Int. J. Dev. Biol. 66: 269 - 275 (2022)
Special Issue: Developmental Biology in Greece
Keratinocyte differentiation and proteolytic pathways in skin (patho) physiology
Original Article | Published: 26 October 2021
Abstract
The epidermis is a stratified epithelium that forms the barrier between the organism and its environment. It is mainly composed of keratinocytes at various stages of differentiation. The stratum corneum is the outermost layer of the epidermis and is formed of multiple layers of anucleated keratinocytes called corneocytes. We aim to highlight the roles of epidermal differentiation and proteolysis in skin diseases. Skin biopsies isolated from Spink5-/- mice, the established model of Netherton syndrome (NS), and from patients with NS, seborrheic dermatitis (SD) and psoriasis, as well as healthy controls, were analyzed by histology and immunohistochemistry. Our results showed that NS, SD, and psoriasis are all characterized by abnormal epidermal differentiation, manifested by hyperplasia, hyperkeratosis, and parakeratosis. At the molecular level, abnormal differentiation is accompanied by increased expression of involucrin and decreased expression of loricrin in NS and psoriasis. Increased epidermal proteolysis associated with increased kallikrein-related peptidases (KLKs) expression is also observed in both NS and psoriatic epidermis. Furthermore, reduced expression of desmosomal proteins is observed in NS, but increased in psoriasis. Since desmosomal proteins are proteolytic substrates and control keratinocyte differentiation, their altered expression directly links epidermal proteolysis to differentiation. In conclusion, abnormal cellular differentiation and proteolysis are interconnected and underlie the pathology of NS, SD and psoriasis.
Keywords
Epidermis, Keratinocytes, Corneocytes, Desmosomal proteins, Proteolysis
Introduction
The skin is the largest organ of the human body and a stratified epithelium with three distinct layers: the epidermis, dermis, and underlying hypodermis. The epidermis constitutes the physical, chemical, and microbial barrier towards the environment; it controls body temperature and water loss and protects against mechanical insults. It is composed of the following four layers defined from the deeper to the outermost surface layer: stratum basale (SB), stratum spinosum (SS), stratum granulosum (SG) and stratum corneum (SC). Each layer is formed by keratinocytes, the less differentiated keratinocytes are found in SB and the terminally differentiated keratinocytes in the SC, known as “mummified” keratinocytes or corneocytes. An additional layer between the SC and the SG is found in soles and palms, where the skin is thicker, and is known as stratum lucidum. During terminal differentiation, the plasma membrane of keratinocytes is replaced by the cornified cell envelope (CE), which is a complex protein-lipid composite, approximately 70% of which is loricrin, to form the corneocytes, which are held together by special intercellular junctions called corneodesmosomes, multiprotein complexes containing desmoglein 1 (DSG1), desmocollin 1 (DSC1) and corneodesmosin (CDSN) (Simpson et al. 2011; Sotiropoulou et al. 2021).
Terminally differentiated keratinocytes are constantly replenished by differentiating keratinocytes moving from the inner skin layers. Physiological shedding of corneocytes (desquamation) is enabled by tightly regulated proteolysis of (corneo)desmosomal proteins via a proteolytic cascade, in which enzymes from the kallikrein-related peptidase (KLK) family play dominant roles (Borgoño et al. 2007; Ovaere et al. 2009; Sotiropoulou and Pampalakis 2012; Bisyris et al. 2021). Aberrantly elevated epidermal proteolysis, as for example is observed in Netherton syndrome and other skin diseases, results in dissociation of the SC from the SG, leading to severe impairment of the skin barrier, which is linked with abnormal differentiation.
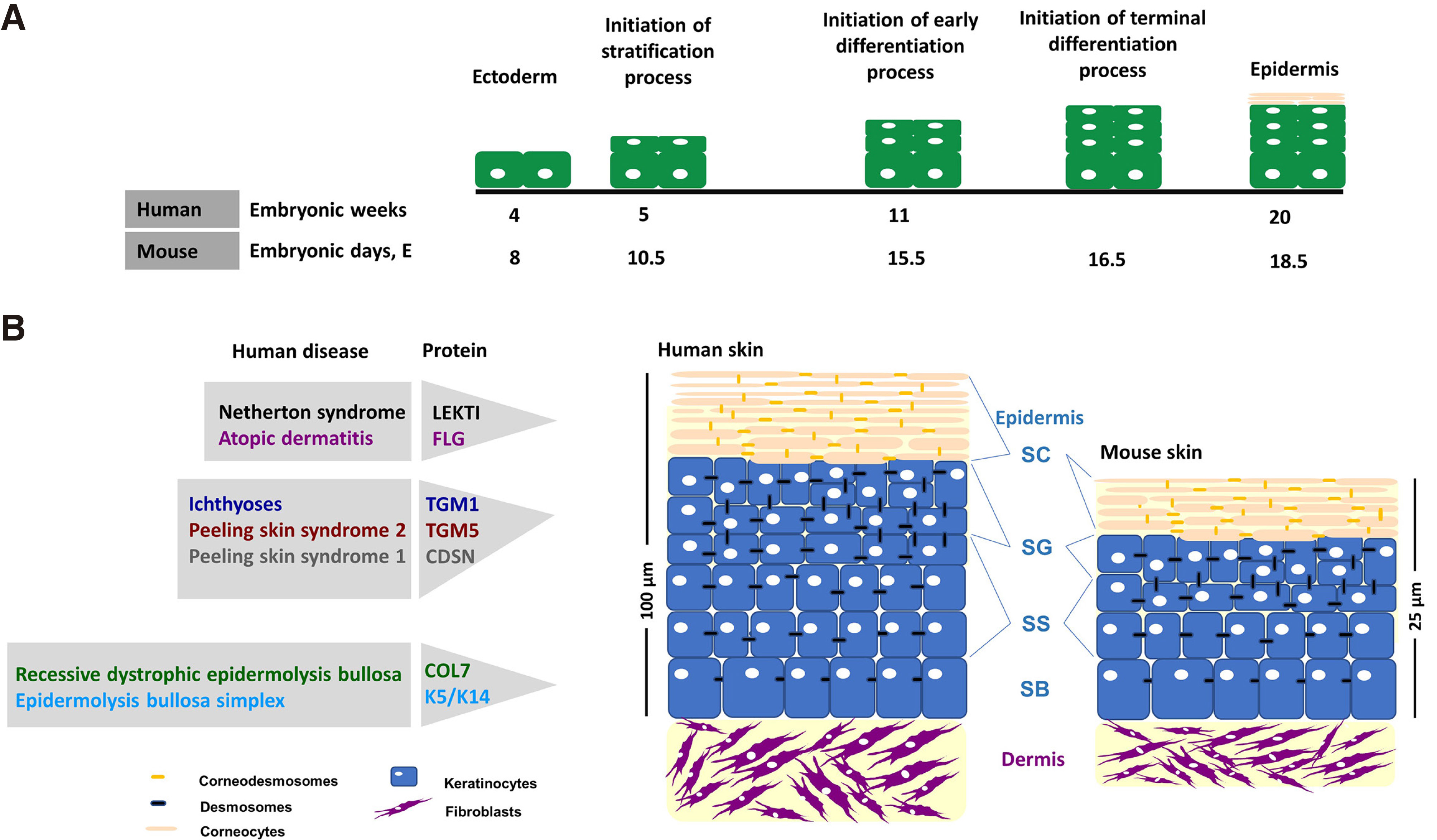
The development of the epidermis in mouse and human embryos is a very similar process, as schematically depicted in Fig. 1. In the implanted human blastocyst, the “epidermis” is first defined on days 7 to 8. At this stage, the primordial epidermis is composed of a single layer of cells and is referred to as indifferent ectoderm. These cells are loosely integrated along basal lamina and form the basal cells. During the first embryonic month, the stratification process is initiated in the epidermis to yield the periderm that will transiently cover the skin until the epidermal differentiation process is complete. During the end of the third month of embryonic development, basal cells will proliferate and generate multiple layers that form the stratum intermedium that will eventually form the SS. Stratum granulosum and stratum corneum form in month six (Holbrock, 1979). In the mouse embryo, the series of events is similar, as depicted in Fig. 1, but significantly faster, and leads to the development of a mature epidermis by day 18.5 (Du et al. 2018).
Fig. 1.
(A) Schematic representation of human and mouse epidermis development. Skin tissue rises from the ectoderm, a single layer of progenitor cells of keratinocytes. At the end of the 4th week of life, the development of human epidermis begins, and by the end of the 5th week, the periderm has been developed lying on the basal layer. Differentiation processes initiate around week 11, and by the 20th week all epidermal layers have been formed. Development of murine epidermis initiates around E8.5 from ectodermal keratinocytes that express keratin 8 and 18. On E10.5, the stratification process initiates and keratinocytes express keratin 5 and 14, and subsequently stratum spinosum is formed. On E15.5, keratinocyte early differentiation initiates to form stratum granulosum. On E16.5, keratinocytes lead to cornification. Finally, by E18.5, stratum corneum is formed. (B) Architecture of human and mouse skin. Human and mouse skin share common histological characteristics consisting of three distinct layers: the epidermis, the dermis underneath, and the hypodermis. A major difference between human and murine skin is the thickness of the tissue: more than 100 μm and less than 25 μm in human and mouse, respectively.
Proper keratinocyte differentiation is essential for normal epidermal development and barrier function, while abnormal differentiation is a feature in various common and rare skin diseases, such as atopic dermatitis, psoriasis, Netherton syndrome, etc. Here, epidermal keratinocyte differentiation is examined in relation to skin inflammatory disorders.
Results and Discussion
Homozygous or compound heterozygous inactivating mutations in the SPINK5 gene encoding the multidomain protease inhibitor LEKTI lead to the rare skin ichthyosis, Netherton syndrome (NS) (OMIM 256500). NS is a devasting, often lethal in neonates, skin syndrome for which there is no specific treatment available. Hallmark clinical manifestations of NS include erythroderma, allergies, generalized skin inflammation, epidermal overdesquamation, and a pathognomonic hair structure, known as bamboo hair. The Spink5-/- mouse model recapitulates NS and is widely used to unravel the molecular mechanisms underlying NS and to aid the development of NS-specific therapeutic approaches. Spink5-/- mice evidence highly compromised integrity of the epidermal barrier as early as embryonic day 18.5 (E18.5), when development of the epidermis is already completed (Descargues et al. 2005).
Epidermal KLKs, mainly KLK5, KLK7 and KLK14, are secreted by keratinocytes as inactive proenzymes (zymogens) in the SG. There, KLK5 can autoactivate and subsequently activate other KLK zymogens. LEKTI is produced by the keratinocytes in the SG as a macromolecular precursor that is proteolytically cleaved by furin to yield the processed fragments with inhibitory effect. Then, the processed LEKTI fragments are secreted in the SG and inhibit the activity of KLKs by forming an enzyme-inhibitor complex. As the KLK-LEKTI complexes diffuse towards the outer skin surface, where the pH is acidic due to the acid mantle, dissociation of LEKTI from the complex takes place and KLKs are spatially activated in the outermost layers of the SC. This allows for controlled corneocyte shedding of only the outer corneocyte layers. In NS, LEKTI deficiency results in unopposed activities of KLKs causing aberrantly enhanced cleavage of the (corneo)desmosomal proteins (DSG1, DSC1, CDSN) at the SG-SC junction leading to severe/pathological epidermal desquamation (Sotiropoulou and Pampalakis 2012).
The Spink5R820X/R820X mouse model used here carries the R820X mutation that introduces a stop codon (Hewett et al. 2005). This mutation mimics the E827X mutation identified in NS patients. Spink5R820XR820X mice do not express Spink5 mRNA probably due to nonsense-mediated decay, therefore, they are homologous knockout, i.e., Spink5-/- (Fig. 2A). Thus, they are referred to as Spink5-/-.
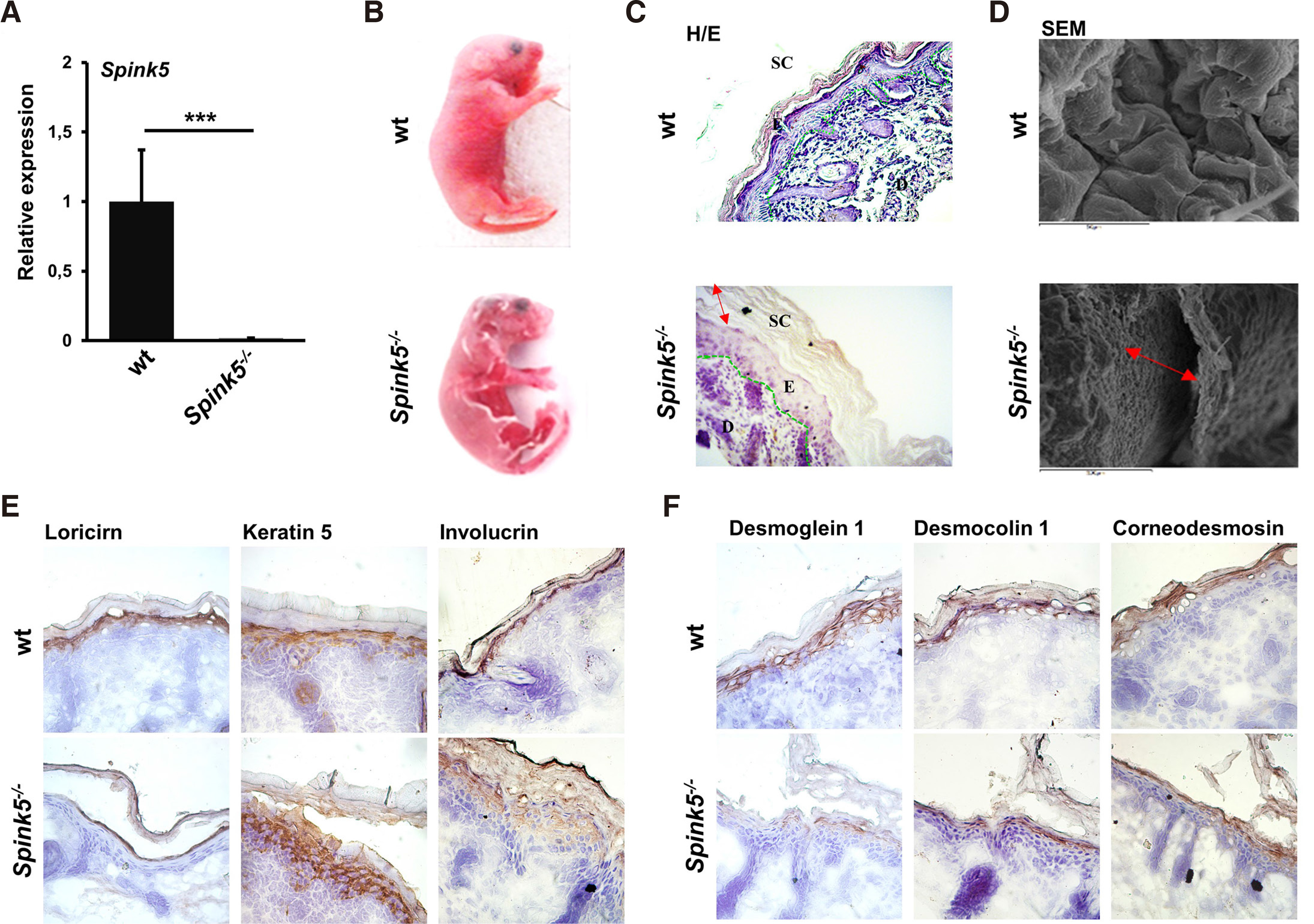
Fig. 2. Skin abnormalities in Spink5-/- mouse model.
(A) The bar graph shows the relative expression of Spink5 quantified by real-time PCR in wild type (wt) and Spink5-/- skin. Spink5 is significantly undetectable in the skin of Spink5-/- mice compared with wt counterparts. The bars represent the mean ± SEM of 4 different mice in each group; *** p ≤ 0.001; expression was normalized using the Hprt1 housekeeping gene. (B) Macroscopic appearance of wt and Spink5-/- mouse model for Netherton syndrome (NS). (C-D) H/E staining and scanning electron microscopy (SEM) of skin from wt and Spink5-/- neonates revealed that skin microstructure is abnormal in Spink5-/- mice. Hyperkeratosis and marked disruption of the SC from the deeper epidermal layers were observed in the Spink5-/- compared with the control. (E) IHC analysis showed changes in expression of differentiation markers including loricrin and involucrin in Spink5-/- mice compared with the wt counterpart. (F) IHC analysis showed that expression of (corneo) desmosomal proteins is reduced in Spink5-/-.
Macroscopically, Spink5-/- mice are easily distinguished from wild type (wt) littermates, on the basis of their generalized overdesquamation, skin redness linked to constitutive skin inflammation, and low body weight, because of dehydration due to a severely defective epidermal barrier, which uniformly leads to neonatal lethality within 5 hours from birth (Fig. 2B). To shed light on the structural basis of these macroscopic changes, H/E staining was performed on skin tissue sections. Histologically, Spink5-/- mice show thickening of the SC, which is loosely attached to the underlying SG (Fig. 2C). Scanning electron microscopy (SEM) performed on the facial area of newborn mice confirmed the extensive detachment of the SC that is compatible with their severe barrier defect (Fig. 2D). The expression of differentiation markers was studied by immunohistochemistry (IHC). Reduced loricrin and increased involucrin immunostaining was observed in Spink5-/- skin indicative of abnormal keratinocyte differentiation (Fig. 2E), a fact also substantiated by the increased expression of keratin 5 in Spink5-/- skin (Fig. 2E). The (corneo)desmosomal proteins DSG1, DSC1, and CDSN, which are major protein targets for proteolysis by KLKs, showed reduced staining intensities in Spink5-/- epidermis (Fig. 2F) which is consistent with the increased proteolytic activities in the epidermis.
The desmosomal protein DSG1 is directly linked with differentiation. Expression of DSG1 occurs when the undifferentiated keratinocytes stop dividing and start to differentiate and form the suprabasal layers. The cytoplasmic domain of DSG1 binds to Erbin that enhances Erbin-SHOC2 interaction, thus diminishing formation of SHOC2-Ras complexes. This results in absence of Erk phosphorylation and induction of keratinocyte differentiation. Consequently, decreased DSG1 leads to reduced expression of differentiation markers (Harmon et al. 2013). Furthermore, DSG1 inhibits EGFR signaling, which is required to maintain keratinocytes in an undifferentiated stage. Thus, DSG1 links epidermal integrity to differentiation and suprabasal morphogenesis (Getsios et al. 2009; Short 2009). Increased DSG1 expression is accompanied by increased corneodesmosomes, in number and length, which confers increased mechanical resistance (Pampalakis et al. 2019).
CDSN, encoding corneodesmosin that is reduced in the epidermis of the Spink5-/- mouse, is localized on human chromosome 6, particularly in PSORS1, which is the major locus for psoriasis susceptibility. CDSN is a key molecule for preservation of corneocyte-corneocyte cohesion and its proteolytic cleavage occurs during the desquamation process (Simon et al. 2001). In lesional psoriatic skin, an almost full-length form of corneodesmosin is present along with reduced degradation of (corneo) desmosomal proteins (Simon et al. 2008).
NS shares many common symptoms with severe dermatitis, multiple allergies and metabolic wasting syndrome (SAM) (Samuelov et al. 2013) and with peeling skin disease (PSD or peeling skin syndrome type B) (Mazereeuw-Hautier et al. 2011) such as food allergies, constitutive skin inflammation, overdesquamation and hypotrichosis. This highlights a “phenotype linkage” between NS-PSD-SAM, underlined by LEKTI deficiency in NS leading to unopposed activities of epidermal KLKs and deficiency of the KLK substrates corneodesmosin in PSD and DSG1 in SAM.
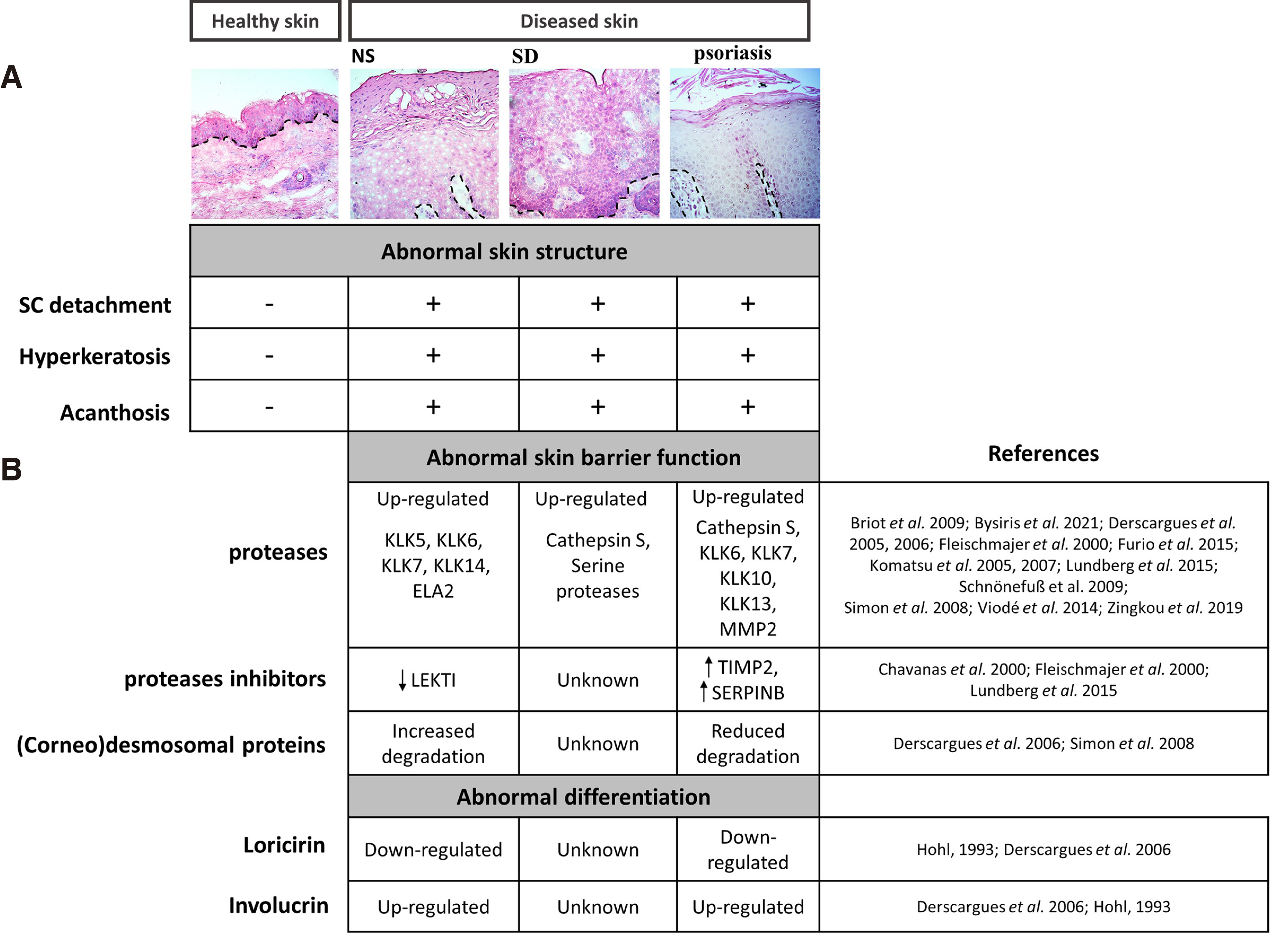
The changes observed in skin microstructure of the pre-clinical model of NS, i.e., in Spink5-/- mice, as well as the aberrant expression of differentiation markers and (corneo) desmosomal proteins, were then investigated by histological staining in skin tissue biopsies obtained from NS, SD, and psoriasis patients (Fig. 3). The NS sample showed similarly to Spink5-/- hyperkeratosis, acanthosis, and SC detachment.
Fig. 3. Histological analysis of NS, SD and psoriatic skin.
(A) Human healthy and disease tissue sections were stained with H/E, followed by microscopic examination. Epidermal hyperplasia and hyperkeratosis were found in all diseased skin. Parakeratosis was observed in NS and psoriatic skin. (B) Summary of the expression of epidermal proteases, endogenous protease inhibitors, (corneo) desmosomal proteins, and markers of epidermal differentiation in NS, SD or psoriasis.
Psoriasis is also an inflammatory skin condition with erythematous scaling patches. As shown in Fig. 3, epidermal hyperplasia and hyperkeratosis were detected that could be a consequence of hyperproliferative epidermis, as illustrated previously by abnormal expression of integrin, which is not confined to the basal layer but extends to the suprabasal layers (Watt, 2002).
On the other hand, SD is a chronic skin disease characterized by recurring inflammation and visible signs of erythematous scaling patches and itching. The affected areas include the face, scalp, upper chest, and other parts of the body that are mainly areas rich in sebaceous glands (Borda et al. 2018). Dandruff is a milder disease that does not involve inflammation and is restricted to the scalp. Histopathological findings in SD include epidermal hyperplasia and hyperkeratosis (Fig. 3). These are probably a result of increased proteolytic activities in the epidermis (Zingkou et al. 2019).
All diseases shown here are characterized by epidermal hyperplasia and hyperkeratosis that are signs of abnormal differentiation. In addition, all three diseases are inflammatory, and chronic inflammation is known to be causative for hyperkeratosis and acanthosis (https://www.ncbi.nlm.nih.gov/books/NBK562206/). In Fig. 3, parakeratosis, namely retention of nuclei at the corneocytes, another sign of abnormal differentiation, is also observed in NS and psoriatic skin.
In addition, all diseases shown here are characterized by aberrantly elevated epidermal proteolysis. Increased levels of cathepsin S have been reported in SD patients, and the expression levels potentially correlate with disease severity and itching (Viodé et al. 2014). In accordance, it was previously shown that cathepsin S provokes a scratching response in mice by activating MrgprC11 (Schnönefuß et al. 2009). Moreover, in SD, high epidermal serine protease activity was encountered (Zingkou et al. 2019). However, deletion of Klk5 seems to exacerbate the symptoms of dandruff (Zingkou et al. 2020). Elevated levels of MMP2 or gelatinase A, a type IV collagenase, and its inhibitor TIMP2, have been found in psoriatic skin, and the active form of MMP2 was abnormally increased in psoriatic skin, as detected by gelatin zymography (Fleischmajer et al. 2000). High levels of KLK6, KLK10 and KLK13 proteases were found in the SC of lesional and nonlesional psoriatic skin (Komatsu et al. 2005; Komatsu et al. 2007). Interestingly, KLK6 can activate the proMMP2 (Pampalakis et al. 2017); thus, overexpression of KLK6 in psoriasis could cause MMP2 overactivation. Using in vivo models, it was demonstrated that KLK6 is a causative protease for psoriasis and inflammatory joint disease and down-regulation of KLK6 expression represses the psoriatic symptoms, e.g. it reverted hyperplasia, etc. (Billi et al. 2020). Imiquimod application in mice results in a psoriasiform phenotype, while Klk6-/-mice show less pronounced symptoms (Iinuma et al. 2017). Proteomics analysis in the skin of KC-Tie2 mice, a pre-clinical model for psoriasis, showed that KLK6, SERPINB and CSTA are increased in skin lesions, and these data were validated in human samples (Lundberg et al. 2015). Analysis of these data as a whole suggests that KLK6 is an important regulator of psoriatic phenotype.
Keratinocyte-keratinocyte interactions are crucial to maintain skin integrity and skin barrier function, and the expression of (corneo) desmosomal proteins is essential for a normal skin microstructure and function. Even moderate alterations in the expression of the protein components of (corneo) desmosomes dramatically compromise skin barrier function. Aberrantly enhanced degradation of (corneo) desmosomal proteins has been reported in NS, as a result of KLK5 hyperactivation leading to severe exfoliation. Transmission electron microscopy (TEM) imaging of NS epidermis revealed several ultrastructure alterations, including decreased numbers of corneodesmosomes with reduced size and presence of numerous lipid droplets in their cytoplasm, thicker plasma membrane in the SG, indicative of premature maturation of the cornified envelope, and abnormal ultrastructure of desmosomes (Descargues et al. 2006). Reduced degradation of (corneo)desmosomal cadherins was observed in psoriasis (Simon et al. 2008), although TEM showed reduced number of desmosomes and increased intercellular space between adjacent keratinocytes that could be causative for loose keratinocyte-keratinocyte cohesions (Fleischmajer et al. 2000). Το our knowledge, expression of (corneo)desmosomal proteins and differentiations markers has not been investigated in SD but only in dandruff, in which retention of corneodesmosomes was found (Singh et al. 2014).
Immunohistochemical analysis of differentiation markers in skin from NS patients revealed moderately increased expression of involucrin, an early differentiation marker during keratinization, and increased expression of loricrin, a late marker of cornification leading to premature cornification of keratinocytes (Descargues et al. 2006). Expression of involucrin is elevated in psoriasis, while loricrin is reduced (Hohl, 1993).
In summary, dysregulated epidermal differentiation process and perturbed epidermal proteolytic activities are present in NS, AD and psoriasis. Collectively, these diseases highlight the fact that strictly controlled epidermal differentiation processes and epidermal proteolytic activities are pivotal for normal skin microstructure, and that even moderate alterations dramatically affect skin structure and skin barrier function. Furthermore, we verified that Spink5-/-mice constitute a reliable pre-clinical mouse model for investigating unexplored facets of NS. It should be noted that Spink5-/- mice are also an ideal model for atopic dermatitis (AD), since NS patients exhibit severe atopic symptoms that are largely mediated by the KLK5 activity (Furio et al. 2015). In this direction, polymorphisms in the SPINK5 gene have been identified in AD patients (Li et al. 2020). Thus, the study of Spink5-/- mice provides important insights into the mechanisms that drive not only NS but also AD pathogenesis, with important implications for the development of new treatments. Further, the Tg-KLK5 mouse is an alternative model for NS that survives to adulthood (12 months), thus allowing the testing of potential pharmacological agents (Furio et al. 2014). Tg-KLK5 mouse recapitulates the major cutaneous hallmarks of NS and displays increased epidermal proteolytic activity, overdesquamation, and skin inflammation. However, it does not exhibit signs of abnormal epidermal terminal differentiation. Epidermal application of the borocyclic compound GSK951, which inhibits KLK5 with an IC50 250 pM in Tg-KLK5 mice, reduced epidermal proteolysis and proinflammatory cytokine expression (Liddle et al. 2021). GSK951 exhibits good epidermal permeability and is expected to be used in NS patients as the first disease-specific treatment.
Materials and Methods
Ethics statement
All patients and healthy donors provided written informed consent for use of the materials for research purposes, before obtaining biopsies. All experiments with mice were performed in accordance with corresponding National and EU laws and institutional guidelines. The protocol was approved by the local committee (Department of Regional Units of Achaia, Region of Western Greece).
Reagents and antibodies
All chemical reagents were obtained from Sigma-Aldrich. The antibodies were as follows: anti-loricrin (Abcam, ab24722), working dilution for immunohistochemistry (IHC) 1/1000; anti-involucrin (Santa Cruz, sc-15230), working dilution for IHC 1/1000; anti-cytokeratin 5 (Abcam, ab24647), working dilution for IHC 1/1000; anti-desmoglein 1 (Santa Cruz, sc-20114), working dilution for IHC 1/300; anti-desmocollin 1 (Santa Cruz, sc18115), working dilution for IHC 1/250; anti-corneodesmosin (CusAb, CSBPA0051241A01HU), working dilution for IHC 1/500.
Real-time PCR
RNA was isolated from neonatal skin tissue using the NucleoSpin RNA Kit (Macherey-Nagel), and 1 μg was transcribed to cDNA with First-Strand cDNA Synthesis Kit (Invitrogen) according to the manufacturers’ instructions. Relative expression analyses were performed using SYBR green (Applied biosystems) and the reactions were conducted using the Step One and Step One Plus Real-Time PCR Systems (Applied biosystems). The primers used were as follows: Spink5-Forward: 5’-GTCAGACTAATACACGCA-3’, Spink5-Reverse: 5’-GCAGGCAGTTCAGAATCG-3’; Hprt1-Forward: 5’-CTGGTTAAGCAGTACAGCCCCA A-3’, Hprt1-Reverse: 5’-CGAGAGGTCCTTTTCACCAGC-3’.
Histology
Skin biopsies were fixed in 3.7% formaldehyde in PBS for 24 h and then embedded in paraffin blocks. Five μm sections were cut with a microtome. Alternatively, sections were embedded in OCT and stored at -80°C. To investigate the morphology of tissues, sections were stained with haematoxylin/eosin (H/E) and observed under a light microscope using Zeiss Zen microscope software.
Immunohistochemistry
Skin cryosections of 5 μm were warmed to room temperature for 10 min, fixed in acetone for 10 min, air-dried for 5 min and hydrated in PBS for 5 min, before blocking of endogenous peroxidase activity in 3% H2O2/PBS for 10 min. Slides were then rinsed in PBS, blocked in 0.3% BSA/PBS-0.1% Triton-X 100 for 5 min and subsequently incubated in 0.3% BSA/PBS containing the diluted primary antibody. Afterwards, slides were washed twice with 0.3% BSA/PBS and incubated for 30 min in 0.3% BSA/PBS containing the secondary HRP-conjugated antibody following by 2 washes in 0.3% BSA/PBS and 1 wash in PBS. Finally, antibody staining was conducted by application of the 1x DAB substrate, and then sections were rinsed in PBS and stained with hematoxylin before being mounted with Eukitt and observed under light microscope using Zeiss Zen microscope software.
Scanning Electron Microscopy
For SEM, neonates were sacrificed, and their heads were fixed in 4% paraformaldehyde/PBS overnight, dehydrated in ethanol solutions (50, 75, 90 and 100%) for 10 min in each step and then dehydrated by incubation in 100% acetone twice for 20 min each. Finally, they were air-dried overnight before being coated with a gold layer of 10 nm and analyzed using a field emission SEM microscope (JOEL, 6300).
Acknowledgements
We would like to thank Dr.Manthoula Valari, MD, PhD (Aghia Sofia Children’s Hospital, Athens, Greece) for providing the NS and SD biopsies and Professor Maria Melachrinou (Department of Pathology, School of Medicine, University of Patras, Rion-Patras, Greece) for the psoriatic skin biopsy. We would also like to thank Professor Andrew McKenzie (MRC Laboratory of Molecular Biology, Cambridge, UK) for kindly providing the Spink5-/- mice, and Dr. Andreas Seferlis (Laboratory of Electron Microscopy and Microanalysis, University of Patras, Greece) for technical assistance with scanning electron microscopy.
References
Billi A. C., Ludwig J. E., Fritz Y., Rozic R., Swindell W. R., Tsoi L. C., Gruzska D., Abdollahi-Roodsaz S., Xing X., Diaconu D., Uppala R., Camhi M. I., Klenotic P. A., Sarkar M. K., Husni M. E., Scher J. U., McDonald C., Kahlenberg J. M., Midura R. J., Gudjonsson J. E., Ward N. L. (2020). KLK6 expression in skin induces PAR1-mediated psoriasiform dermatitis and inflammatory joint disease. Journal of Clinical Investigation 130: 3151-3157.
Bisyris E., Zingkou E., Kordopati G. G., Matsoukas M., Magriotis P. A., Pampalakis G., Sotiropoulou G. (2021). A novel theranostic activity-based probe targeting kallikrein 7 for the diagnosis and treatment of skin diseases. Chemical Communications 57: 6507-6510.
Borda L. J., Perper M., Keri J. E. (2018). Treatment of seborrheic dermatitis: a comprehensive review. Journal of Dermatological Treatment 30: 158-169.
Borgoño C. A., Michael I. P., Komatsu N., Jayakumar A., Kapadia R., Clayman G. L., Sotiropoulou G., Diamandis E. P. (2007). A Potential Role for Multiple Tissue Kallikrein Serine Proteases in Epidermal Desquamation. Journal of Biological Chemistry 282: 3640-3652.
Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A. D., Bonafé J.L., Wilkinson J., Taïeb A., Barrandon Y., Harper J. I., de Prost Y., Hovnanian A. (2000). Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nature Genetics 25: 141-142.
Descargues P., Deraison C., Prost C., Fraitag S., Mazereeuw-Hautier J., D'Alessio M., Ishida-Yamamoto A., Bodemer C., Zambruno G., Hovnanian A. (2006). Corneodesmosomal Cadherins Are Preferential Targets of Stratum Corneum Trypsin- and Chymotrypsin-like Hyperactivity in Netherton Syndrome. Journal of Investigative Dermatology 126: 1622-1632.
Descargues P., Deraison C., Bonnart C., Kreft M., Kishibe M., Ishida-Yamamoto A., Elias P., Barrandon Y., Zambruno G., Sonnenberg A., Hovnanian A. (2005). Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nature Genetics 37: 56-65.
Du H., Wang Y., Haensel D., Lee B., Dai X., Nie Q. (2018). Multiscale modeling of layer formation in epidermis. PLOS Computational Biology 14: e1006006.
Fleischmajer R., Kuroda K., Hazan R., Gordon R. E., Lebwohl M. G., Sapadin A. N., Unda F., Iehara N., Yamada Y. (2000). Basement Membrane Alterations in Psoriasis are Accompanied by Epidermal Overexpression of MMP-2 and its Inhibitor TIMP-2. Journal of Investigative Dermatology 115: 771-777.
Furio L., Pampalakis G., Michael I. P., Nagy A., Sotiropoulou G., Hovnanian A. (2015). KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome. PLOS Genetics 11: e1005389.
Furio L., de Veer S., Jaillet M., Briot A., Robin A., Deraison C., Hovnanian A. (2014). Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. Journal of Experimental Medicine 211: 499-513.
Fortugno P., Furio L., Teson M., Berretti M., El Hachem M., Zambruno G., Hovnanian A., D'Alessio M. (2012). The 420K LEKTI variant alters LEKTI proteolytic activation and results in protease deregulation: implications for atopic dermatitis. Human Molecular Genetics 21: 4187-4200.
Getsios S., Simpson C. L., Kojima S., Harmon R., Sheu L. J., Dusek R. L., Cornwell M., Green K. J. (2009). Desmoglein 1–dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. Journal of Cell Biology 185: 1243-1258.
Harmon R. M., Simpson C. L., Johnson J. L., Koetsier J. L., Dubash A. D., Najor N. A., Sarig O., Sprecher E., Green K. J. (2013). Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation. Journal of Clinical Investigation 123: 1556-1570.
Hewett D. R., Simons A. L., Mangan N. E., Jolin H. E., Green S. M., Fallon P. G., McKenzie A. N.J. (2005). Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Human Molecular Genetics 14: 335-346.
Hohl D., Piérard G. E. (1993). Expression Patterns of Loricrin in Dermatological Disorders. The American Journal of Dermatopathology 15: 20-27.
Holbrook K. A. (1979). Human Epidermal Embryogenesis. International Journal of Dermatology 18: 329-356.
Iinuma S., Kishibe M., Saito N., Igawa S., Honma M., Bando Y., Yoshida S., Ishida-Yamamoto A. (2017). Kallikrein-related peptidase 6 promotes psoriasiform skin inflammation through a protease-activated receptor 2-independent mechanism. Experimental Dermatology 26: 289-291.
Komatsu N., Saijoh K., Toyama T., Ohka R., Otsuki N., Hussack G., Takehara K., Diamandis E.P. (2005). Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. British Journal of Dermatology 153: 274-281.
Komatsu N., Saijoh K., Kuk C., Shirasaki F., Takehara K., Diamandis E.P. (2007). Aberrant human tissue kallikrein levels in the stratum corneum and serum of patients with psoriasis: dependence on phenotype, severity and therapy. British Journal of Dermatology 156: 875-883.
Li Y., Li Y., Li W., Guo X., Zhou S., Zheng H. (2020). Genetic polymorphisms in serine protease inhibitor Kazal-type 5 and risk of atopic dermatitis. Medicine 99: e21256.
Liddle J., Beneton V., Benson M., Bingham R., Bouillot A., Boullay A.B., Brook E., Cryan J., Denis A., Edgar E., Ferrie A., Fouchet M.H., Grillot D., Holmes D. S., Howes A., Krysa G., Laroze A., Lennon M., McClure F., Moquette A., Nicodeme E., Santiago B., Santos L., Smith K. J., Thorpe J. H., Thripp G., Trottet L., Walker A. L., Ward S. A., Wang Y., Wilson S., Pearce A. C., Hovnanian A. (2021). A Potent and Selective Kallikrein-5 Inhibitor Delivers High Pharmacological Activity in Skin from Patients with Netherton Syndrome. Journal of Investigative Dermatology 141: 2272-2279.
Lundberg K. C., Fritz Y., Johnston A., Foster A. M., Baliwag J., Gudjonsson J. E., Schlatzer D., Gokulrangan G., McCormick T. S., Chance M. R., Ward N. L. (2015). Proteomics of Skin Proteins in Psoriasis: From Discovery and Verification in a Mouse Model to Confirmation in Humans. Molecular & Cellular Proteomics 14: 109-119.
Mazereeuw-Hautier J., Leclerc E.A., Simon M., Serre G., Jonca N. (2011). A novel mutation in CDSN causes peeling skin disease in a patient from Morocco. British Journal of Dermatology 165: 1152-1155.
Ovaere P., Lippens S., Vandenabeele P., Declercq W. (2009). The emerging roles of serine protease cascades in the epidermis. Trends in Biochemical Sciences 34: 453-463.
Pampalakis G., Zingkou E., Kaklamanis L., Spella M., Stathopoulos G. T., Sotiropoulou G. (2019). Elimination of KLK5 inhibits early skin tumorigenesis by reducing epidermal proteolysis and reinforcing epidermal microstructure. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1865: 165520.
Reddy V. B., Sun S., Azimi E., Elmariah S. B., Dong X., Lerner E. A. (2015). Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nature Communications 6: 7864.
Samuelov L., Sarig O., Harmon R. M., Rapaport D., Ishida-Yamamoto A., Isakov O., Koetsier J. L., Gat A., Goldberg I., Bergman R., Spiegel R., Eytan O., Geller S., Peleg S., Shomron N., Goh C. S. M., Wilson N. J., Smith F. J. D., Pohler E., Simpson M. A., McLean W. H. I., Irvine A. D., Horowitz M., McGrath J. A., Green K. J., Sprecher E. (2013). Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nature Genetics 45: 1244-1248.
Schönefuß A., Wendt W., Schattling B., Schulten R., Hoffmann K., Stuecker M., Tigges C., Lübbert H., Stichel C. (2010). Upregulation of cathepsin S in psoriatic keratinocytes. Experimental Dermatology 19: e80-e88.
Short B. (2009). Desmoglein directs differentiation. Journal of Cell Biology 185: 1130-1130.
Simon M., Tazi-Ahnini R., Jonca N., Caubet C., Cork M.J., Serre G. (2008). Alterations in the desquamation-related proteolytic cleavage of corneodesmosin and other corneodesmosomal proteins in psoriatic lesional epidermis. British Journal of Dermatology 159: 77-85.
Simon M., Jonca N., Guerrin M., Haftek M., Bernard D., Caubet C., Egelrud T., Schmidt R., Serre G. (2001). Refined Characterization of Corneodesmosin Proteolysis during Terminal Differentiation of Human Epidermis and Its Relationship to Desquamation. Journal of Biological Chemistry 276: 20292-20299.
Simpson C. L., Patel D. M., Green K. J. (2011). Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nature Reviews Molecular Cell Biology 12: 565-580.
Singh B., Haftek M., Harding C.R. (2014). Retention of corneodesmosomes and increased expression of protease inhibitors in dandruff. British Journal of Dermatology 171: 760-770.
Sotiropoulou G., Zingkou E., Pampalakis G. (2021). Redirecting drug repositioning to discover innovative cosmeceuticals. Experimental Dermatology 30: 628-644.
Sotiropoulou G., Pampalakis G. (2012). Targeting the kallikrein-related peptidases for drug development. Trends in Pharmacological Sciences 33: 623-634.
Viodé C., Lejeune O., Turlier V., Rouquier A., Casas C., Mengeaud V., Redoulès D., Schmitt A.M. (2014). Cathepsin S, a new pruritus biomarker in clinical dandruff/seborrhoeic dermatitis evaluation. Experimental Dermatology 23: 274-275.
Watt F. M. (2002). NEW EMBO MEMBER'S REVIEW: Role of integrins in regulating epidermal adhesion, growth and differentiation. The EMBO Journal 21: 3919-3926.
Zingkou E., Pampalakis G., Kiritsi D., Valari M., Jonca N., Sotiropoulou G. (2019). Activography reveals aberrant proteolysis in desquamating diseases of differing backgrounds. Experimental Dermatology 28: 86-89.
Zingkou E., Pampalakis G., Sotiropoulou G. (2020). Exacerbated dandruff in the absence of kallikrein‐related peptidase 5 protease. The Journal of Dermatology 47: 311-313.