Int. J. Dev. Biol. 67: 57 - 63 (2023)
Characterization of the developing axolotl nasal cavity supports multiple evolution of the vertebrate choana
Open Access | Original Article | Published: 1 August 2023
Abstract
All tetrapods (mammals, birds, reptiles, and amphibians) share the ability to breathe with their mouths closed due to the formation of choanae, which are openings that allow communication between the nasal and oral cavities. In most fishes, the nasal cavities serve a strictly olfactory function, possessing incurrent and excurrent nares that lie outside of the mouth and therefore, never communicate with the respiratory system. It is not until the evolution of tetrapods, in which the nasal cavities consistently open into the mouth, that they are used both for olfaction and for respiration. However, this developmental transition is poorly understood, with no consensus on the evolutionary origin of the choana in various groups despite decades of debate. Here, we use high-contrast 3D imaging in conjunction with histology and apoptotic cell analysis in non-mineralized embryonic tissues to study the formation of the choana in the axolotl (Ambystoma mexicanum), an aquatic salamander species. We show that the axolotl choana forms from an extension of the embryonic nasal sac, which pushes through intervening mesenchyme and connects with the palate epithelium of the oral cavity, eventually breaking through. This mechanism differs from caecilians, mammals and reptiles, where fusion across a bucconasal groove plays an active role in choana formation. Nevertheless, caecilians, mammals and axolotls converge on the development of a transient epithelial tissue that has to break down in order to develop a patent choana, adding another twist to the intriguing arguments on the evolutionary history of the choana.
Keywords
Ambystoma, axolotl embryo, choana, craniofacial development, nasal cavity, oral cavity, bucconasal groove
Introduction
The ability to breathe through the nose is due to a significant milestone in the evolution of the tetrapod respiratory system: the communication between the nasal and oral cavities through choanae. The choana is one of the first components of the tetrapod body plan to evolve. First occurring in extinct fish relatives of the tetrapods at least 380 million years ago (30 million years before limb development), it is crucial to the understanding of the fish–tetrapod transition (Janvier, 2004; Zhu and Ahlberg, 2004) and is considered a “preadaptation” to terrestriality (Triques and Christoffersen, 2018). Notwithstanding its importance in tetrapod life history, there is still no consensus on the developmental or evolutionary origin of the choana despite more than a century of debate (Bertmar, 1969; Hinsberg, 1901; Hinsberg, 1902; Janvier, 2004; Panchen and Smithson, 1987; Rosen et al., 1981).
In most fishes, the nasal cavity contains an incurrent, anterior nostril and excurrent, posterior nostril, both of which are external to the oral cavity and allow for olfaction but no communication with the respiratory system (Bertmar, 1969; Pashchenko and Kasumyan, 2017; Zeiske et al., 2009). There are representatives both in Actinopterygii (ray-finned fishes) and Chondrichthyes (sharks, skates, rays, and chimaeras) where the excurrent nostril opens into the oral cavity (Allis, 1917; Allis, 1932; Atz, 1952a; Atz, 1952b; Bertmar, 1968). However, fish choanae are the exception and not the rule, being largely used by inactive, bottom-dwelling species to improve water circulation over the sensory cells of the nasal cavities during olfaction (Jankowski, 2013; Parsons, 1971). One group that has elicited particular scrutiny on the topic is the lungfishes, which are closer relatives to tetrapods and possess nostrils that open into the mouth. However, homology between lungfish and tetrapod choanae has been rejected due to major differences in their relationships to the surrounding nerves, bones and sensory line canals (Arnason et al., 2004; Janvier, 2004). Therefore, the tetrapod choana is understood to be a homoplastic structure relative to those in the aforementioned fish lineages.
Extant tetrapods are categorized into two groups: amniotes (mammals, birds, and reptiles) and anamniotes (amphibians), which differ in several anatomical characteristics, including the embryonic developmental of their craniofacial regions. Amniotes develop craniofacial prominences that grow out and fuse around bucconasal grooves running between the nasal and oral cavities, simultaneously forming an intact upper jaw, as well as their choanae (Jiang et al., 2006). Amphibians, on the other hand, show more diversity. Caecilians (Order: Gymnophiona) utilize a similar mechanism to amniotes where nasal and oral cavities communicate through bucconasal grooves, which then serve as the foundation for the choana as facial prominences fuse around them to complete the process (Brauer, 1899; Hinsberg, 1902). The majority of frogs and salamanders, on the other hand, develop disconnected oral and nasal cavities that eventually communicate through choanae without the involvement of bucconasal grooves (Schneider, 1935; Tsui and Pan, 1946). Regardless, all tetrapods eventually converge upon a final configuration of separate nasal and oral cavities that communicate with each other through open choanae.
Here, we present a study of the formation of the nasal cavity and choana in the Mexican axolotl (Ambystoma mexicanum), a commonly studied salamander species used as a research model in laboratories across the globe (Voss et al., 2009). Using X-ray micro computed tomography (microCT) as well as histology and fluorescence microscopy for the detection of cellular apoptosis, we show that the axolotl initiates nasal cavity and choana formation in a similar manner to what has previously been described for other urodelans (salamanders and newts) and anurans (frogs and toads). The choana in the axolotl forms from an epithelial extension of the posterior nasal sac, which pushes through the intervening mesenchyme and connects with the palate epithelium; eventually thinning and breaking up without the death of the constituent cells. While the developmental mechanism differs from caecilians, mammals and crocodilians, the axolotl does go through a stage where epithelial tissue obstructing the putative choana has to break down in order to facilitate communication between the nasal and oral cavities.
Results
3D analysis of developing nasal and oral cavities
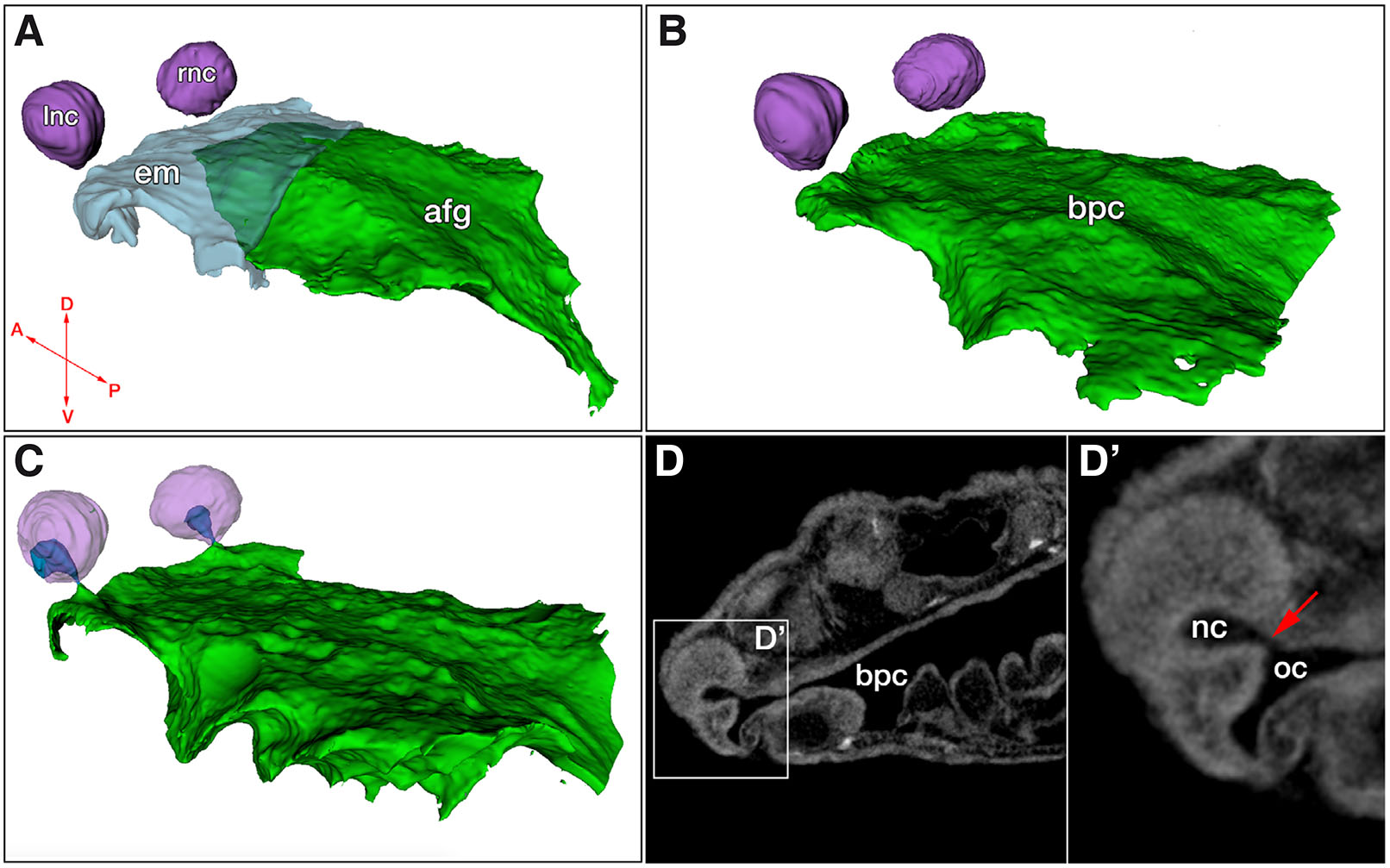
To examine the relationship of the developing nasal cavities to the oral cavity in 3-dimensional space, we used microCT scans of embryos in developmental stages 39 – 47. Deepening nasal pits eventually form rounded sacs disjointed from the developing oral cavity and from each other (Fig. 1A, purple). The oral cavity, on the other hand, forms from the anterior foregut, with a hollow buccopharyngeal segment that is continuous with the gut (Fig. 1, green) and an anterior segment that is filled with foregut endoderm-derived epithelial cells (Fig. 1A, light blue); a developmental phenomenon previously described by Takahama et al., (1988) in Hynobius tokyoensis and Soukup et al., (2008) in the axolotl. As embryonic growth progresses and oral endoderm mass breaks down, the hollow buccopharyngeal segment expands to form an entirely open oral cavity that lies directly beneath the developing nasal sacs (Fig. 1B). The growing nasal sacs ultimately approach the oral cavity and their epithelia make contact (Fig. 1C), eventually forming a communication between them through patent choanae (Fig. 1C - dark blue, D,D’).
Fig. 1. X-ray micro computed tomography (MicroCT) analysis reveals independent development and subsequent connection of the oral and nasal cavities of the axolotl.
(A) Segmentation of a stage 39 embryo showing left and right nasal cavities, as well as the endodermal mass in the oral cavity (light blue) and the open segment of the anterior foregut (green). (B) Stage 43 embryo showing loss of endodermal mass in the oral cavity and expansion of the open buccopharyngeal cavity underneath the nasal cavities. (C) Stage 44 embryo showing contact between the oral and nasal cavities (dark blue). (D,D’) Virtual section from stage 44 embryo showing open choana (red arrow). Abbreviations: afg, anterior foregut; bpc, buccopharyngeal cavity; em, endodermal mass of oral cavity; lnc, left nasal cavity; nc, nasal cavity; oc, oral cavity; rnc, right nasal cavity. Arrows in (A): A, anterior; P, posterior; D, dorsal; V, ventral.
Histological analysis of developmental progression in the oronasal region
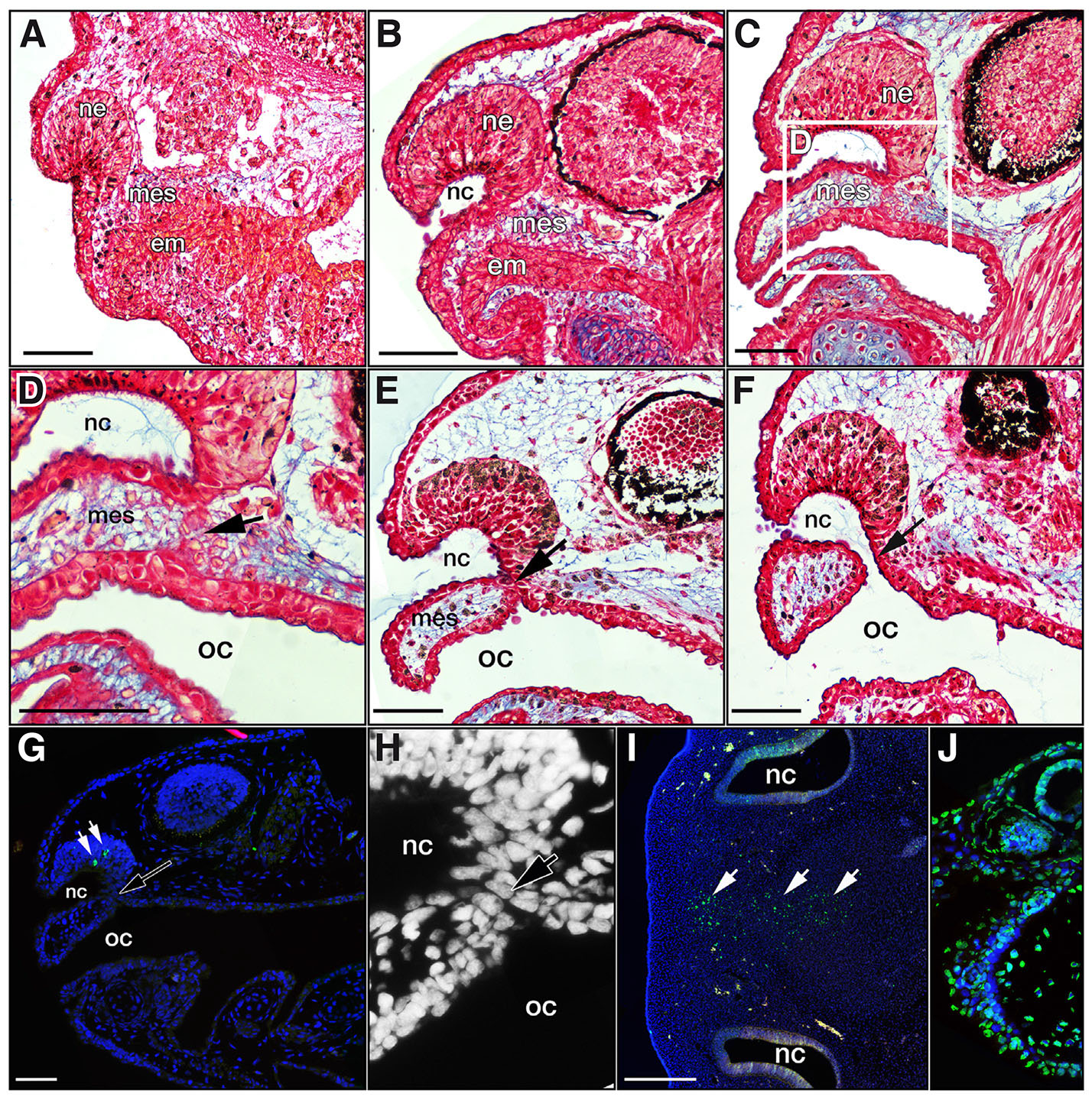
In order to gain a better understanding of the soft-tissue dynamics underlying the microCT observations, we decided to study histological sections from comparable embryonic stages. At stages 39 and 40, the nasal pit is distinct and nasal epithelium progressively thickens in the posterior direction (Fig. 2 A,B). During this period, the nasal sac remains physically separated from the putative oral cavity with a discrete, intervening mesenchymal tissue layer in between (Fig. 2 A,B). Around stages 40-41, the nasal and oral epithelia begin to approach one another at the future site of the choana, despite the oral cavity still being filled with endodermal tissue (Fig. 2B). At Stage 42, epithelial tissue from both the oral and nasal cavities begins to infiltrate the region and make contact with each other (Fig. 2 C,D). The growth of epithelia towards each other, along with the displacement of intervening mesenchyme, eventually leads to the formation of an epithelial bridge between the oral and nasal cavities, demarcating the putative choana (Fig. 2 E,H). This tissue is reminiscent of the mammalian oronasal membrane (also known as the bucconasal membrane or lamina oronasalis). Around stage 44-45, this tissue breaks down, creating an open communication between the nasal and oral cavities (Fig. 2F).
Fig. 2. Histological analysis delineating the development of the choana.
Sagittal sections of axolotl embryos. (A,B) Stage 39 and stage 40 embryos respectively, showing early and later stages of endodermal mass in the oral cavity, visibly separated by mesenchyme from the nasal epithelium. (C,D) Stage 42 embryo where the endodermal mass has cleared, leaving a patent oral cavity. Epithelia from the nasal and oral cavities have begun to cross the intervening mesenchyme and mix together. (E) Stage 43, mesenchyme has been completely displaced by an epithelial bridge between the oral and nasal cavities (black arrow). (F) At Stage 44-45, the epithelial bridge has broken down and the choana is completely open (black arrow). (G) Representative image of TUNEL assay on Stage 43 embryo showing TUNEL-positive cells (in green; white arrows) in the nasal epithelium, but none in the thinning oronasal membrane. (H) Close-up view of the developing choana region from panel (G) in DAPI channel only, revealing details of the very few, large cells that make up the epithelial membrane in the axolotl (black arrow). (I) Transverse section of stage 29 chicken embryo showing TUNEL-positive apoptotic cells in the frontonasal mass (white arrows). (J) Axolotl section showing strong TUNEL signal after DNase treatment, intended as as a second positive control for TUNEL analysis. Abbreviations: em, endodermal mass of oral cavity; ne, nasal epithelium; nc, nasal cavity; mes, mesenchyme; oc, oral cavity. Scale bars indicate 100μm.
Perforation of the choana
Since the mammalian oronasal membrane is understood to break down through apoptosis (Weingaertner et al., 2006), we decided to check for the presence of apoptotic cells during choana opening in the axolotl. TUNEL analysis did not detect any apoptotic cells in the choana region of sagittal sectioned embryos at any of the stages of development examined (stages 41 – 44), suggesting that cell death was not involved in the breakdown of the oronasal membrane (Fig. 2G). In general, very few apoptotic cells were detected in the axolotl embryos as can be observed in Fig. 2G. As a positive control, transverse sections of stage 29 chicken embryos were placed on the same slides as experimental samples and exhibited TUNEL-positive apoptotic cells in the center of the frontonasal mass (Fig. 2I), a region previously reported to be TUNEL-positive by Abramyan et al., (2015). As an additional positive control, an axolotl section on the same slide was treated with DNase I and exhibited strong TUNEL signal (Fig. 2J).
In X. laevis, Dickinson and Sive (2006) describe a “burst” of apoptosis prior to the opening of the oral cavity during buccopharyngeal membrane breakdown, crediting this process with the reduction of cells in the region and not necessarily the perforation of the membrane per se. To test for early apoptosis in the axolotl (prior to the choana breaking through), we treated live embryos with the pan-caspase inhibitor z-VAD-fmk, from stage 39 until stage 43. Of 11 treatment animals and 10 control animals, all exhibited normal development of the oronasal region, including the approach and merger of the epithelia at the site of the putative choana, with no visible deviation in developmental processes when assessed using histological analysis. If axolotls use apoptosis very sparingly, z-VAD-fmk should have no effect, as we found to be the case. This experiment indicates that caspase-mediated apoptosis is not responsible for the formation or eventual perforation of the developing choana in the axolotl. Unfortunately, since there are very few TUNEL-positive cells in axolotl embryos in generally, we were unable to quantify the efficacy of z-VAD-fmk in reducing apoptosis in the axolotl.
Discussion
Here, we analyzed the development of the choana in the axolotl, an enigmatic salamander species that has been studied as a research model by generations of scientists. The homology of the vertebrate choana has been a topic of debate among scientists for decades (Arnason et al., 2004; Bertmar, 1966; Bertmar, 1969; Janvier, 2004; Parsons, 1971; Rosen et al., 1981). These debates largely revolve around the fact that in some groups the primitive nasal and oral cavities communicate through bucconasal grooves which later form choanae within the ectodermal region, whereas in others, choanae form de novo, opening directly into the oral endoderm from the nasal sac. As described in this study, the axolotl falls into the latter category. There were no bucconasal grooves or fusion observed in the axolotl, with the developmental mechanism largely following what has historically been described for anurans and urodelans (Hinsberg, 1901; Schneider, 1935; Tsui, 1946; Tsui and Pan, 1946).
However, this mode of development is not shared by all amphibians, as caecilians have long been known to develop a bucconasal groove with medial and lateral processes that fuse across the groove to develop choanae (Brauer, 1899; Hinsberg, 1902), similar to what has been described for amniotes (Abramyan et al., 2015); apparently leaving the anurans and urodelans as the odd group within tetrapods with a derived developmental mechanism of choana formation (Fig. 3). In fact, the phylogenetic distribution of the bucconasal groove has historically led researchers to propose that this structure was likely found in the ancestor to all tetrapods but was subsequently either lost or reduced in frogs and salamanders (Allis, 1932). While most authors agree that bucconasal grooves are restricted to amniotes and caecilians (Hinsberg, 1901; Medvedeva, 1961; Schneider, 1935; Tsui, 1946; Tsui and Pan, 1946), Bertmar (1966) describes the formation of choanae in Hynobius retardatus as involving postnasal grooves, which he proclaims as being remnants of the original tetrapodan bucconasal groove. He further states that he has also observed such postnasal grooves in Hynobius nebulosus and the Cryptobranchus japonicas (Japanese giant salamander - Andrias japonicus). Interestingly, Bertmar’s observations are supported by earlier, independent observation of similar grooves by Watanabe (1936) in Onychodactylus japonicus and Hynobius lichenatus as well as Kawagoe (1932) in the Japanese giant salamander. It is possible that vestigial bucconasal grooves remain in members of the Cryptobranchoidea clade (otherwise known as primitive salamanders), as all of the abovementioned species belong to this group. Axolotls, on the other hand, belong to Salamandroidea, which are known as the advanced salamanders. Using 3D segmentation and histology, we show that in the axolotl, the communication between the nasal and oral cavities is initiated deep in the nasal sac, away from any superficial postnasal groove that would have developed. In this, our results differ significantly from the description of the superficial choana formation in Hynobius retardatus by Bertmar (1966).
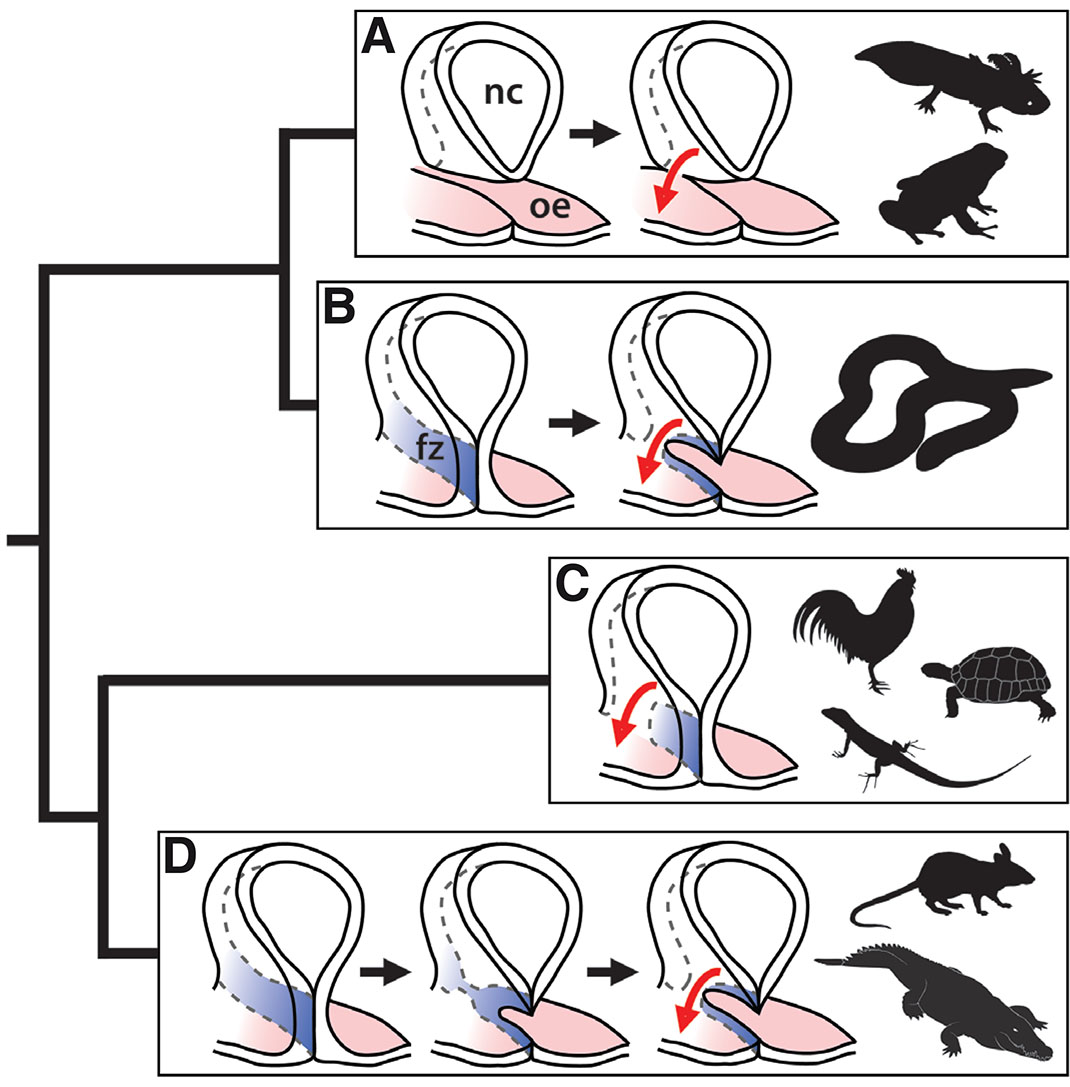
Fig. 3. Tetrapod phylogeny showing the distribution of embryonic choana development mechanisms.
(A) Frogs and salamanders develop isolated nasal cavities that eventually connect with the oral epithelium, leading to the formation of an epithelial membrane that breaks to form the choana. (B) Caecilians develop medial and lateral processes that fuse across a bucconasal groove, temporarily obstructing the choana with fused tissue (blue), which later breaks down to develop a patent choana (as described by Hinsberg, 1902). (C) In birds, turtles and squamates, craniofacial prominences fuse across the bucconasal groove, but leave an open choana posterior to the fusion zone. (D) Crocodilians and mammals develop medial and lateral processes that fuse across a bucconasal groove, forming a nasal fin that physically separates the nasal and oral cavities. As development progresses, the posterior nasal fin thins to form an oronasal membrane that eventually breaks, forming a patent choana. fz, fusion zone; nc, nasal cavity; oe, oral ectoderm. Illustration modified from (Kurosaka, 2019); silhouette images from www.phylopic.org
A second trait that sporadically appears across the tetrapod phylogeny is the formation of a transient epithelial tissue covering the nascent choana. In amphibians, Hinsberg (1902) described the caecilian choana as first closing and then reopening. Similarly, in mammals and crocodilians, the prominences fuse along their entire length, forming an epithelial seam between them called the nasal fin that completely closes the putative choana (Abramyan et al., 2015; Jiang et al., 2006). Later, the posterior segment of the nasal fin is reduced in thickness, forming an oronasal membrane that eventually breaks and creates a patent choana (Fig. 3) (Gaare and Langman, 1977; Hasso and Kim-Miller, 2006; Piotrowski et al., 2011; Rudé et al., 1994). In birds, turtles, and squamates, on the other hand, there is no formation of a nasal fin or oronasal membrane. Instead, the craniofacial prominences grow in a U-shape around the bucconasal groove (Abramyan et al., 2015). This facilitates formation of an open choana from the outset, allowing communications between the nose and mouth throughout development (Abramyan and Richman, 2015).
In studying the rat oronasal membrane, Weingaertner et al., (2006) found that apoptosis was responsible for its breakdown during the formation of a patent choana. However, we did not detect apoptosis during choana formation in the axolotl, suggesting a different mechanism of tissue breakdown is at play. One possible mechanism could involve the growth of the embryo causing enough tension to physically pull apart the cells in the axolotl choana (Miller et al., 1993; Watanabe et al., 1984), while another possibility may be the molecular loss of cell adhesion factors, leading to dissociation (Waterman and Balian, 1980). A detail to note of the epithelial membranes found in mammal, crocodilians, and caecilians, is that it arise due to the fusion of free epithelia, while in the axolotl, the oronasal membrane arises through the fusion of the basal surfaces of nasal and oral epithelia, in a different process than the aforementioned groups. This may account for the differences in the mechanism of breakdown as well.
In conclusion, the results we present in this manuscript describe a mechanism of nasal cavity and choana formation in axolotl which is distinct from what has been described in most other tetrapods, even including some salamanders. Without dismissing the historical observations of remnant bucconasal grooves in caecilians and cryptobranchoid salamanders, and assuming bucconasal grooves are indeed ancestral to all tetrapods, a clearer picture begins to emerge that places the configuration of the nasal sacs, the oral cavity, and the choanae described in this study, into context. Bertmar (1966) describes Hynobius retardatus as developing its choanae from three portions: a vestigial bucconasal groove, an epithelial process extending from the nasal sac, and an epithelial process extending from the oral cavity. In the axolotl, we show here that only the latter two processes are involved, having dispensed with any involvement of a bucconasal groove in the formation of the choana. Therefore, we propose two alternative scenarios for the evolution of the urodelan choana: (a) a stepwise progression where the nasal and oral epithelial processes evolved in stem salamander and worked with the existing bucconasal groove to form the choana. This mechanism is then retained in cryptobranchoid salamanders, while advanced salamanders such as axolotl only utilize the epithelial processes, allowing the bucconasal groove to be lost; (b) the enlargement and/or repositioning of the nasal cavities in axolotl and related species eventually perforates the roof of the mouth, forming a new connection with the oral cavity in the posterior nasal sac independent of the bucconasal groove. Such a mechanism has been previously proposed by Bertmar (1969) and Panchen (1967) for other instances of de novo choana evolution such as in the fish genus Astroscopus. This would eliminate the need for bucconasal grooves, eventually allowing them to degenerate or be lost. Thus, a “new” choana would form that is independent of the bucconasal groove-related structure in tetrapods.
Materials and Methods
Axolotl embryos
Ambystoma mexicanum embryos were obtained from the Ambystoma Genetic Stock Center (RRID:SCR_006372) and reared in spring water at 18°C. The use of pre-feeding larvae does not require a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan. However, studies adhered to the compliance standards of the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals. Embryo stages 43 and under were staged according to Bordzilovskaya and Dettlaff (1979). Embryos stages 44 and over were staged according to Atkins et al., (2020).
MicroCT scanning and segmentation
Samples were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) overnight and then transferred to 70% ethanol through a graded ethanol series. Samples were then stained with 0.3% phosphotungstic acid (Sigma-Aldrich) in 70% ethanol overnight at room temperature on a low speed shaker and then embedded in 0.5% agarose inside of a 0.2 ml PCR tube. For scanning, specimens were placed in a 9 mm diameter specimen holder and scanned using a µCT100 system (Scanco Medical, Bassersdorf, Switzerland). Scan settings: voxel size 3.3 µm, 70 kVp, 85 µA, 0.5 mm AL filter, 1500 projections and integration time 1000 ms. Scans were analyzed using open-source software. MicroView (Parallax Innovations Inc., Ilderton, ON, Canada) was used for reorientation and cropping, while 3D Slicer version 4.2.2 (http://www.slicer.org) was used for segmentation by hand. Figures were compiled in Adobe Photoshop CS6 and Adobe Illustrator CS6 (Adobe Systems Inc., San Jose, CA). The following stages and numbers were scanned and segmented: Stage 39 (n=2), Stage 41 (n=4), Stage 42 (n=4), Stage 43 (n=4), Stage 44 (n= 4), Stage 45 (n=4), Stage 46 (n=2), Stage 47 (n=2).
Histology
Samples were fixed and transferred to 70% ethanol as described for microCT, embedded in paraffin, sectioned at 7 μm thickness, and mounted on slides. Sections were stained with picrosirius red to highlight skeletal and soft tissue and alcian blue to highlight cartilage (Buchtová et al., 2007). Bright-field images were captured using a Nikon Eclipse E800 microscope with a CoolSNAP-EZ CCD camera (Photometrics, Tucson, AZ) and NIS-Elements BR v. 4.12.01 software (Nikon, Melville, NY). Different samples were used for histology and microCT. The following stages and numbers were sectioned: Stage 39 (n=3), Stage 40 (n=4), Stage 42 (n=4), Stage 43 (n=4), Stage 44-45 (n= 4).
Apoptosis detection
Unstained histological sections were also utilized for TUNEL (terminal deoxynucleotide transferase dUTP nick end labeling) assay to detect apoptotic cells using the ApopTag Fluorescein In situ Apoptosis Detection Kit (EMD Millipore - S7110) according to manufacturer’s instructions. Specimens were cover-slipped using Vectashield mounting medium for fluorescence with DAPI (Vector Laboratories). Fluorescence images were captured using a Nikon Eclipse Ts2R inverted microscope mounted with a CoolSNAP Dyno monochrome CCD camera (Photometrics) and NIS-Elements BR v. 5.02 software (Nikon). The following stages and numbers were analyzed using TUNEL: Stage 41 (n=3), Stage 42 (n=4), Stage 43 (n=4), Stage 44 (n= 3). Chicken embryo used as a positive control was staged according to Hamburger and Hamilton (1992). DNase treated positive control was circled with a solvent-resistant histology pen and treated with DNase I for 1 minute at RT on the same slide as experimental samples.
Apoptosis inhibition
To block apoptosis during the development of the nasal and oral cavities, the pan-caspase inhibitor, z-vad-fmk (benzyloxycarbonyl Val–Ala–Asp (O-methyl) fluoromethyl ketone) (Sigma - CAS 187389-52-2 – Cat No: 627610-1MG) was used at a concentration of 300 μM (Dickinson and Sive, 2006). Devitellinized embryos (n=11) were treated in a 100ml petri dish filled with 7.13 ml 300 μM solution of z-vad-fmk (dissolved in DMSO) at 18°C beginning at stage 39 until stage 43. Devitellinized control embryos (n=10) were reared in a 100ml petri dish filled with 0.7% solution of DMSO/spring water in 7.13 ml total volume.
Acknowledgements
We would like to thank Michelle Lynch for assistance with microCT scan optimization. We would also like to thank two anonymous reviewers for their suggestions, which substantially improved the manuscript. Research reported in this publication was supported by startup funds for JA, Research Initiation & Development Grant to JA from UM-Dearborn under Award Number U080676, as well as by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30 AR069620. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The University of Michigan School of Dentistry microCT Core is funded in part by NIH/NCRR S10RR026475-01.
References
Abramyan J., Richman J. M. (2015). Recent insights into the morphological diversity in the amniote primary and secondary palates. Developmental Dynamics 244: 1457-1468.
Abramyan J., Thivichon-Prince B., Richman J. M. (2015). Diversity in primary palate ontogeny of amniotes revealed with 3D imaging. Journal of Anatomy 226: 420-433.
Allis E. P. (1917). The Lips and the Nasal Apertures in the Gnathostome Fishes, and Their Homologues in the Higher Vertebrates. Proceedings of the National Academy of Sciences 3: 73-78.
Allis E. P., (1932). Concerning the Nasal Apertures, the Lachrymal Canal and the Bucco-Pharyngeal Upper Lip. Journal of anatomy 66: 650-658.
Arnason U., Gullberg A., Janke A., Joss J., Elmerot C. (2004). Mitogenomic analyses of deep gnathostome divergences: a fish is a fish. Gene 333: 61-70.
Atkins J. B., Houle L., Cantelon A. S., Maddin H. C. (2020). Normal development in Ambystoma mexicanum : A complementary staging table for the skull based on Alizarin red S staining . Developmental Dynamics 249: 656-665.
Atz J. W. (1952a). Internal nares in the teleost, Astroscopus. The Anatomical Record 113: 105-115.
Atz J. W. (1952b). Narial Breathing in Fishes and the Evolution of Internal Nares. The Quarterly Review of Biology 27: 366-377.
Bertmar G. (1966). On the Ontogeny and Homology of the Choanal Tubes and Choanae in Urodela. Acta Zoologica 47: 43-59.
Bertmar G., (1968). Lungfish phylogeny. In Nobel Symposium. , Stockholm.
Bertmar G. (1969). The vertebrate nose, remarks on its structural and functional adaptation and evolution. Evolution 23: 131-152.
Bordzilovskaya N. P., Dettlaff T. A., (1979). Table of stages of the normal development of axolotl embryos. Axolotl Newsletter 7: 2-22.
Brauer A., (1899). Beiträge zur Kenntniss der Entwicklung und Anatomie der Gymnophionen. Zoologische Jahrbucher, Abteilung fur Anatomie und Ontogenie der Tiere 12: 477-508.
Buchtová M., Boughner J. C., Fu K., Diewert V. M., Richman J. M. (2007). Embryonic development of Python sebae – II: Craniofacial microscopic anatomy, cell proliferation and apoptosis. Zoology 110: 231-251.
Dickinson A. J.G., Sive H. (2006). Development of the primary mouth in Xenopus laevis. Developmental Biology 295: 700-713.
Gaare J. D., Langman J. (1977). Fusion of nasal swellings in the mouse embryo: Surface coat and initial contact. American Journal of Anatomy 150: 461-475.
Hamburger V., Hamilton H. L. (1992). A series of normal stages in the development of the chick embryo. Developmental Dynamics 195: 231-272.
Hasso A.N., Kim-Miller M.J. (2006). Imaging of Craniofacial and Sinonasal Anomalies. The Neuroradiology Journal 19: 413-426.
Hinsberg V. (1901). Die entwicklung der nasenhöhle bei amphibien. Archiv für Mikroskopische Anatomie 58: 411-482.
Hinsberg V. (1902). Die Entwicklung der Nasenhöhle bei Amphibien. Archiv für Mikroskopische Anatomie 60: 369-385.
Jankowski R., (2013). The evo-devo origin of the nose, anterior skull base and midface. Springer, Paris.
Janvier P. (2004). Wandering nostrils. Nature 432: 23-24.
Jiang R., Bush J. O., Lidral A. C. (2006). Development of the upper lip: Morphogenetic and molecular mechanisms. Developmental Dynamics 235: 1152-1166.
Kawagoe I. (1932). Über die Entwicklungsgeschichte des Geruchsorgans von Megalobdtrachus hiponicus. Folia Anatomica Japonica 10: 655-720_4.
Kurosaka H. (2019). Choanal atresia and stenosis: Development and diseases of the nasal cavity. WIREs Developmental Biology 8: e336.
Medvedeva I. M., (1961). On the qeustion of the origin of the choanae in urodels. Supplements of the SSSR's academy of science 137: 468-471.
Miller S. A., Favale A. M., Knohl S. J. (1993). Role for differential cell proliferation in perforation and rupture of chick pharyngeal closing plates. The Anatomical Record 237: 408-414.
Panchen A. L. (1967). The nostrils of choanate fishes and early tetrapods. Biological Reviews 42: 374-419.
Panchen A. L., Smithson T. R. (1987). Character diagnosis, fossils and the origin of tetrapods. Biological Reviews 62: 341-436.
Parsons T. S. (1971). Anatomy of Nasal Structures from a Comparative Viewpoint. In Olfaction. (Ed. Beidler Lloyd M.) Springer Berlin Heidelberg, Berlin, Heidelberg.
Pashchenko N. I., Kasumyan A. O. (2017). Development of the olfactory organ in the ontogeny of carps (Cyprinidae). Journal of Ichthyology 57: 136-151.
Piotrowski A., Woźniak W., Bruska M., (2011). Early development of the human palate in stages 16 and 17. Folia Morphol (Warsz) 70: 29-32.
Rosen D. E., Forey P. L., Gardiner B. G., Patterson C., (1981). Lungfishes, tetrapods, paleontology, and plesiomorphy. Bulletin of the AMNH 167: 163-275.
Rudé F. P., Anderson L., Conley D., Gasser R. F. (1994). Three-dimensional reconstructions of the primary palate region in normal human embryos. The Anatomical Record 238: 108-113.
Schneider P. P. (1935). Über die Primitiv-Entwicklung der Nase in der Reihe der Wirbeltiere. Zeitschrift für Anatomie und Entwicklungsgeschichte 104: 61-78.
Soukup V., Epperlein H.H., Horácek I., Cerny R. (2008). Dual epithelial origin of vertebrate oral teeth. Nature 455: 795-798.
Takahama H., Sasaki F., Watanabe K. (1988). Morphological changes in the oral (Buccopharyngeal) membrane in urodelan embryos: Development of the mouth opening. Journal of Morphology 195: 59-69.
Triques M. L., Christoffersen M. L. (2018). Arguments for replacing the concept of preadaptation by exaptation at the origin of terrestriality in Vertebrata. Biological Journal of the Linnean Society 123: 235-246.
Tsui C. (1946). Memoirs: Development of Olfactory Organ in Rana nigromaculata. Journal of Cell Science s2-87: 61-90.
Tsui C., Pan T. (1946). Memoirs: The Development of the Olfactory Organ of Kaloula borealis (Barbour) as compared with that of Rana nigromaculata Hallowell. Journal of Cell Science s2-87: 299-316.
Voss S. R., Epperlein H. H., Tanaka E. M. (2009). Ambystoma mexicanum , the Axolotl: A Versatile Amphibian Model for Regeneration, Development, and Evolution Studies . Cold Spring Harbor Protocols 2009: pdb.emo128.
Watanabe K., Sasaki F., Takahama H. (1984). The ultrastructure of oral (buccopharyngeal) membrane formation and rupture in the anuran embryo. The Anatomical Record 210: 513-524.
Watanabe M. (1936). Über die Entwicklung des Geruchsorgans von Rhacophorus schlegelii. Zeitschrift für Anatomie und Entwicklungsgeschichte 105: 519-561.
Waterman R. E., Balian G. (1980). Indirect immunofluorescent staining of fibronectin associated with the floor of the foregut during formation and rupture of the oral membrane in the chick embryo. The Anatomical Record 198: 619-635.
Weingaertner J., Proff P., Bienengraeber V., Gedrange T., Fanghaenel J., Lotz K. (2006). In vivo study of apoptosis as a creative agent of embryonic development of the primary nasal duct in rats. Journal of Cranio-Maxillofacial Surgery 34: 3-7.
Zeiske E., Bartsch P., Hansen A. (2009). Early Ontogeny of the Olfactory Organ in a Basal Actinopterygian Fish: Polypterus. Brain, Behavior and Evolution 73: 259-272.
Zhu M., Ahlberg P. E. (2004). The origin of the internal nostril of tetrapods. Nature 432: 94-97.