Ambystoma) . Proceedings of the Zoological Society of London 119: 609-632.
Freitas R., Zhang G.J., Cohn M. J. (2006). Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 442: 1033-1037.
Garriock R. J., Krieg P. A. (2007). Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Developmental Biology 304: 127-140.
Géraudie J. (1988). Fine structural peculiarities of the pectoral fin dermoskeleton of two Brachiopterygii, Polypterus senegalus and Calamoichthys calabaricus (Pisces, Osteichthyes). The Anatomical Record 221: 455-468.
Gilbert S. F., Bender G., Betters E., Yin M., Cebra-Thomas J. A. (2007). The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Integrative and Comparative Biology 47: 401-408.
Gilbert S. F., Loredo G. A., Brukman A., Burke A. C. (2001). Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evolution and Development 3: 47-58.
Gillis J. A., Alsema E. C., Criswell K. E. (2017). Trunk neural crest origin of dermal denticles in a cartilaginous fish. Proceedings of the National Academy of Sciences 114: 13200-13205.
Goldberg S., Venkatesh A., Martinez J., Dombroski C., Abesamis J., Campbell C., Mccalipp M., Bellard M. E. (2020). The development of the trunk neural crest in the turtle Trachemys scripta . Developmental Dynamics 249: 125-140.
Graveson A. C. (1993). Neural Crest Contributions to the Development of the Vertebrate Head. American Zoologist 33: 424-433.
Graveson A. C., Armstrong J. B. (1987). Differentiation of cartilage from cranial neural crest in the axolotl (Ambystoma mexicanum). Differentiation 35: 16-20.
Graveson A. C., Hall B. K. (1995). The relationship between migration and chondrogenic potential of trunk neural crest cells in Ambystoma mexicanum. Roux's Archives of Developmental Biology 204-204: 477-483.
Green S. A., Uy B. R., Bronner M. E. (2017). Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature 544: 88-91.
Hall B. K. (2015). Part XV. Evolutionary Skeletal Biology. In Bones and Cartilage (Second Edition). Elsevier BV.
Häming D., Simoes-Costa M., Uy B., Valencia J., Sauka-Spengler T., Bronner-Fraser M. (2011). Expression of Sympathetic Nervous System Genes in Lamprey Suggests Their Recruitment for Specification of a New Vertebrate Feature. PLoS ONE 6: e26543.
Hirata M., Ito K., Tsuneki K. (1997). Migration and Colonization Patterns of HNK-1-Immunoreactive Neural Crest Cells in Lamprey and Swordtail Embryos. Zoological Science 14: 305-312.
Ho L., Symes K., Yordán C., Gudas L. J., Mercola M. (1994). Localization of PDGF A and PDGFRα mRNA in Xenopus embryos suggests signalling from neural ectoderm and pharyngeal endoderm to neural crest cells. Mechanisms of Development 48: 165-174.
Holtfreter J. (1933). Der Einfluss von Wirtsalter und verschiedenen Organbezirken auf die Differenzierung von angelagertem Gastrulaektoderm. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen 127: 619-775.
Holtfreter J. (1935). Morphologische Beeinflussung von Urodelenektoderm bei xenoplastischer Transplantation. Wilhelm Roux Archiv für Entwicklungsmechanik der Organismen 133: 367-426.
Hörstadius S. O. (1950). The neural crest: Its properties and derivatives in the light of experimental research. Oxford University Press, London.
Hörstadius S. O., Sellman S. (1946). Experimentelle Untersuchungen über die Determination des knorpeligen Kopfskelletes bei Urodelen. Almquist & Wiksell.
Hutchins E. J., Kunttas E., Piacentino M. L., Howard A. G.A., Bronner M. E., Uribe R. A. (2018). Migration and diversification of the vagal neural crest. Developmental Biology 444: S98-S109.
Ido A., Ito K. (2006). Expression of chondrogenic potential of mouse trunk neural crest cells by FGF2 treatment. Developmental Dynamics 235: 361-367.
Joseph N. M., Mukouyama Y., Mosher J. T., Jaegle M., Crone S. A., Dormand E.L., Lee K.F., Meijer D., Anderson D. J., Morrison S. J. (2004). Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 131: 5599-5612.
Kague E., Gallagher M., Burke S., Parsons M., Franz-Odendaal T., Fisher S. (2012). Skeletogenic Fate of Zebrafish Cranial and Trunk Neural Crest. PLoS ONE 7: e47394.
Kaucka M., Adameyko I. (2019). Evolution and development of the cartilaginous skull: From a lancelet towards a human face. Seminars in Cell & Developmental Biology 91: 2-12.
Khannoon E. R., Alvarado C., de Bellard M. E. E. (2021). Early Development of The Trunk Neural Crest Cells in the Egyptian Cobra Naja H. Haje. Research Square Platform LLC, Preprint .
Kleinman H. K., Martin G. R. (2005). Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology 15: 378-386.
Krmpotic C. M., Nishida F., Galliari F. C., Pombo M. T., Acuña F., Barbeito C. G., Carlini A. A. (2021). The Dorsal Integument of the Southern Long-Nosed Armadillo Dasypus hybridus (Cingulata, Xenarthra), and a Possible Neural Crest Origin of the Osteoderms. Discussing Evolutive Consequences for Amniota. Journal of Mammalian Evolution 28: 635-645.
Krotoski D. M., Fraser S. E., Bronner-Fraser M. (1988). Mapping of neural crest pathways in Xenopus laevis using inter- and intra-specific cell markers. Developmental Biology 127: 119-132.
Le Douarin N., Kalcheim C. (2011). The Neural Crest. Cambridge University Press (CUP), Cambridge.
Le Douarin N. M., Teillet M. A., Le Lièvre C. (1977). Influence of the tissue environment on the differentiation of neural crest cells. Soc Gen Physiol Ser 32: 11-27.
Le Douarin N. M., Teillet M. A. (1974). Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Developmental Biology 41: 162-184.
Lee R. T. H., Knapik E. W., Thiery J. P., Carney T. J. (2013a). An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 140: 2923-2932.
Lee R. T. H., Thiery J. P., Carney T. J. (2013b). Dermal fin rays and scales derive from mesoderm, not neural crest. Current Biology 23: R336-R337.
Lumsden A. G. S. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 103: 155-169.
Lwigale P. Y., Conrad G. W., Bronner-Fraser M. (2004). Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development 131: 1979-1991.
Lyson T. R., Bever G. S., Scheyer T. M., Hsiang A. Y., Gauthier J. A. (2013). Evolutionary Origin of the Turtle Shell. Current Biology 23: 1113-1119.
McCauley D. W., Bronner-Fraser M. (2003). Neural crest contributions to the lamprey head. Development 130: 2317-2327.
McGonnell I. M., Graham A. (2002). Trunk Neural Crest Has Skeletogenic Potential. Current Biology 12: 767-771.
Mongera A., Nüsslein-Volhard C. (2013). Scales of fish arise from mesoderm. Current Biology 23: R338-R339.
Morrison S. J., White P. M., Zock C., Anderson D. J. (1999). Prospective Identification, Isolation by Flow Cytometry, and In Vivo Self-Renewal of Multipotent Mammalian Neural Crest Stem Cells. Cell 96: 737-749.
Nagashima H., Kuraku S., Uchida K., Kawashima-Ohya Y., Narita Y., Kuratani S. (2011). Body plan of turtles: an anatomical, developmental and evolutionary perspective. Anatomical Science International 87: 1-13.
Nagashima H., Shibata M., Taniguchi M., Ueno S., Kamezaki N., Sato N. (2014). Comparative study of the shell development of hard- and soft-shelled turtles. Journal of Anatomy 225: 60-70.
Nakamura H., Ayer-Le Lievre C. S. (1982). Mesectodermal capabilities of the trunk neural crest of birds. Development 70: 1-18.
Newth D. R. (1956). On the Neural Crest of the Lamprey Embryo. Development 4: 358-375.
Ramos-Hryb A. B., Da-Costa M. C., Trentin A. G., Calloni G. W. (2013). Matrigel supports neural, melanocytic and chondrogenic differentiation of trunk neural crest cells. The International Journal of Developmental Biology 57: 885-890.
Raven C. P. (1931). Zur entwicklung der Ganglienleiste. I. Die Kinematik der Ganglienleistenentwicklung bei den Urodelen. Wilhelm Roux' Archiv für Entwicklungsmechanik der Organismen 125: 210-292.
Rothstein M., Bhattacharya D., Simoes-Costa M. (2018). The molecular basis of neural crest axial identity. Developmental Biology 444: S170-S180.
Sadaghiani B., Thiébaud C. H. (1987). Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Developmental Biology 124: 91-110.
Schatteman G.C., Morrison-Graham K., van Koppen A., Weston J.A., Bowen-Pope D.F. (1992). Regulation and role of PDGF receptor alpha-subunit expression during embryogenesis. Development 115: 123-131.
Serra J. A. (1946). Histochemical Tests for Proteins and Amino Acids; The Characterization of Basic Proteins. Stain Technology 21: 5-18.
Shah N. M., Groves A. K., Anderson D. J. (1996). Alternative Neural Crest Cell Fates Are Instructively Promoted by TGFβ Superfamily Members. Cell 85: 331-343.
Shepherd I., Eisen J. (2011). Development of the Zebrafish Enteric Nervous System. In The Zebrafish: Cellular and Developmental Biology, Part B. Elsevier.
Shimada A., Kawanishi T., Kaneko T., Yoshihara H., Yano T., Inohaya K., Kinoshita M., Kamei Y., Tamura K., Takeda H. (2013). Trunk exoskeleton in teleosts is mesodermal in origin. Nature Communications 4: 1639.
Simoes-Costa M., Bronner M. E. (2016). Reprogramming of avian neural crest axial identity and cell fate. Science 352: 1570-1573.
Smith M., Hickman A., Amanze D., Lumsden A., Thorogood P. (1994). Trunk neural crest origin of caudal fin mesenchyme in the zebrafish Brachydanio rerio. Proceedings of the Royal Society of London. Series B: Biological Sciences 256: 137-145.
Smith M. M. (1991). Putative Skeletal Neural Crest Cells in Early Late Ordovician Vertebrates from Colorado. Science 251: 301-303.
Smith M. M., Hall B. K. (1993). Evolutionary Biology. (Ed. Hecht M. K., MacIntyre R. J., Clegg M. T.) Springer, Boston.
Smith M. M., Hall B. K. (1990). Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biological Reviews 65: 277-373.
Sobkow L., Epperlein H.H., Herklotz S., Straube W. L., Tanaka E. M. (2006). A germline GFP transgenic axolotl and its use to track cell fate: Dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Developmental Biology 290: 386-397.
Suzuki T., Haga Y., Takeuchi T., Uji S., Hashimoto H., Kurokawa T. (2003). Differentiation of chondrocytes and scleroblasts during dorsal fin skeletogenesis in flounder larvae. Development, Growth and Differentiation 45: 435-448.
Takahama H., Kinoshita T., Sasaki F. (1992). Structural and Endocytotic Differences of Fibroblasts and Macrophages in the Tail Fin of Amphibian Larvae during Metamorphosis. Archives of Histology and Cytology 55: 437-448.
Takakura N., Yoshida H., Ogura Y., Kataoka H., Nishikawa S., Nishikawa S.I. (1997). PDGFRα Expression During Mouse Embryogenesis: Immunolocalization Analyzed by Whole-mount Immunohistostaining Using the Monoclonal Anti-mouse PDGFRα Antibody APA5. Journal of Histochemistry & Cytochemistry 45: 883-893.
Tang W., Li Y., Li A., Bronner M. E. (2021). Clonal analysis and dynamic imaging identify multipotency of individual Gallus gallus caudal hindbrain neural crest cells toward cardiac and enteric fates. Nature Communications 12: 1894.
Taniguchi Y., Kurth T., Medeiros D. M., Tazaki A., Ramm R., Epperlein H.H. (2015). Mesodermal origin of median fin mesenchyme and tail muscle in amphibian larvae. Scientific Reports 5: 11428.
Taufer C. R., Rodrigues-Da-Silva M. A., Calloni G. W. (2020). PuraMatrix allows differentiation of a broad repertoire of neural and mesenchymal phenotypes from trunk neural crest. The International Journal of Developmental Biology 64: 433-443.
Trentin A., Glavieux-Pardanaud C., Le Douarin N. M., Dupin E. (2004). Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proceedings of the National Academy of Sciences 101: 4495-4500.
Tucker A. S., Slack J. M. W. (2004). Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Developmental Dynamics 230: 461-467.
Twitty V. C., Bodenstein D. (1941). Experiments on the determination problem I. The roles of ectoderm and neural crest in the development of the dorsal fin in Amphibia. II. Changes in ciliary polarity associated with the induction of fin epidermis. Journal of Experimental Zoology 86: 343-380.
Voeltzkow A., Döderlein L. (1901). Beiträge zur Entwicklungsgeschichte der Reptilian III. Zur Frage nach Bildung der Bauchcrippen. Abh Senkenbergischen Naturforsch Gesselsch 26: 313-336.
Woerdeman M. W. (1946). Induction of dorsal fins by neural crest in Amblystoma mexicanum. In Experimental embryology in the Netherlands. (Ed. Woerdeman M. W., Raven C. P.) Elsevier Publishing Co, New York.
Zalc A., Sinha R., Gulati G. S., Wesche D. J., Daszczuk P., Swigut T., Weissman I. L., Wysocka J. (2021). Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science 371: eabb4776.
Zhao X., Zhang S., Spirio L. (2005). PuraMatrix: Self-Assembling Peptide Nanofiber Scaffolds. In Scaffolding In Tissue Engineering. (Ed. Ma P. X., Elisseeff J.) CRC Press, Boca Raton.