Int. J. Dev. Biol. 66: 285 - 296 (2022)
Special Issue: Developmental Biology in Greece
Age-dependent modulation of short-term neuronal dynamics in the dorsal and ventral rat hippocampus
Original Article | Published: 22 November 2021
Abstract
Brain aging is associated with alterations in the behavioral capacity to process information, due to mechanisms that are still largely unclear. Short-term neuronal activity dynamics are basic properties of local brain networks profoundly involved in neural information processing. In this study, we investigated the properties of short-term changes in excitatory synaptic transmission and neuronal excitation in the CA1 field of dorsal and ventral hippocampal slices from young adult and old rats. We found that short-term synaptic plasticity (i.e. short-term dynamics of input to CA1 circuit) does not significantly differ between young and old dorsal or ventral hippocampus. However, short-term dynamics of hippocampal output differ markedly between young and old rats. Notably, age-dependent alterations in short-term neuronal dynamics were detected mainly in the dorsal hippocampus. Thus, the dorsal hippocampus of young rats can detect and facilitate transmission of 1-30 Hz input and depress transmission of higher-frequency input. In contrast, the old dorsal hippocampus appears unable to transmit information in a frequency-dependent discriminatory manner. Furthermore, the amplification of steady-state output at frequencies < 40 Hz is considerably lower in the old than the young dorsal hippocampus. The old ventral hippocampus did not show major alterations in short-term processing of neural information, though under conditions of intense afferent activation, neuronal output of the ventral hippocampus is depressed at steady-state more in old than in young rats. These results suggest that aging is accompanied by alterations in neural information processing mainly in the dorsal hippocampus, which displays a narrower dynamic range of frequency-dependent transient changes in neuronal activity in old compared with young adult rats. These alterations in short-term dynamics may relate to deficits in processing ongoing activity seen in old individuals.
Keywords
Aging, Hippocampus, Neuronal Dynamics, Septotemporal, Short-Term Plasticity
Introduction
Aging is a complex biological phenomenon that impacts brain function in various non-uniform ways (Samson and Barnes 2013). Previous evidence suggests that physiological aging of the brain is often associated with a decline in some cognitive functions including everyday memory, sensory information processing and working memory (Salthouse et al. 1989; Craik and Jennings 1992; Kausler 1994; de Dieuleveult et al. 2017). The hippocampus is a brain structure that is particularly vulnerable during aging (Bettio et al. 2017), and normal aging people often show deficits in hippocampus-dependent memory (Rapp and Heindel 1994; Foster et al. 2012). Also, the hippocampus features prominently as a brain structure with particularly enhanced ability for plastic changes in synaptic transmission and local circuit dynamics (Bliss et al. 2007). Thus, the hippocampus displays a very wide range of forms of activity-dependent neural plasticity, including phenomena of short-term synaptic plasticity. i.e. transient changes in synaptic transmission that last from tens of milliseconds to several minutes (Zucker and Regehr 2002; Regehr 2012), and long-term synaptic plasticity, i.e. changes in synaptic transmission that can last from about one hour to at least several weeks (Nicoll and Roche 2013). Classically, in studies of aging, considerable attention has been paid to forms of long-term changes in synaptic plasticity in the hippocampus, mainly because of their conceptual similarities to time-corresponding hippocampus-dependent forms of memory (Martin and Morris 2002). Research during the past four decades has suggested that normal aging is accompanied by changes in synaptic plasticity in the hippocampus, mainly expressed as a reduced capacity for long-term potentiation of excitatory synaptic transmission; for recent reviews, see (Foster 2002; Burke and Barnes 2006; Gray and Barnes 2015; Kumar 2020).
In addition to basic roles that phenomena of long-term synaptic plasticity appear to play in neural information processing and behavior (Dringenberg 2020), accumulating evidence shows that short-term changes in synaptic effectiveness and neuronal activity, i.e. frequency-dependent transient changes in synaptic transmission and neuronal excitation upon repeated activation, are also significantly involved in neural information processing, including temporal filtering, synaptic input diversification and dynamic gain control (Abbott and Regehr 2004; Jackman and Regehr 2017). Importantly, recent evidence shows that mechanisms that determine the properties of short-term neural dynamics (STND) play important roles in processing ongoing activity (Yang and Xu-Friedman 2015). Hence, considering the aging-related deficits in sensory information processing (de Dieuleveult et al. 2017), one can hypothesize that short-term neuronal dynamics in the hippocampus may be altered during aging. Short-term changes in synaptic transmission and neuronal excitation have previously been examined in some aging studies; however, the results of these studies do not allow a consistent conclusion to be drawn, likely due to the limited range of experimental conditions under which STND were studied; see for instance (Landfield and Lynch 1977; Ouanounou et al. 1999; Ramsey et al. 2004). Therefore, the effects of aging on STND in the hippocampus have yet to be sufficiently clarified.
Importantly, though the hippocampus is susceptible to the aging process, it may not, however, be affected homogeneously along its longitudinal axis, as recent studies suggest (Yassa et al. 2011; Damoiseaux et al. 2016; Salami et al. 2016; Reichel et al. 2017; Dalton et al. 2019; Stark et al. 2020). Functional diversification along the longitudinal axis (also called the septotemporal or dorsoventral axis) of the hippocampus is a concept supported by a substantial and rapidly growing body of evidence. According to this concept, different segments along the septotemporal axis of the hippocampus play discrete roles in several brain functions, most notably spatial cognition, memory and emotionality (Kheirbek et al. 2013; Bannerman et al. 2014; Strange et al. 2014; Tannenholz et al. 2014; Gulyaeva 2018). Interestingly, a recent study reports that intrahippocampal and extrahippocampal functional connectivity are reduced specifically in the anterior human hippocampus (that corresponds to the ventral hippocampus in rodents) of older adults that appears to contribute significantly to age associated memory impairment (Stark et al. 2020). Hence, investigation of possible differences in the responsiveness to aging of distinct segments of the hippocampus along its long axis will help us to better understand the mechanisms underlying age-related declines in hippocampus-dependent functions. However, the effects of aging on STND with respect to the longitudinal axis of the hippocampus have never been studied before. Notably, distinct segments along the septotemporal axis of the hippocampus display remarkably different properties of STND (Papatheodoropoulos 2015; Kouvaros and Papatheodoropoulos 2016; Milior et al. 2016; Babiec et al. 2017; Papaleonidopoulos et al. 2017; Dubovyk and Manahan-Vaughan 2018; Koutsoumpa and Papatheodoropoulos 2019; Koutsoumpa and Papatheodoropoulos 2021), which may contribute to specific ways of information processing performed by discrete segments of the hippocampus. Possible differential age-associated alterations of STND along the hippocampus may contribute to specific cognitive impairments seen in the elderly.
In the present study, we examined STND, applying short trains of stimulation pulses covering a wide range of frequencies (0.1 – 100 Hz) in slices from the dorsal and the ventral hippocampus, prepared from young adult and old rats. In addition, we examined STND under different levels of local circuit activation. We report that aging is accompanied by frequency-dependent, segment-specific alterations in short-term neuronal excitation but not short-term synaptic plasticity. Possible implications of these findings are discussed.
Results
Short-term changes in excitatory synaptic transmission (i.e., short-term synaptic plasticity) and short-term changes in neuronal excitation (i.e., short-term dynamics of neuronal output) in dorsal and ventral hippocampal slices were studied and compared between young and old rats under similar experimental conditions. There is prior evidence that the magnitude of the first response in variable-frequency stimulation trains strongly influences the properties of short-term changes in both synaptic transmission and neuronal excitation (Koutsoumpa and Papatheodoropoulos 2019). Specifically, regarding short-term synaptic plasticity, the magnitude of the first fEPSP in a ten-pulse train largely determines whether the steady-state synaptic response to the stimulation train will be facilitation or depression. Similarly, the intensity of stimulation current greatly affects the response of the local network to repeated activation. Notably, the dependence of short-term dynamics on the initial conditions of afferent stimulation is far more evident in the dorsal compared with the ventral hippocampus (Koutsoumpa and Papatheodoropoulos 2019). It is, therefore, imperative to keep initial conditions similar when searching for the effects of a factor ( age, in the present study) on short-term synaptic/neuronal dynamics. We therefore proceeded to study short-term dynamics in the hippocampus of young and old rats as follows: to study short-term changes in fEPSP, we applied frequency stimulation at three different stimulation current intensities, producing subthreshold, suprathreshold and submaximal responses, respectively; then, we averaged the responses collected under these three experimental conditions to obtain the final values for a particular slice. To study short-term changes in PS, we applied frequency stimulation at two different stimulation current intensities, producing PS with an amplitude less than 2 mV and PS with an amplitude equal to or greater than 2 mV, respectively. Given that frequency stimulation trains starting with low and high amplitude PS mostly produce steady-state facilitation and depression, respectively, we decided to present results obtained under low and high intensity of stimulation separately. Only slices with similar initial fEPSPs and PSs from young and old rats were included in the final analysis. Specifically, we studied short-term synaptic plasticity in young and old rats that showed an initial fEPSP of 1.2 ± 0.09 mV/ms (41/27) and 1.2 ± 0.06 mV/ms (33/14) in dorsal and 1.17 ± 0.12 mV/ms (24/15) and 1.16 ± 0.08 mV/ms (24/15) in ventral hippocampal slices, respectively. Correspondingly, we studied short-term changes in neuronal excitation under conditions of afferent stimulation producing a PS less than 2 mV and specifically 1.04 ± 0.05 mV (28/18) and 1.05 ± 0.05 mV (31/14) in the dorsal hippocampus and 1.05 ± 0.04 mV (27/18) and 1.04 ± 0.06 mV (24/15) in the ventral hippocampus of young and old rats, respectively. Furthermore, we studied short-term changes in neuronal excitation under conditions of stimulation producing a PS equal to or greater than 2 mV; specifically 4.72 ± 0.36 mV (22/16) and 4.58 ± 0.34 mV (31/14) in the dorsal hippocampus and 4.34 ± 0.35 mV (17/12) and 3.89 ± 0.36 mV (21/15) in the ventral hippocampus of young and old rats, respectively.
Short-term synaptic plasticity in dorsal and ventral hippocampus does not change during aging
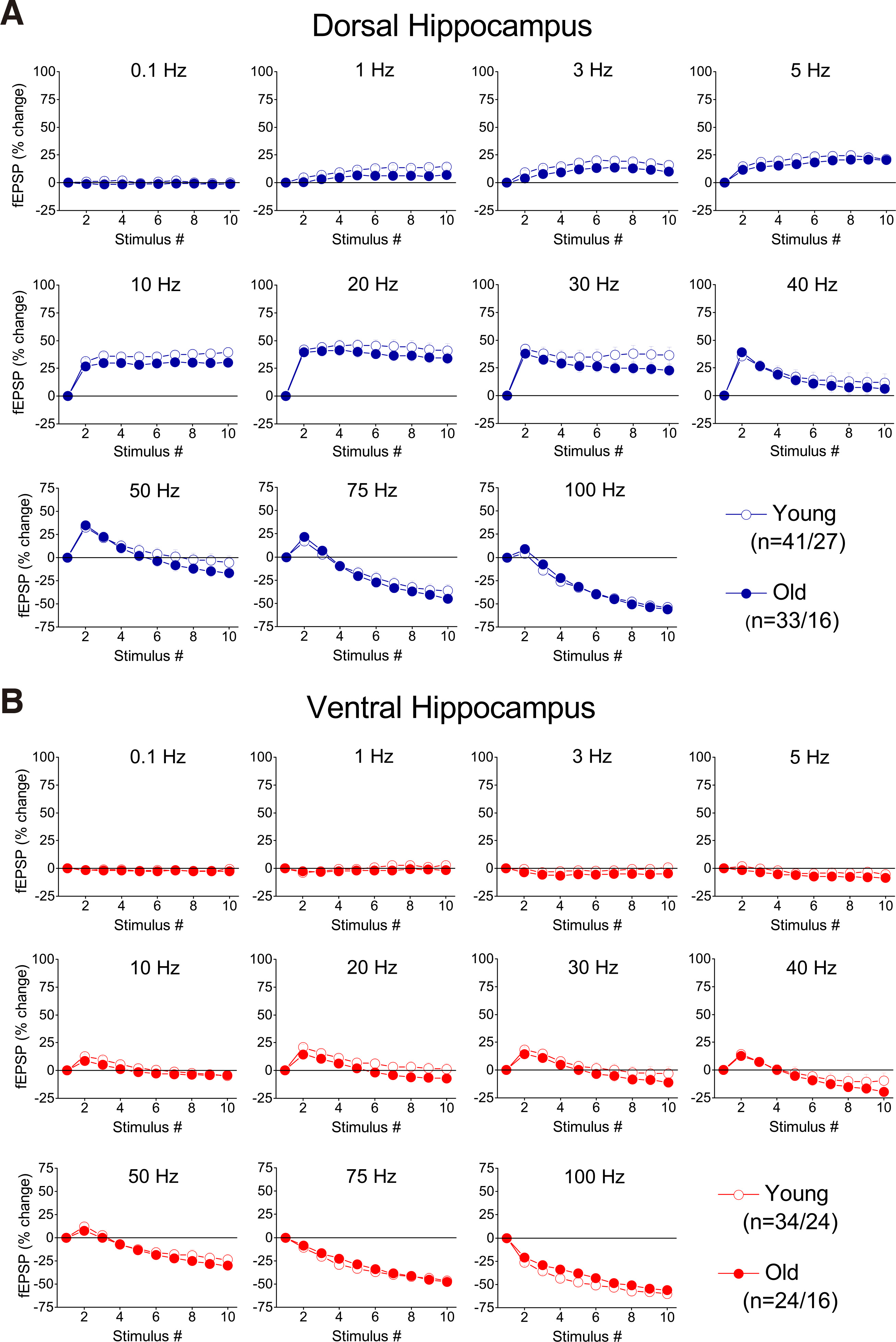
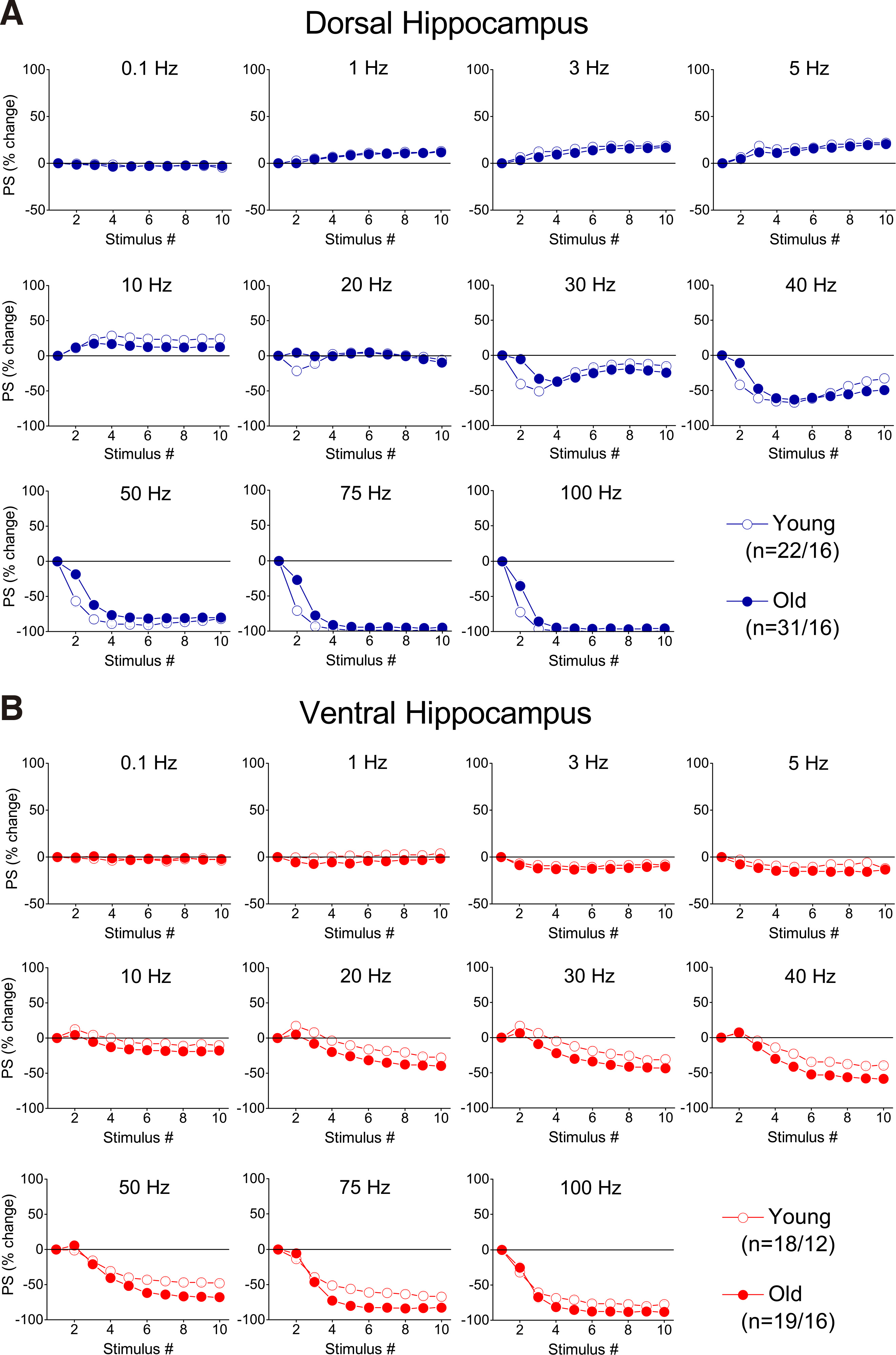
Application of frequency stimulation in hippocampal slices from young rats showed that short-term synaptic plasticity greatly differs between the dorsal and ventral hippocampus, confirming the results from previous reports (Papaleonidopoulos et al. 2017; Koutsoumpa and Papatheodoropoulos 2019). Specifically, stimulation in dorsal slices induced a steady-state facilitation when delivered at frequencies 1-40 Hz, and a steady-state depression when delivered at higher frequencies (Fig. 2A and Fig. 3A). In contrast, the ventral hippocampus responded with steady-state depression at frequencies equal to or greater than 40 Hz, and no significant change at lower stimulation frequencies (Fig. 2B and Fig. 3B). Regarding the second response in a train (onset response), the dorsal hippocampus showed facilitation across 1-100 Hz, while the ventral hippocampus showed facilitation at a narrower range of frequencies (20-50 Hz), and depression at higher frequencies (Fig. 3 and Fig. 4). We then compared the responses between young and old dorsal (Fig. 3A and Fig. 4A) and ventral hippocampus (Fig. 3B and Fig. 4A). We observed no significant interaction between age and frequency-dependent short-term synaptic plasticity, in either the dorsal (MANOVA, F (100, 5554) = 1.13, p>0.1; Wilk’s Λ = 0.866) or the ventral hippocampus (MANOVA, F (100, 4258) = 1.08, p>0.1; Wilk’s Λ = 0.836). Furthermore, we did not find any significant age-related difference for the onset and steady-state response, in either the dorsal or the ventral hippocampus (independent t-test inside each segment of the hippocampus and at individual stimulation frequencies, p>0.05) (Fig. 4B). These results suggested that aging does not significantly affect short-term synaptic plasticity in either segment of the hippocampus.
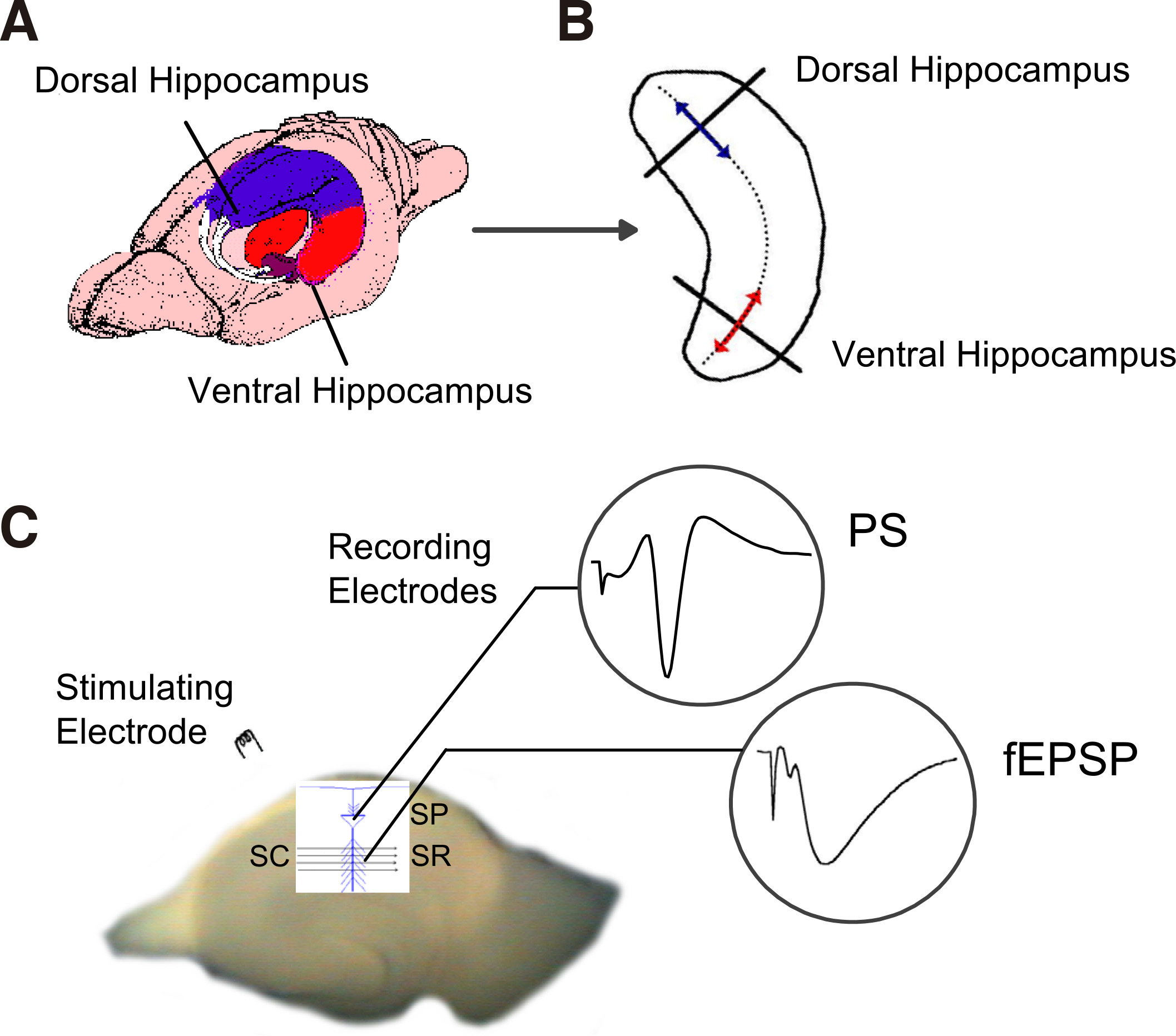
Fig. 1. Methods used to prepare transverse slices from the dorsal and the ventral segment of the hippocampus and to record field excitatory population potentials.
(A) Schematic drawing of the rat brain illustrating the hippocampus and the dorsal (blue) and ventral (red) segments of the structure. (B) Contour of a left-hemisphere hippocampus illustrating the method used to prepare thin slices (500 μm) cutting the structure transversely (thick lines) to its long axis (dotted line through the structure). The dorsal and the ventral portions (segments) of the hippocampus used to prepare slices are indicated by lines with arrowheads (blue and red, respectively). (C) A photograph of a transverse hippocampal slice illustrating the method used to stimulate Schaffer collaterals (SC) by a bipolar stimulating electrode and record field excitatory postsynaptic potentials (fEPSP) from the stratum radiatum (SR) and population spikes (PS) from the stratum pyramidale (SP) of CA1 field.
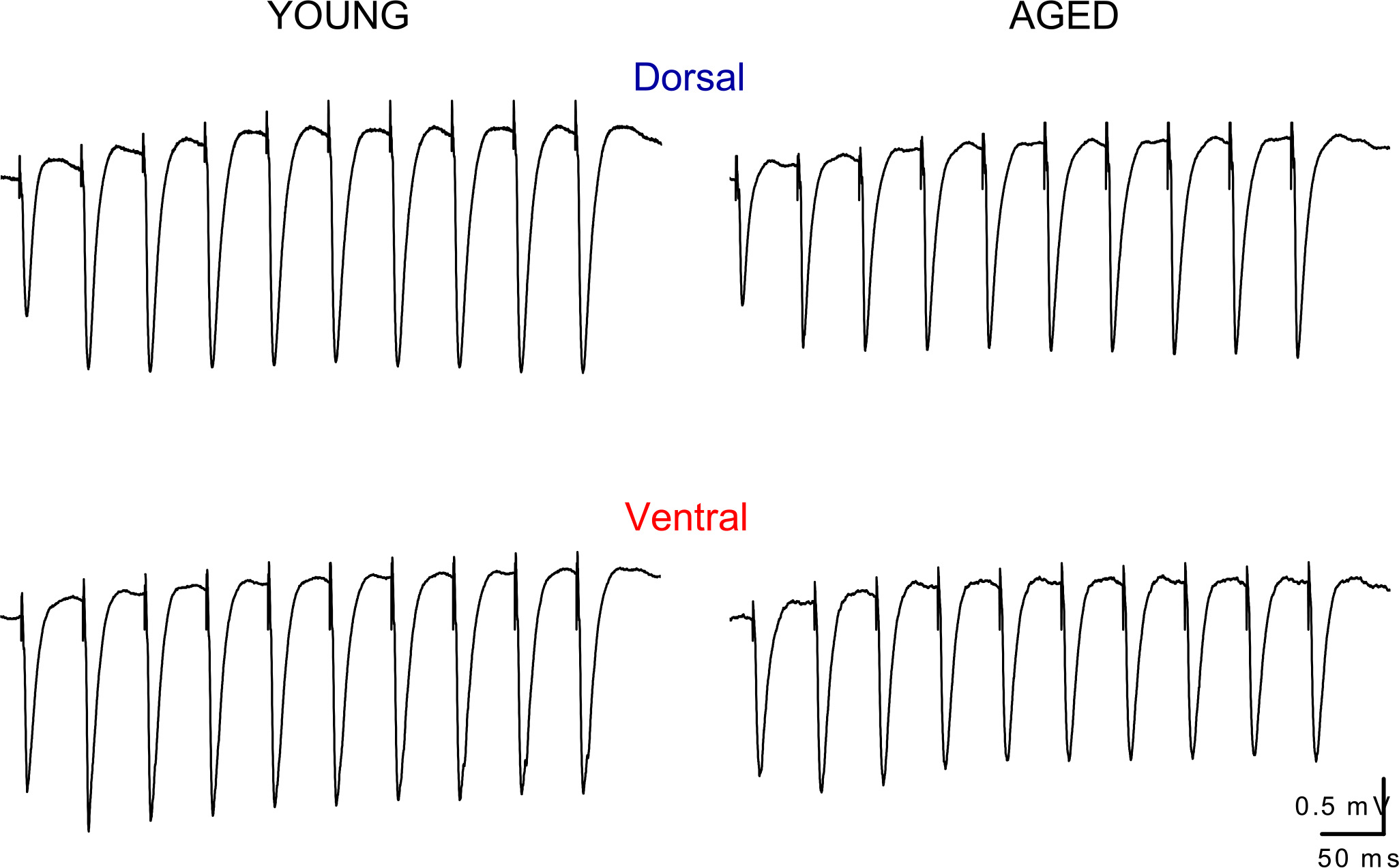
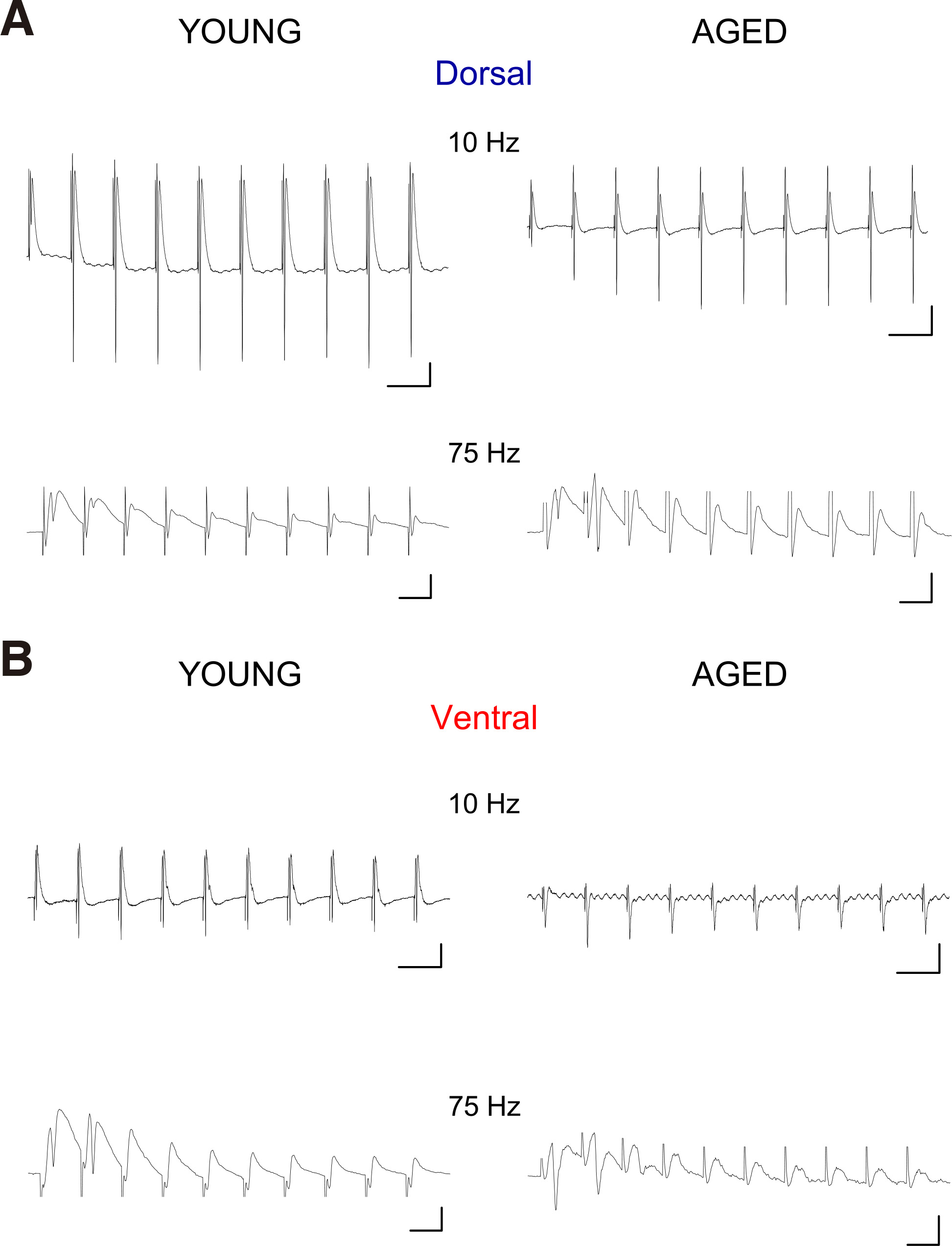
Fig. 2. Representative traces of fEPSPs recorded during repetitive stimulation of Schaffer collaterals in dorsal and ventral slices obtained from a young and an old rat.
Examples of responses evoked by trains of 10 pulses applied at the frequencies of 20 Hz are shown. Note that the responses recorded from aged rats are similar with those obtained from young individuals, either in the dorsal or the ventral hippocampus. Stimulation artifacts are truncated for clarity.
Fig. 3. Percent changes of fEPSP evoked by a ten-pulse stimulation train applied at different frequencies in dorsal and ventral hippocampal slices from young and aged rats.
Each graph shows percent changes of fEPSP as a function of stimulation time (i.e. number of pulses). Results for the different stimulation frequencies are presented in different graphs.
Fig. 4. Short-term synaptic plasticity (i.e. transient changes in fEPSP during repetitive afferent stimulation) does not significantly differ between young and aged rats, in either the dorsal or the ventral hippocampus.
(A) Cumulative 3D graphs illustrating in a compact manner percent changes of fEPSP across both stimulation frequency and stimulation time. The highest and the lowest value of each 3D plot (corresponding to intense facilitation and depression respectively) are marked by red and blue color respectively, as indicated in the color scale bar. Data in this figure are the same as in Fig. 3. (B) Comparison of short-term synaptic plasticity between young and aged dorsal and ventral hippocampus. Percentages for fEPSPs produced by the 2nd pulse (onset response) and averages of fEPSP percentages produced by the 8th – 10th pulses (steady-state responses), plotted as a function of stimulation frequency are shown. Frequency stimulation did not reveal any difference between young and aged rats, either for the onset or steady-state response in the dorsal or the ventral hippocampus.
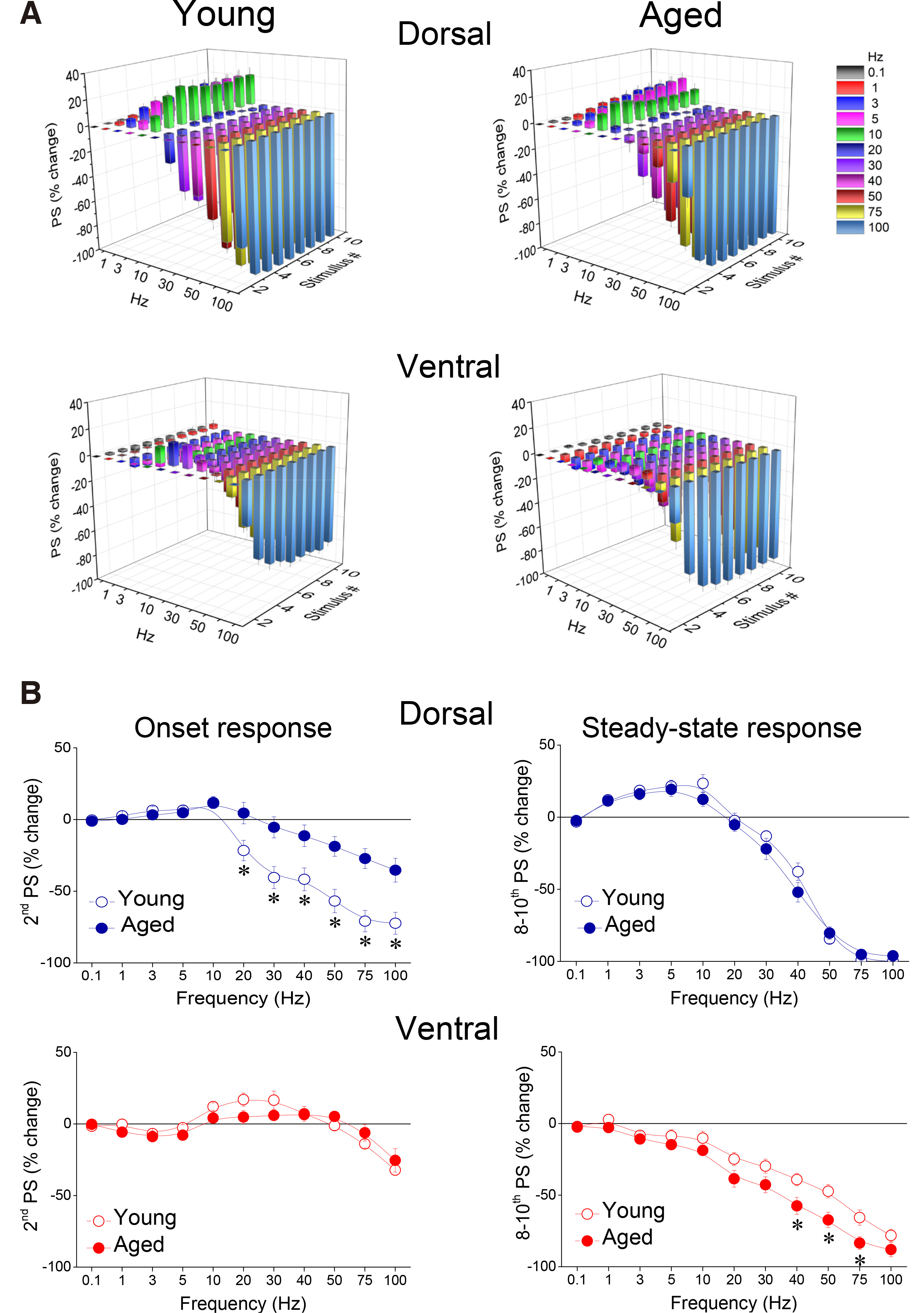
Short-term dynamics of neuronal excitation changes during aging more in dorsal than in ventral hippocampus in a frequency-dependent manner
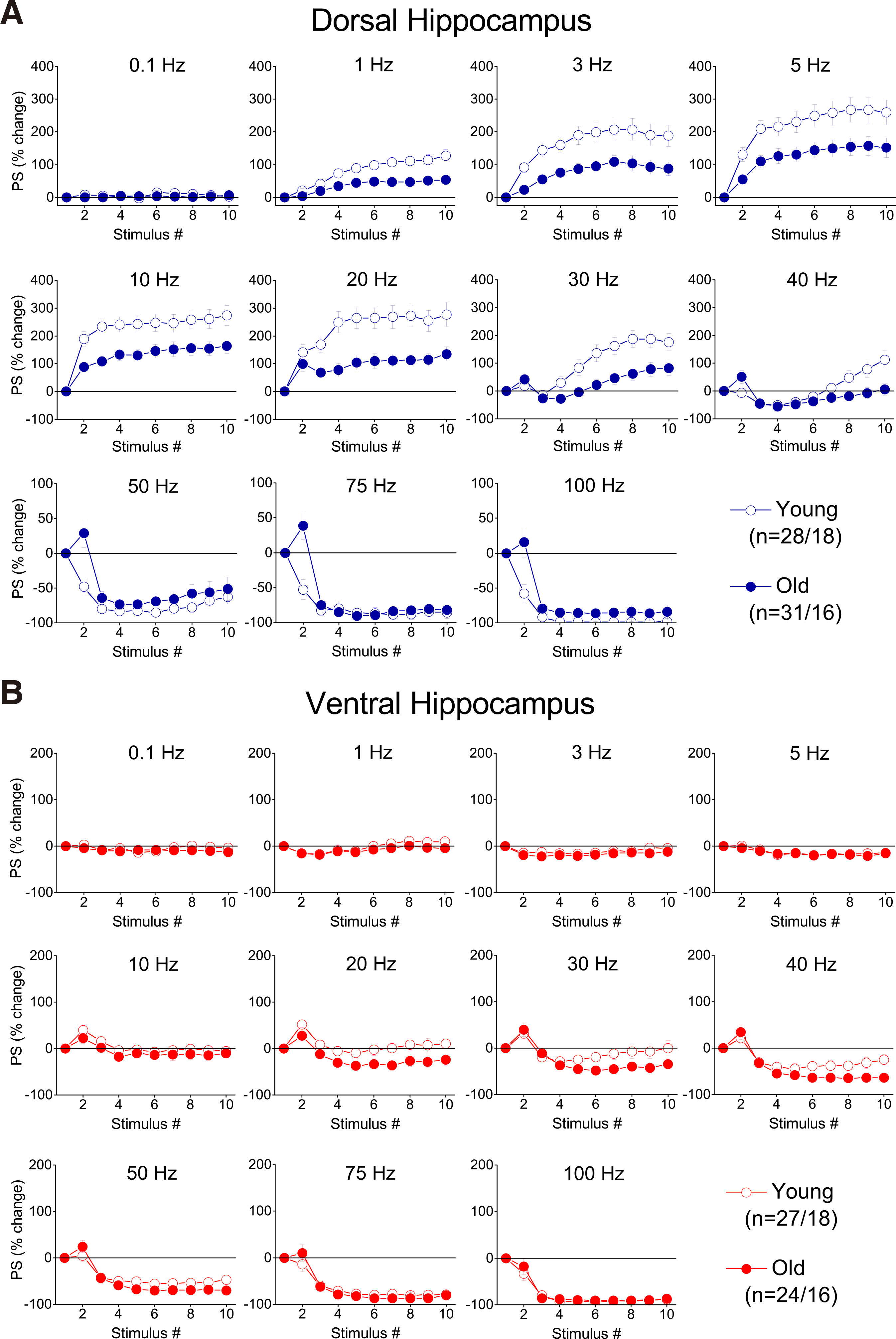
As described above, we studied short-term modulation of neuronal excitation under two different conditions of intensity of afferent stimulation, relatively low and high, producing a PS < 2 mV and a PS ≥ 2 mV, respectively. Previous observations have shown that relatively low and high levels of initial excitation of hippocampal circuit produce mainly facilitation and depression of steady-state responses, respectively (Koutsoumpa and Papatheodoropoulos 2019). In line with these observations, we found that frequency stimulation of low intensity produced strong facilitation of steady-state neuronal responses in the young dorsal hippocampus, at frequencies 1-40 Hz, and depression at 50-100 Hz (Fig. 5 and Fig. 6). Steady-state responses in the ventral hippocampus of young rats either displayed no significant change when evoked at 0.1-30 Hz, or were depressed when evoked at higher frequencies (40-100 Hz), (Fig. 5 and Fig. 6).
Fig. 5. Representative traces of PSs evoked by trains of ten pulses applied at Schaffer collaterals in dorsal (A) and ventral slices (B) obtained from a young and an aged rat.
Examples are shown for the stimulation frequencies of 10 Hz and 75 Hz. Calibration bars: 0.5 mV in all graphs, 100 ms in graphs of 10 Hz and 10 ms in graphs of 75 Hz. Stimulation artifacts are truncated for clarity.
Fig. 6. Percent changes of PS evoked by a ten-pulse train applied at different frequencies and starting with a low excitation level (PS<2 mV), in dorsal and ventral hippocampal slices from young and aged rats.
Percentages of PS are plotted against stimulation time (i.e. number of pulses). Results for the different stimulation frequencies are presented in different graphs. Note that age-dependent differences in short-term neuronal dynamics are observed in the dorsal but not the ventral hippocampus.
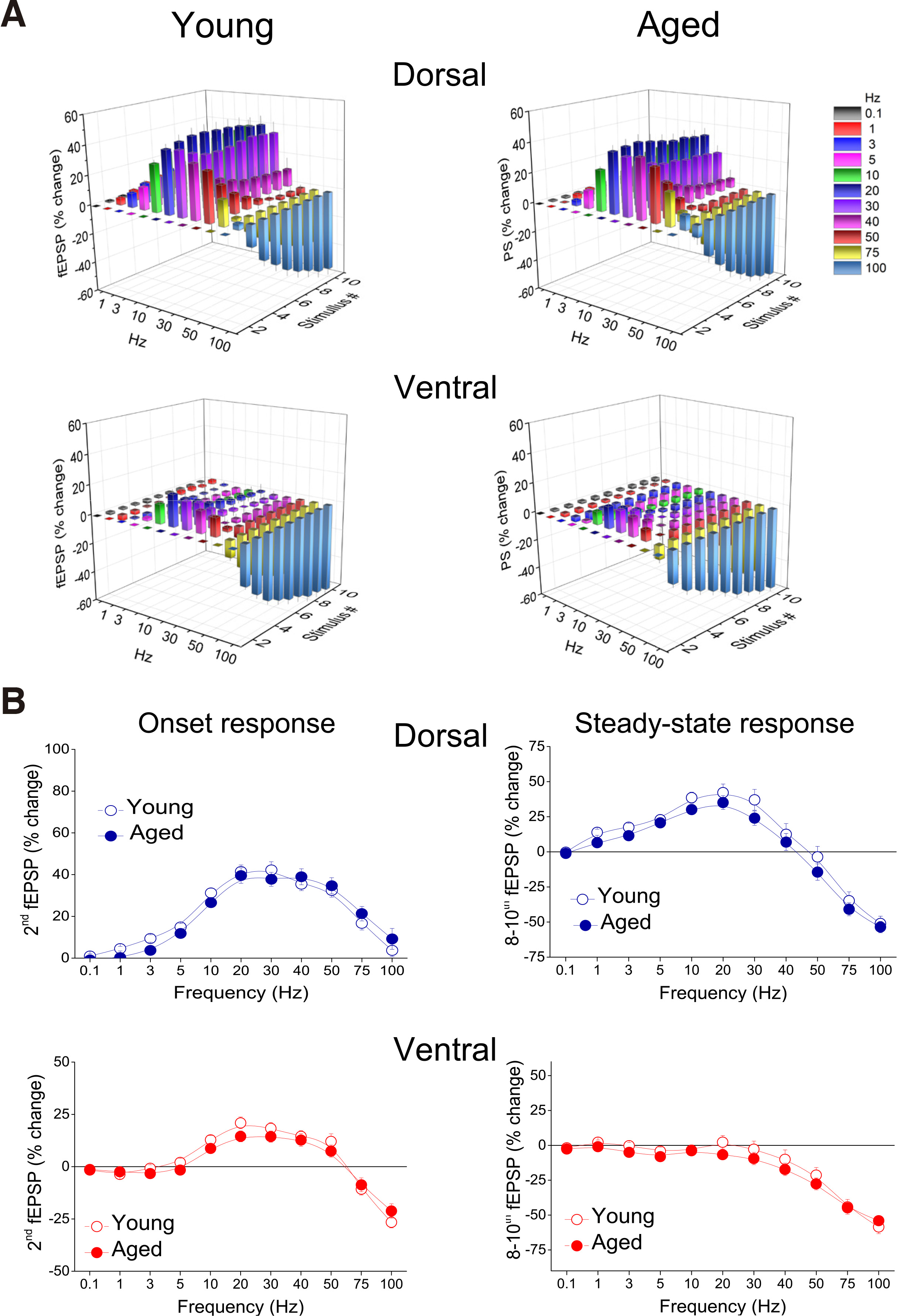
Comparing short-term dynamics between young and old hippocampus, we found that the dorsal hippocampus of old rats was unable to reach the facilitation score displayed by young rats (Fig. 6A and Fig. 7A); therefore, aging significantly affected the frequency-dependent properties of STND in the dorsal hippocampus under these experimental conditions (MANOVA, F(90, 3883) = 2.91, p<0.0005; Wilk’s Λ = 0.642). Specifically, facilitation of steady-state responses was significantly lower in the old compared with the young dorsal hippocampus (independent t-test at individual stimulation frequencies between 1 and 40 Hz, p<0.05) (Fig. 7B, Steady-state response). Furthermore, facilitation of the onset response was significantly lower in old compared with young dorsal hippocampus at stimulation frequencies 1-20 Hz (independent t-test at individual stimulation frequencies, p<0.05) (Fig. 7B, Onset response). Notably, at higher stimulation frequencies (50-100 Hz), significant depression was observed in the young dorsal hippocampus (paired t-test, p<0.05), but not in ventral hippocampus, which continued to show facilitation (comparison between young and old dorsal hippocampus, independent t-test, p<0.05). Steady-state depression produced in the dorsal hippocampus at high stimulation frequencies (50-100 Hz) was similar between young and old rats (independent t-test, p>0.05). Remarkably, aging did not significantly affect short-term dynamics of neuronal excitation in the ventral hippocampus (MANOVA, F (90, 3225) = 0.988, p>0.5; Wilk’s Λ = 0.832), (Fig. 6B and Fig. 7A). More specifically, neither onset nor steady state responses were significantly changed in the ventral hippocampus of old vs young rats (independent t-test at individual stimulation frequencies, p>0.05).
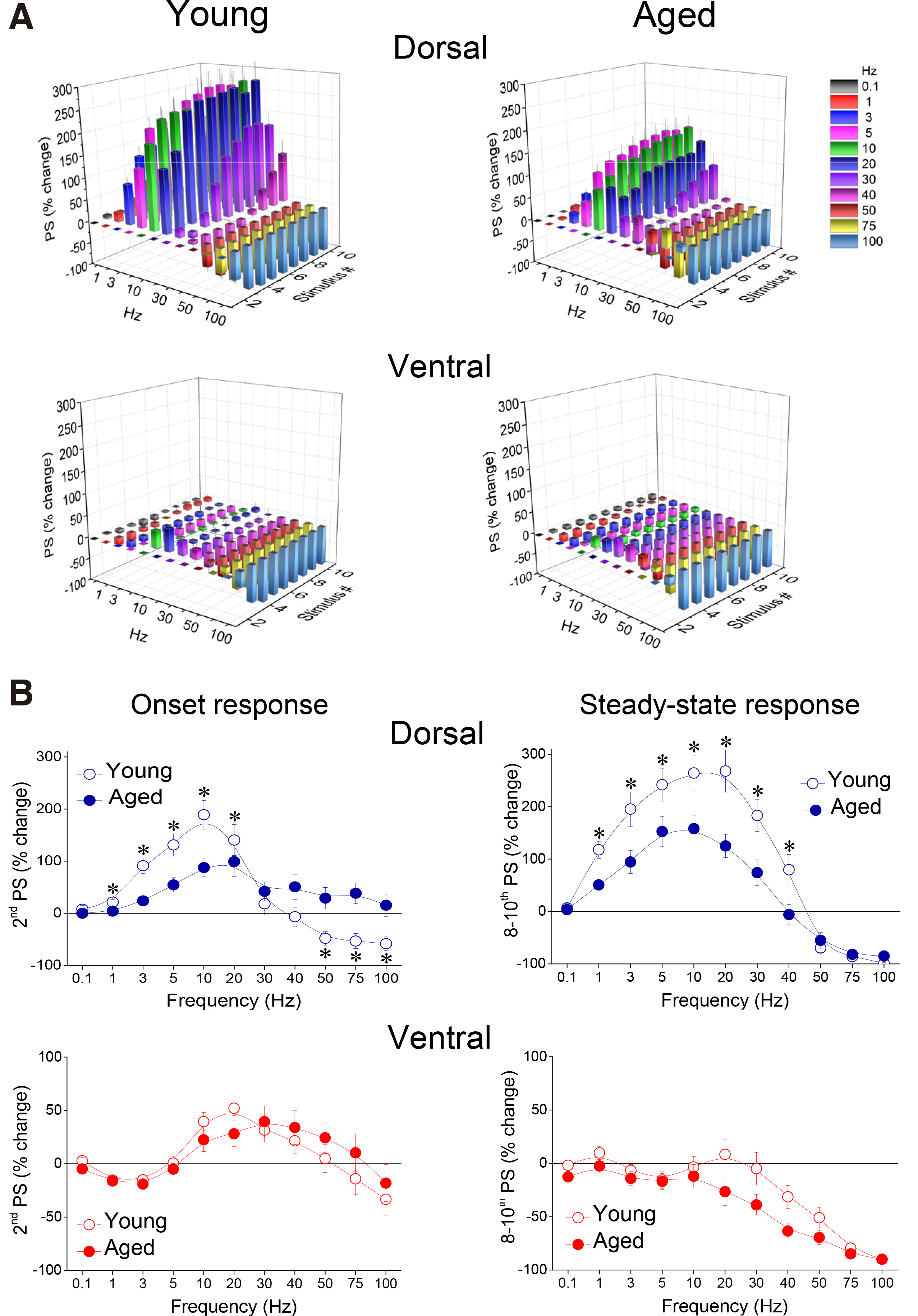
Fig. 7 Short-term dynamics of neuronal excitation (i.e. transient changes in PS) induced by stimulation evoking an initial PS with an amplitude less than 2 mV, was significantly altered in a frequency-dependent manner in the dorsal hippocampus, but not ventral hippocampus, of aged vs young rats.
(A) Cumulative 3D graphs illustrating percent changes of fEPSP across both stimulation frequency and stimulation time. The highest and the lowest value of each 3D plot (corresponding to intense facilitation and depression respectively) are marked by red and blue color respectively, as indicated in the color scale bar. Data in this figure are the same as in Fig. 6. (B) Comparison of short-term dynamics of neuronal excitation between young and aged dorsal and ventral hippocampus. Are shown percentages for PSs produced by the 2nd pulse (onset response) and averages of PS percentages produced by the 8th – 10th pulses (steady-state responses), plotted as a function of stimulation frequency. Asterisks indicate statistically significant differences between young and aged rats at p<0.05 (independent t-test at individual frequencies). Note that the facilitation induced by a wide range of stimulation frequencies in steady-state responses of the dorsal hippocampus is higher in young than in aged rats. Moreover, the second response in the dorsal hippocampus from young rats (onset response) facilitates at relatively low frequencies (< 40 Hz) and depressed at high stimulation frequency (> 40 Hz), while only facilitates in the dorsal hippocampus of aged individuals.
Under conditions of high-intensity stimulation producing PS ≥ 2 mV, the facilitation of both onset and steady-state responses was markedly attenuated compared with the facilitation seen under conditions of low-intensity stimulation, in both segments of the hippocampus (Fig. 8 and Fig. 9A). We found that aging significantly interacted with STND in both the dorsal (MANOVA, F (90, 3551) = 1.71, p<0.0005; Wilk’s Λ = 0.75) and the ventral hippocampus (MANOVA, F (90, 2513) = 1.38, p<0.05; Wilk’s Λ = 0.721). More specifically, the onset response in the dorsal hippocampus showed significantly stronger inhibition in young compared with old rats (at 20-100 Hz, independent t-test, p<0.05) (Fig. 9B, Dorsal), while steady-state depression was stronger in the ventral hippocampus of old compared with young rats (at 40-75 Hz, independent t-test, p<0.05) (Fig. 9B, Ventral). We did not detect any other significant age-dependent difference in short-term neuronal dynamics of the dorsal or ventral hippocampus under conditions of high-intensity stimulation. The combined patterns of short-term changes in synaptic transmission and neuronal excitation observed in the dorsal and ventral hippocampus of young and old rats are illustrated in Fig. 10.
Fig. 8. Percent changes of PS evoked by a ten-pulse train applied at different frequencies and starting with a high excitation level (PS=>2 mV), in dorsal and ventral hippocampal slices from young and aged rats.
Percentages of PS are plotted against stimulation time (i.e. number of pulses). Results for the different stimulation frequencies are presented in different graphs. Note that age-dependent differences in short-term neuronal dynamics are observed in the dorsal but not the ventral hippocampus.
Fig. 9. Short-term dynamics of neuronal excitation induced by stimulation evoking an initial PS with an amplitude equal to or greater than 2 mV, was significantly altered in a frequency-dependent and time-dependent manner in both dorsal and ventral hippocampus of aged compared with young rats.
(A) Cumulative 3D graphs illustrating percent changes of fEPSP across both stimulation frequency and stimulation time. The highest and the lowest value of each 3D plot (corresponding to intense facilitation and depression respectively) are marked by red and blue color respectively, as indicated in the color scale bar. Data in this figure are the same as in Fig. 8. (B) Comparison of short-term dynamics of neuronal excitation between young and aged dorsal and ventral hippocampus. Are shown percentages for PSs produced by the 2nd pulse (onset response) and averages of PS percentages produced by the 8th – 10th pulses (steady-state responses), plotted as a function of stimulation frequency. Asterisks indicate statistically significant differences between young and aged rats at p<0.05 (independent t-test at individual frequencies). Note that strong stimulation intensity (i.e. evoking PS => 2 mV) applied at relatively high frequencies produces a greater depression of onset response in the dorsal hippocampus of young vs aged rats and a greater depression in the ventral hippocampus of aged vs young rats.
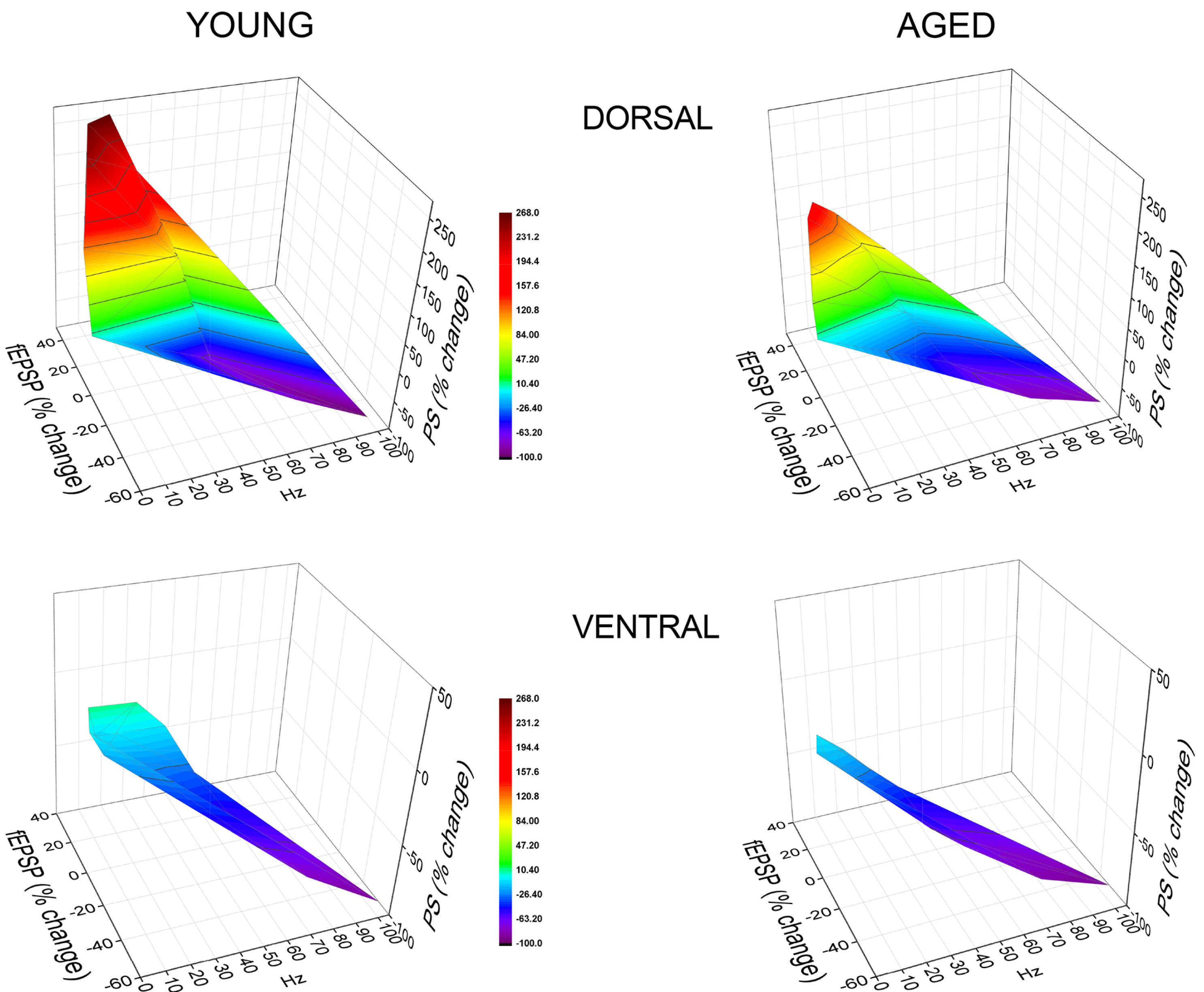
Fig. 10. The range of short-term neuronal dynamics differs between young and aged hippocampus.
3D graphs illustrating the dynamic range of transient changes in synaptic input (fEPSP) and neuronal output (PS) in the dorsal and ventral hippocampus of young and aged rats. The graphs show the combined steady-state changes in fEPSP and PS as a function of stimulus frequency. Data regarding PS are obtained at a stimulation current intensity of low strength. The highest and the lowest value of each 3D plot (corresponding to intense facilitation and depression respectively) are marked by red and blue color respectively, as indicated in the color scale bar. Note that the dorsal hippocampus of young rats demonstrates higher scores of steady-state output facilitation compared with the dorsal hippocampus of aged rats, despite similar corresponding short-term changes in synaptic input in the two age groups. In contrast, the dynamic range of steady-state responses in the ventral hippocampus did not significantly differ between young and aged individuals.
Discussion
This study presents a detailed characterization of frequency-dependent short-term changes in synaptic transmission and neuronal excitation in the young adult and old dorsal and ventral CA1 hippocampal field. The main findings of the present study are the following:
1). Aging does not affect short-term synaptic plasticity in either the dorsal or the ventral hippocampus.
2). Aging is accompanied by frequency-dependent alterations in short-term dynamics of CA1 neuronal output, more in the dorsal than in the ventral hippocampus.
3). The dorsal hippocampus of old rats displays reduced short-term facilitation of steady-state neuronal output.
4). The dorsal hippocampus of old rats displays reduced paired-pulse inhibition of excitation.
5). Under conditions of strong afferent activation, the ventral hippocampus of old rats displays increased steady-state inhibition.
It is noted here that there has never been a comparative study of the effects of aging on short-term synaptic plasticity or short-term dynamics of neuronal excitation in the dorsal and ventral hippocampus. Past studies in the dorsal or middle part of the hippocampus have shown a tendency of the young vs the old CA1 hippocampal circuit to facilitation of excitatory synaptic transmission following relatively prolonged stimulation applied at a fixed frequency. Specifically, long trains of Schaffer collateral stimulation (35-315 pulses) applied at the frequency of 7 Hz produced a greater facilitation of fEPSP in young than in old rats (Landfield et al. 1986). In another study, fEPSP was facilitated in hippocampal slices from young but not old rats, after 16 stimuli at 1 Hz (Ouanounou et al. 1999). Greater facilitation of intracellularly recorded EPSP was also observed in young vs old hippocampus following the delivery of long trains of suprathreshold but not subthreshold stimulation pulses (>40) at 7 Hz, (Thibault et al. 2001). In the present study, using a physiologically relevant stimulation consisting of only ten pulses and a wide range of stimulation frequencies, a pattern resembling normal firing activity of CA3 and CA1 pyramidal cells (Ranck 1973; Wong and Prince 1981; Johnston and Brown 1984; Fenton and Muller 1998), we did not observe any significant effect of age upon short-term synaptic dynamics, in either the dorsal or the ventral hippocampus. It therefore seems likely that age-dependent differences in short-term synaptic plasticity may occur under prolonged but not short periods of afferent activity in the hippocampus.
In contrast to the results on fEPSP, short-term dynamics of hippocampal output markedly differ between young and old rats. Furthermore, age-dependent alteration in short-term neuronal dynamics was more pronounced in the dorsal than in ventral hippocampus. Previous studies have reported no age-related difference in paired-pulse facilitation of PS at inter-pulse intervals 4-200 ms (corresponding to 5-50Hz), and a greater facilitation of PS in young vs old hippocampus only following long trains of stimulation pulses (≥150 pulses delivered at 12-15Hz), (Landfield and Lynch 1977; Landfield et al. 1978). However, in another study, prolonged stimulation at 7 Hz failed to show a significant effect of age on frequency potentiation or depression of PS (Landfield et al. 1986). In contrast to these results, we show that in young vs old dorsal hippocampus, the paired-pulse facilitation of PS is greater at inter-pulse intervals corresponding to 1-20 Hz and paired-pulse inhibition of PS is greater at inter-pulse intervals corresponding to 50-100 Hz. Furthermore, the amplification of steady-state output (PS) at frequencies 1-40 Hz is significantly higher in the young than the old dorsal hippocampus. A higher facilitation of PS at 40 Hz has previously been observed in the CA3 field of young vs old dorsal hippocampus (Villanueva-Castillo et al. 2017). In addition, we show that conditions of intense activation of the local network reveal a higher frequency-dependent inhibition of PS in old vs young ventral hippocampus.
Several mechanisms that contribute to determining the properties of short-term dynamics can be involved in differentiation of age-dependent short-term facilitation in neuronal excitability, including facilitation of excitatory synaptic transmission and mechanisms of disinhibition (Leung and Fu 1994). However, the age-associated differences in short-term neuronal excitability observed here could not be explained by short-term changes in excitatory synaptic transmission, since aging does not affect short-term synaptic plasticity. An increased GABAA receptor-mediated transmission can support greater depression of excitation at relatively high frequencies of neural activity and greater facilitation of excitation under conditions of disinhibition, at relatively lower frequencies (Michelson et al. 1989; Margineanu and Wülfert 2000; Papatheodoropoulos et al. 2002; Koutsoumpa and Papatheodoropoulos 2019). Thus, most probably, the reduced inhibition of the onset responses (or absence of inhibition at low intensities of afferent activation) and the lower facilitation of steady-state responses in the aged dorsal hippocampus could be attributed to the previously demonstrated reduced GABAA receptor-mediated synaptic inhibition in the dorsal hippocampus of old compared with young rodents and other species (Papatheodoropoulos and Kostopoulos 1996; Potier et al. 2006; Rissman et al. 2006; Hoekzema et al. 2012; Kouvaros et al. 2015). Conspicuously, however, frequency depression of PS is higher in the old vs young ventral hippocampus, permitting the hypothesis that the pattern of age-associated changes in GABAergic transmission in the ventral hippocampus may differ from that in the dorsal hippocampus. However, to the best of our knowledge, data on GABAergic transmission in the aged ventral hippocampus are still missing.
Possible implications
Regarding young adults, the results of the present study confirm previous observations that under conditions of synaptic activation of moderate strength, the dorsal hippocampus can detect and facilitate transmission of relatively low frequency input (< 50 Hz) and depress transmission of higher-frequency input, while synapses in the ventral hippocampus mostly depress with little sensitivity to frequency of afferent activity (Papaleonidopoulos et al. 2017; Koutsoumpa and Papatheodoropoulos 2019). The present findings suggest that the CA1 circuit of the dorsal hippocampus of old rats appears unable to transmit incoming information in a frequency-dependent discriminatory manner, as evidenced by the facilitation of onset responses across the entire range of stimulation frequencies. This may have important implications in dorsal hippocampus-dependent functions including spatial cognition (Hampson et al. 1999; Cenquizca and Swanson 2007; Moser et al. 2017). Interestingly, a recent study shows that a significant decrease in dorsal hippocampus volume correlates with decline in spatial learning in old mice (Reichel et al. 2017). In contrast to the conspicuous aging-associated alterations in STND in the dorsal hippocampus, the aged ventral hippocampus shows much less pronounced alterations in short-term processing of neural information. The changes in the ventral hippocampus consisted of increased depression of steady-state neuronal output (at ≥ 40Hz) under conditions of intense afferent activation, suggesting intensified suppression of high-frequency information in this segment of old hippocampus. Furthermore, the distinct vulnerability of dorsal and ventral hippocampus, as shown here, supports the concept of localized aging-related changes in brain structure and function (Small 2001) and underlines the need to include at least the two extreme, opposite segments of the structure when studying possible actions of physiological or pathological factors upon the hippocampal function.
We conclude that aging is associated with reduced frequency-dependent dynamic range of the short-term changes in neuronal excitability more in the dorsal than in the ventral hippocampus. This diversification may contribute to alterations in behaviorally observed information processing displayed by old individuals.
Materials and Methods
Animals and hippocampal slice preparation
Young adult (3-4 months) and old (20-32 months) Wistar rats were used in this study. Rats were housed at the SPF Laboratory of Experimental Animals of the Department of Medicine, University of Patras (licence No: EL-13-BIOexp-04), under stable conditions of light-dark cycle (12/12 h), temperature (20-22 oC) and had free access to food and water. The treatment of animals and all experimental procedures were conducted in accordance with the European Communities Council Directive Guidelines for the care and use of laboratory animals (2010/63/EU – European Commission). Furthermore, animal treatment and experimental procedures were approved by the Protocol Evaluation Committee of the Department of Medicine of the University of Patras and the Directorate of Veterinary Services of the Achaia Prefecture of Western Greece Region (reg. number: 203173/1049, 22/08/2014). In addition, every effort was made to minimize the number of animals used. Hippocampal slices from the dorsal and the ventral segment of the structure were prepared as previously described (Papatheodoropoulos and Kostopoulos 2000; Koutsoumpa and Papatheodoropoulos 2019). Specifically, animal was decapitated under deep anaesthesia with diethyl-ether, and the brain was removed after opening the cranium. The brain was placed in ice-cold (2-4 oC) standard artificial cerebrospinal fluid (ACSF) containing, in mM: 124 NaCl, 4 KCl, 2 CaCl2, 2 MgSO4, 26 NaHCO3, 1.25 NaH2PO4 and 10 glucose. ACSF was equilibrated with 95% O2 and 5% CO2 gas mixture at a pH=7.4. The hippocampi were then exposed and placed on the disc of a McIlwain tissue chopper. Transverse 500 µm-thick slices were prepared from the dorsal (septal) and the ventral (temporal) segment of the hippocampus extending between 1.0 mm and 3.0 mm from the dorsal and the ventral end, as previously described. Immediately after their preparation, slices were transferred to an interface type recording chamber where they were constantly perfused with standard medium of the same composition as described above and at a rate of ~1.5 ml/min. Slices were continuously humidified with a mixed gas consisting of 95% O2 and 5% CO2 at a constant temperature of 30±0.5 oC. Tissue stimulation and recording started at least one and a half hours after their placement in the chamber.
Electrophysiological recordings, Data Processing and Analysis
Evoked field population potentials were recorded from the middle of the CA1 field following electrical stimulation of the Schaffer collaterals. Field excitatory postsynaptic potential (fEPSP) and population spike (PS) were recorded from the middle stratum radiatum and stratum pyramidale, respectively. Electrical stimulation consisted of current pulses of a fixed duration (100 μs) and variable amplitude (20 to 260 μA) applied through a bipolar platinum/iridium electrode with a wire diameter of 25 μm and an inter-wire distance of 100 μm (World Precision Instruments, USA). Recordings were performed using a 7 μm-thick carbon fiber (Kation Scientific, Minneapolis, USA) positioned 350 μm from the stimulation electrode. As a rule, simultaneous recordings of fEPSP and PS were made from an individual slice. Baseline stimulation was delivered every thirty seconds using a current intensity that elicited a just subthreshold fEPSP based on input-output curves between stimulation intensity and evoked responses. Short-term changes in fEPSP and PS were studied using a frequency stimulation protocol as previously described (Koutsoumpa and Papatheodoropoulos 2019). Specifically, the frequency stimulation protocol consisted of a sequence of ten consecutive pulses delivered at varying frequency between 0.1 and 100 Hz. The number of pulses falls into the range of naturally occurring spike trains in CA3 cells (Fenton and Muller 1998). Consecutive trains of pulses, delivered during the application of frequency stimulation paradigm, were separated by a two-minute interval to allow synapses to return to baseline level. Furthermore, stimulation trains of different frequency were applied in random fashion during each experiment. Frequency stimulation used to study short-term changes in fEPSP was applied at three intensities of stimulation current, while frequency stimulation used to study short-term changes in PS was applied at two intensities of stimulation current. Specifically, regarding the study of fEPSP, we adjusted the stimulation current intensity to alternatively produce a subthreshold fEPSP of about 0.5 mV/ms, a suprathreshold fEPSP that evoked a PS of 0.5-1.0 mV, and a PS of 75% of its maximum amplitude. We call the three stimulation intensities that produced three different levels of local network activation subthreshold, suprathreshold and submaximal, respectively. Regarding the study of PS,we adjusted the stimulation current intensity to produce a PS with amplitude either less than 2 mV (low intensity stimulation) or equal to or greater than 2 mV (high intensity stimulation).
Signals were amplified 500 times, band-pass filtered at 0.5Hz–2kHz using Neurolog amplifiers (Digitimer Limited, UK), digitized at 10 kHz and stored on a computer disk for off-line analysis using the CED 1401-plus interface and the Signal6 software (Cambridge Electronic Design, Cambridge, UK). The fEPSP was quantified by the maximum slope of its initial rising phase; slope was measured in a time window of one millisecond immediately after the appearance of the presynaptic fiber volley. PS was quantified by its amplitude measured by the length of the projection of the minimum peak on the line connecting the two maxima peaks of the PS waveform. The effects of frequency stimulation on fEPSP and PS were quantified as the percentage change of each of the nine consecutive responses evoked by the consecutive pulses with respect to the first response in the train. Steady-state response was estimated by averaging the last three responses (i.e. 8th-10th).
The following tests were used for statistical comparisons: paired t-test, independent t-test and multivariate general linear model (MANOVA). The values in the text and figures express meanS.E.M. Statistical analyses were performed using the number of slices. Furthermore, the number of slices and animals used in the analysis (slices/animals) is given throughout the text.
Acknowledgements
G. Tsotsokou is a recipient of a Ph.D. fellowship from Andreas Mentzelopoulos Foundation of the University of Patras.
Abbreviations
fEPSP, field Excitatory Postsynaptic Potential ; MANOVA, Multivariate Analysis of Variance ; PS, Population Spike ; STND, Short-Term Neural Dynamics ;References
Abbott L. F., Regehr W. G. (2004). Synaptic computation. Nature 431: 796-803.
Babiec W. E., Jami S. A., Guglietta R., Chen P. B., O'Dell T. J. (2017). Differential Regulation of NMDA Receptor-Mediated Transmission by SK Channels Underlies Dorsal-Ventral Differences in Dynamics of Schaffer Collateral Synaptic Function. The Journal of Neuroscience 37: 1950-1964.
Bannerman D. M., Sprengel R., Sanderson D. J., McHugh S. B., Rawlins J. N. P., Monyer H., Seeburg P. H. (2014). Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews Neuroscience 15: 181-192.
Bettio L. E.B., Rajendran L., Gil-Mohapel J. (2017). The effects of aging in the hippocampus and cognitive decline. Neuroscience & Biobehavioral Reviews 79: 66-86.
Bliss T V, Collingridge G L, Morris R, (2007). Synaptic Plasticity in the Hippocampus. In The Hippocampus Book. (Ed. Andersen P., Morris R., Amaral D., Bliss T., O'keefe J., ) Oxford University Press, Oxford.
Burke S. N., Barnes C. A. (2006). Neural plasticity in the ageing brain. Nature Reviews Neuroscience 7: 30-40.
Cenquizca L. A., Swanson L. W. (2007). Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews 56: 1-26.
Craik F, Jennings J, (1992). Human memory. In The handbook of aging and cognition. (Ed. Craik S. T., ) Lawrence Erlbaum Associates, Inc., Hillsdale, NJ, England.
Dalton M. A., McCormick C., De Luca F., Clark I. A., Maguire E. A. (2019). Functional connectivity along the anterior–posterior axis of hippocampal subfields in the ageing human brain. Hippocampus 29: 1049-1062.
Damoiseaux J. S., Viviano R. P., Yuan P., Raz N. (2016). Differential effect of age on posterior and anterior hippocampal functional connectivity. NeuroImage 133: 468-476.
de Dieuleveult A. L., Siemonsma P. C., van Erp J. B. F., Brouwer A.M. (2017). Effects of Aging in Multisensory Integration: A Systematic Review. Frontiers in Aging Neuroscience 9: 80.
Dringenberg H. C. (2020). The history of long‐term potentiation as a memory mechanism: Controversies, confirmation, and some lessons to remember . Hippocampus 30: 987-1012.
Dubovyk V., Manahan-Vaughan D. (2018). Less means more: The magnitude of synaptic plasticity along the hippocampal dorso-ventral axis is inversely related to the expression levels of plasticity-related neurotransmitter receptors. Hippocampus 28: 136-150.
Fenton A. A., Muller R. U. (1998). Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proceedings of the National Academy of Sciences 95: 3182-3187.
Foster T. C., (2002). Regulation of synaptic plasticity in memory and memory decline with aging. In Plasticity in the Adult Brain: From Genes to Neurotherapy. Elsevier.
Foster T. C., DeFazio R. A., Bizon J. L. (2012). Characterizing cognitive aging of spatial and contextual memory in animal models. Frontiers in Aging Neuroscience 4: 12.
Gray D.T., Barnes C.A. (2015). Distinguishing adaptive plasticity from vulnerability in the aging hippocampus. Neuroscience 309: 17-28.
Gulyaeva N. V. (2018). Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochemical Research 44: 1306-1322.
Hampson R. E., Simeral J. D., Deadwyler S. A. (1999). Distribution of spatial and nonspatial information in dorsal hippocampus. Nature 402: 610-614.
Hoekzema E., Rojas S., Herance R., Pareto D., Abad S., Jiménez X., Figueiras F. P., Popota F., Ruiz A., Flotats N., Fernández F. J., Rocha M., Rovira M., Víctor V. M., Gispert J. D. (2012). In vivo molecular imaging of the GABA/benzodiazepine receptor complex in the aged rat brain. Neurobiology of Aging 33: 1457-1465.
Jackman S. L., Regehr W. G. (2017). The Mechanisms and Functions of Synaptic Facilitation. Neuron 94: 447-464.
Johnston D., Brown T. H. (1984). The synaptic nature of the paroxysmal depolarizing shift in hippocampal neurons. Annals of Neurology 16: S65-S71.
Kausler D., (1994). Learning and memory in normal aging. Academic Press.
Kheirbek M. A., Drew L. J., Burghardt N. S., Costantini D. O., Tannenholz L., Ahmari S. E., Zeng H., Fenton A. A., Hen R. (2013). Differential Control of Learning and Anxiety along the Dorsoventral Axis of the Dentate Gyrus. Neuron 77: 955-968.
Koutsoumpa A., Papatheodoropoulos C. (2019). Short-term dynamics of input and output of CA1 network greatly differ between the dorsal and ventral rat hippocampus. BMC Neuroscience 20: 35.
Koutsoumpa A., Papatheodoropoulos C. (2021). Frequency‐dependent layer‐specific differences in short‐term synaptic plasticity in the dorsal and ventral CA1 hippocampal field. Synapse 75: e22199.
Kouvaros S., Kotzadimitriou D., Papatheodoropoulos C. (2015). Hippocampal sharp waves and ripples: Effects of aging and modulation by NMDA receptors and L-type Ca2+ channels. Neuroscience 298: 26-41.
Kouvaros S., Papatheodoropoulos C. (2016). Major dorsoventral differences in the modulation of the local CA1 hippocampal network by NMDA, mGlu5, adenosine A2A and cannabinoid CB1 receptors. Neuroscience 317: 47-64.
Kumar A. (2020). Calcium Signaling During Brain Aging and Its Influence on the Hippocampal Synaptic Plasticity. In Calcium Signaling. (Ed. Islam Md. Shahidul) Springer International Publishing, Cham.
Landfield P. W., Lynch G. (1977). Impaired Monosynaptic Potentiation in in Vitro Hippocampal Slices From Aged, Memory-deficient Rats. Journal of Gerontology 32: 523-533.
Landfield P. W., McGaugh J. L., Lynch G. (1978). Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Research 150: 85-101.
Landfield P. W., Pitler T. A., Applegate M. D. (1986). The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. Journal of Neurophysiology 56: 797-811.
Leung L. S., Fu X.W. (1994). Factors affecting paired-pulse facilitation in hippocampal CA1 neurons in vitro. Brain Research 650: 75-84.
Margineanu D. G., Wülfert E., (2000). Differential paired-pulse effects of gabazine and bicuculline in rat hippocampal CA3 area. Brain research bulletin 51: 69-74.
Martin S.J., Morris R.G.M. (2002). New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus 12: 609-636.
Michelson H. B., Kapur J., Lothman E. W. (1989). Reduction of paired pulse inhibition in the CA1 region of the hippocampus by pilocarpine in naive and in amygdala-kindled rats. Experimental Neurology 104: 264-271.
Milior G., Di Castro M. A., Sciarria L. P., Garofalo S., Branchi I., Ragozzino D., Limatola C., Maggi L. (2016). Electrophysiological Properties of CA1 Pyramidal Neurons along the Longitudinal Axis of the Mouse Hippocampus. Scientific Reports 6: 38242.
Moser E. I., Moser M.B., McNaughton B. L. (2017). Spatial representation in the hippocampal formation: a history. Nature Neuroscience 20: 1448-1464.
Nicoll R. A., Roche K. W. (2013). Long-term potentiation: Peeling the onion. Neuropharmacology 74: 18-22.
Ouanounou A., Zhang L., Charlton M. P., Carlen P. L. (1999). Differential Modulation of Synaptic Transmission by Calcium Chelators in Young and Aged Hippocampal CA1 Neurons: Evidence for Altered Calcium Homeostasis in Aging. The Journal of Neuroscience 19: 906-915.
Papaleonidopoulos V., Trompoukis G., Koutsoumpa A., Papatheodoropoulos C. (2017). A gradient of frequency-dependent synaptic properties along the longitudinal hippocampal axis. BMC Neuroscience 18: 79.
Papatheodoropoulos C. (2015). Striking differences in synaptic facilitation along the dorsoventral axis of the hippocampus. Neuroscience 301: 454-470.
Papatheodoropoulos C., Asprodini E., Nikita I., Koutsona C., Kostopoulos G. (2002). Weaker synaptic inhibition in CA1 region of ventral compared to dorsal rat hippocampal slices. Brain Research 948: 117-121.
Papatheodoropoulos C., Kostopoulos G. (1996). Age-related Changes in Excitability and Recurrent Inhibition in the Rat CA1 Hippocampal Region. European Journal of Neuroscience 8: 510-520.
Papatheodoropoulos C., Kostopoulos G., (2000). Decreased ability of rat temporal hippocampal CA1 region to produce long-term potentiation.. Neuroscience letters 279: 177-180.
Potier B., Jouvenceau A., Epelbaum J., Dutar P. (2006). Age-related alterations of GABAergic input to CA1 Pyramidal neurons and its control by nicotinic acetylcholine receptors in rat hippocampus. Neuroscience 142: 187-201.
Ramsey M.M., Weiner J.L., Moore T.P., Carter C.S., Sonntag W.E. (2004). Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience 129: 119-127.
Ranck J. B. (1973). Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. Experimental Neurology 41: 462-531.
Rapp P. R., Heindel W. C. (1994). Memory systems in normal and pathological aging. Current Opinion in Neurology 7: 294-298.
Regehr W. G. (2012). Short-Term Presynaptic Plasticity. Cold Spring Harbor Perspectives in Biology 4: a005702-a005702.
Reichel J.M., Bedenk B.T., Czisch M., Wotjak C.T. (2017). Age-related cognitive decline coincides with accelerated volume loss of the dorsal but not ventral hippocampus in mice. Hippocampus 27: 28-35.
Rissman R. A., Nocera R., Fuller L. M., Kordower J. H., Armstrong D. M. (2006). Age-related alterations in GABAA receptor subunits in the nonhuman primate hippocampus. Brain Research 1073-1074: 120-130.
Salami A., Wåhlin A., Kaboodvand N., Lundquist A., Nyberg L. (2016). Longitudinal Evidence for Dissociation of Anterior and Posterior MTL Resting-State Connectivity in Aging: Links to Perfusion and Memory. Cerebral Cortex 26: 3953-3963.
Salthouse T. A., Mitchell D. R., Skovronek E., Babcock R. L. (1989). Effects of adult age and working memory on reasoning and spatial abilities.. Journal of Experimental Psychology: Learning, Memory, and Cognition 15: 507-516.
Samson R. D., Barnes C. A. (2013). Impact of aging brain circuits on cognition. European Journal of Neuroscience 37: 1903-1915.
Small S. A. (2001). Age-Related Memory Decline. Archives of Neurology 58: 360-364.
Stark S. M., Frithsen A., Stark C., (2021). Age-related alterations in functional connectivity along the longitudinal axis of the hippocampus and its subfields. Hippocampus 31: 11-27.
Strange B. A., Witter M. P., Lein E. S., Moser E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15: 655-669.
Tannenholz L., Jimenez J. C., Kheirbek M. A. (2014). Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Frontiers in Behavioral Neuroscience 8: 147.
Thibault O., Hadley R., Landfield P. W. (2001). Elevated Postsynaptic [Ca 2+ ] i and L-Type Calcium Channel Activity in Aged Hippocampal Neurons: Relationship to Impaired Synaptic Plasticity . The Journal of Neuroscience 21: 9744-9756.
Villanueva-Castillo C., Tecuatl C., Herrera-López G., Galván E. J. (2017). Aging-related impairments of hippocampal mossy fibers synapses on CA3 pyramidal cells. Neurobiology of Aging 49: 119-137.
Wong R. K., Prince D. A. (1981). Afterpotential generation in hippocampal pyramidal cells.. Journal of Neurophysiology 45: 86-97.
Yang H., Xu-Friedman M. A. (2015). Skipped-Stimulus Approach Reveals That Short-Term Plasticity Dominates Synaptic Strength during Ongoing Activity. Journal of Neuroscience 35: 8297-8307.
Yassa M. A., Mattfeld A. T., Stark S. M., Stark C. E. L. (2011). Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences 108: 8873-8878.
Zucker R. S., Regehr W. G. (2002). Short-Term Synaptic Plasticity. Annual Review of Physiology 64: 355-405.