Int. J. Dev. Biol. 69: 71 - 79 (2025)
RNA and proteins extracted from the regenerating tail of lizards determine inhibition of cancer cell proliferation in vitro
Open Access | Original Article | Published: 13 June 2025
Abstract
Recent studies suggest that tail regeneration in lizards begins with a tumor-like stage usually termed regenerative blastema. Oncogenes and tumor suppressors are activated in blastema cells, resulting in a balanced cell proliferation that does not turn the blastema into a tumor. This outgrowth elongates forming new tissues and tail. We previously showed that physiological extracts from regenerating lizard tissues inhibit the growth of cancer cells in vitro within 2-4 days of administration, demonstrating that the growing lizard blastema contains regulatory molecules which can also influence human cancer cells. The molecules responsible for this inhibition were not identified in that initial study. In the present experimental study, after specific extractions of RNAs and/or proteins from the regenerating tail of lizard, we have confirmed the inhibition of breast cancer cell vitality in vitro within 2-3 days from their addition to the culture medium. Proteolysis or heat denaturation of proteins abolished the inhibitory effect. RNA delivered to breast cancer cells in vitro through lipid vesicles (liposomes) showed the highest inhibition of cancer cells vitality. Cell degeneration, detected by microscopy, revealed that RNA is more effective than proteins extracted from regenerating tissues. The present observations further suggest that RNAs coding for known tumor suppressor proteins, and non-coding RNAs that are highly expressed in the regenerating tail, may be key inhibitors (tumor suppressors) of blastema and cancer cell proliferation. The evolution of a mechanism for the self-remission of tumor growth in lizards remains uncertain, but continuing study of this reptile may help uncover natural mechanisms for tumor growth inhibition.
Keywords
lizard, tail regeneration, tissue extracts, proteins, RNAs, cancer inhibition
Introduction
While regulated cell proliferation and differentiation allow a harmonious development, growth and regeneration in animals, deregulation of proliferation leads to tumor formation and cancer (Brockes, 1998; Smetana et al., 2013; Fior, 2014; Boilly et al., 2017; Sarig and Tzahor, 2017; Wong and Whited, 2020). A unique case of balanced cell proliferation in amniotes, leading to the regeneration of a voluminous organ, the tail, occurs in lizards (Bellairs and Bryant, 1985; Fisher et al., 2012; Alibardi, 2014; Hutchins et al., 2014; Gilbert et al., 2015; Lozito and Tuan, 2016; Jacyniak et al., 2017; Ranadive et al., 2018). In natural conditions, this process most commonly follows the loss of part of the tail after a predatory attack or combats among males during the mating season, or for other causes. Numerous cells from the injured tissue of the tail are activated and some cells also dedifferentiate near the stump surface of the tail and release proliferating cells that form a regenerative blastema (Quattrini, 1954; Alibardi, 2014, 2017; Londono et al., 2017). The blastema is mainly composed of mesenchymal cells and fibroblasts in contact with the tissues of the tail stump and is delimited by a thick wound epidermis (Fig. 1).
Fig. 1. Histology of regenerating cones.
(A) Sagittal histological section of a cone as continuation of stump tissues, showing the main component tissues. Hematoxylin-Eosin stain. Bar, 100 µm. (B) Fibroblasts composing most of the connective tissues and the apical blastema. Toluidine blue stain. Bar, 10 µm. (C) DAPI-fluorescence revealing the main regenerating tissues and providing an indication of differences in cell density in a regenerating cone. Dashes underline the epidermis. Bar, 100 µm. (D) The apical ependymal ampulla. Bar, 10 µm. (E) A regenerating muscle within a cone, at this stage mainly composed of pre-fusing myoblasts to form myotubes. Bar, 10 µm. Abbreviations: a, adipose tissue; bl, blastema (mesenchyme); bv, blood vessels (capillaries); ca, regenerating cartilage; e, ependymal tube/ampulla; mu, regenerating and segmented muscles; ne, regenerating nerve; w, wound (regenerating) epidermis.
Research from the last 10 years has identified several genes involved in this successful regrowth of the tail, an organ counting for 1/4th to 1/7th of the entire body mass of some lizard species (Hutchins et al., 2014, 2016; Liu et al., 2015; Vitulo et al., 2017; Murawala et al., 2018; Xu et al., 2020; Degan et al., 2021; Nagumantri et al., 2021; Patel et al., 2022; Degan and Alibardi, 2023). Transcriptome analyses so far conducted for lizards during tail regeneration have evidenced the up-regulation of numerous coding and non-coding genes of the wnt, fgf, egf etc. pathways, and also SnoRNAs and miRNAs. The comparison between genes expressed in the regenerating tail with those of the scarring limb in the same lizard (Podarcis muralis), has allowed the detection of several differentially expressed genes in the tail that are not activated in the limb (Vitulo et al., 2017). In the lizard P. muralis, these genes belong to two main gene categories: oncogenes and tumor suppressors (Vitulo et al., 2017; Alibardi, 2019, 2021, 2022; Degan et al., 2021; Degan and Alibardi, 2023). The latter studies indicated a possible involvement of some RNAs in the process of regeneration and cancer growth inhibition. This information initially derived from the detection of many coding and non-coding RNAs, SnoRA (SnoRNA), SnoRD (SNORD), and scaRNA (Small Cajal Body RNA), exclusively upregulated in the regenerating tail but not in the scarring limb (Vitulo et al., 2017).
The above studies have indicated that the regenerating blastema initially is equivalent to a small tumor where oncogene activity prevails over that of tumor suppressors and that the elongation of the blastema into a new tail derives from a prevalence of tumor suppressor over oncogene activity which addresses the formation of a new tail and not of a tumor outgrowth (Alibardi, 2023). Therefore, it has been suggested that during the evolution of tail repair and regeneration, lizards have evolved a mechanism to control cell proliferation, addressing the regeneration of the tail and avoiding cell deregulation and cancer, a spontaneous case of cancer remission (Radha and Lopus, 2021). This information has suggested that cells isolated from a regenerating tail collectively contain tumor-suppressor molecules that might also inhibit deregulated cells such as cancer cells. Initially, this hypothesis has been experimentally tested in vitro by applying raw tissue extracts in Ringer fluid, from the normal and regenerating tail of the lizard P. muralis, and evaluating their effect on cancer cells in culture. This has been determined by analyzing the proliferation and vitality of two lines of human cancer cells from breast and prostate cancers (Greco et al., 2023). This preliminary and explorative research indicated that a physiological extract from regenerating tail tissues significantly lowers cell vitality of breast and prostate cancer cells (25-35%), and that numerous cancer cells degenerate within 3-4 days from the beginning of administration of the protein extract. In a previous study, using a physiological and a protein extract form the normal tail of a gecko lizards, no effect was observed on normal human cells cultivated in vitro while an inhibition was shown on human cancer cells cultivated in vitro (Jeong et al., 2012). In our previous study, we only detected inhibition from physiological extracts from the regenerating but not from the normal tail (Greco et al., 2023). However, direct proof that the inhibitory effect derived from proteins, RNA, or even protein-RNA complexes, or smaller molecules extracted in the physiological solution was not established in that study.
To improve information on the inhibitory molecules, in the present study we have specifically examined the nature of the molecules that might be involved in the negative effect, in particular proteins or RNAs extracted from normal or regenerating lizard tissues. The present experimental results indicate that the isolated RNAs, and also isolated proteins, likely determine the inhibition of breast cancer cell viability in vitro.
Results
Histological observations show the main tissues present in the blastema-cone
Here we briefly report a description of the regenerating tail cone of the lizard P. muralis to provide general information on the tissues from which RNA and proteins were extracted in the samples that were utilized in the present study. After the formation of a flat or rounded regenerative blastema from 8-12 days post-autotomy, the blastema grew into a regenerating cone, attached to the stump (normal tail) tissues. The new tissues were in continuation with the normal epidermis, vertebral column, original spinal cord, muscles, nerves, connective and fat tissues (detailed descriptions in Quattrini, 1954; Alibardi, 2014, 2017). In the cone blastema, the vertebral column was replaced with a cartilaginous tube containing a thin ependymal tube, the continuation of the original spinal cord (Fig. 1A). The cartilage was surrounded by a loose connective tissue with blood vessels and nerves, and more externally by segmented muscles and a multi-layered wound (regenerating) epidermis. Most cells of the cone appeared as fibroblast-like cells and in the 0.5-1.0 mm long apex, the blastema, they were mainly represented from more irregularly shaped mesenchymal cells (Fig. 1 B,C). The regenerating ependyma was terminated by the apical part of the regenerating cone with a dilated ampulla (Fig. 1D), and numerous myoblasts were present in the forming segmental muscles (Fig. 1 C,E). Therefore, these are the main types of cells from which proteins and RNA were extracted in the following experiments, with a prevalence of fibroblast-like cells.
Experimental results show an inhibitory effect of proteins and RNAs on cancer cell vitality
Initially, we confirmed the inhibition of cancer cell vitality after administration of physiological extracts (Greco et al., 2023). In the following experiments where proteins were extracted, denatured by boiling or enzymatically digested by proteinase-K, and subsequently administered to breast cancer cells at different concentrations, including one at high concentration (400 µg/ml), no inhibition of proliferation rate was observed (Fig. 2, see the lines of different colors (green, black, orange and red, corresponding to extracted tail areas in the lizard drawings), that was not significantly different from the control-CTR, blue line.
Fig. 2. Plot showing no inhibition on cell vitality from normal tail tissues.
Plot showing that extracted proteins at high concentrations (400 µg/ml) from stump tail (ST, green/black lines and tail areas in the lizard drawings) or regenerating tail (RT, red/orange lines and tail blastemas) tissues do not exert any inhibition on the proliferation rate of cancer cells at 1-3 days after denaturation (boiling) or Proteinase-K degradation, in comparison to controls (untreated, blue line). Data was normalized to that of the control group.
Another experiment was conducted by the administration of RNA extracted from the stump or regenerating tails tissues. To test the efficacy of the treatment, two different concentrations of RNA (2 pmol, low conc., and 5 pmol, high conc.) were utilized for incorporating the extracted RNAs into liposomes that were delivered to cancer cells. The two concentrations were chosen in order to maximize the use of the available RNAs extracted from normal and from regenerating tissues. The control cells treated with empty liposomes and the stump tissues at different RNA concentrations, high or low, did not show any significant variation in the proliferation rate of cancer cells after 3 days (Fig. 3, lines light blue, black and green and corresponding tail areas in the lizard drawings). As opposed, higher RNA concentration derived from regenerating tissues (5 pmol), showed a significant inhibition of cancer cell proliferation (about 40%, Fig. 2B red line and blastema in the lizard drawing). A weak inhibition but not statistically significant, was actually also noted (about 10%) from high RNA concentrations extracted from stump tissues (Fig. 3, green line and tail area of the lizard drawing). Lower concentrations of RNA from regenerating tail (2 pmol), also did not show any inhibition (Fig. 3, orange line and blastema in the lizard drawing).
Fig. 3. Plot showing cell vitality inhibition of extracted RNAs from regenerating tissues.
Absence of inhibition in controls (CTR Transfection, treated with empty liposomes, blue line) and from normal tail (stump, ST, green/black lines and tail areas in the lizard drawings) at high (5 pmol, green line) or low (2 pmol, black line) RNA concentrations. No inhibition was also observed using low concentration of RNA-liposome, derived from the regenerating tail (2 pmol, orange line and blastema in the lizard drawing). In contrast, significant inhibition was detected (35-45%, red line) after the administration of a relatively high RNA-liposome concentration (5 pmol) derived from the regenerating tail (red line and blastema in the lizard drawing). Data was normalized to that of the control group. P-value, ** p ≤ 0.001 vs. CTR.
Microscopic observations show cell damage in cultured cells
The qualitative microscopic control of In vitro cancer cells after 3 days of culture, showed a high cell density and viable cells, many of which appeared flat-attached to the substrate in untreated culture medium (Fig. 4 A,B), in Ringer (Fig. 4C), or in cell culture treated with denatured proteins by boiling or protease treatment (Fig. 4D). In contrast, the number of cells appeared diminished in the culture treated with native proteins (not denatured), especially at high concentration (Fig. 4 E,F). In the latter, numerous cells appeared detached from the Petri dish substrate, and few were still flat-attached to the substrate, others were shrunken and their cytoplasm contained granular inclusions while sparse cells debris were also observed, more commonly than in control cultures.
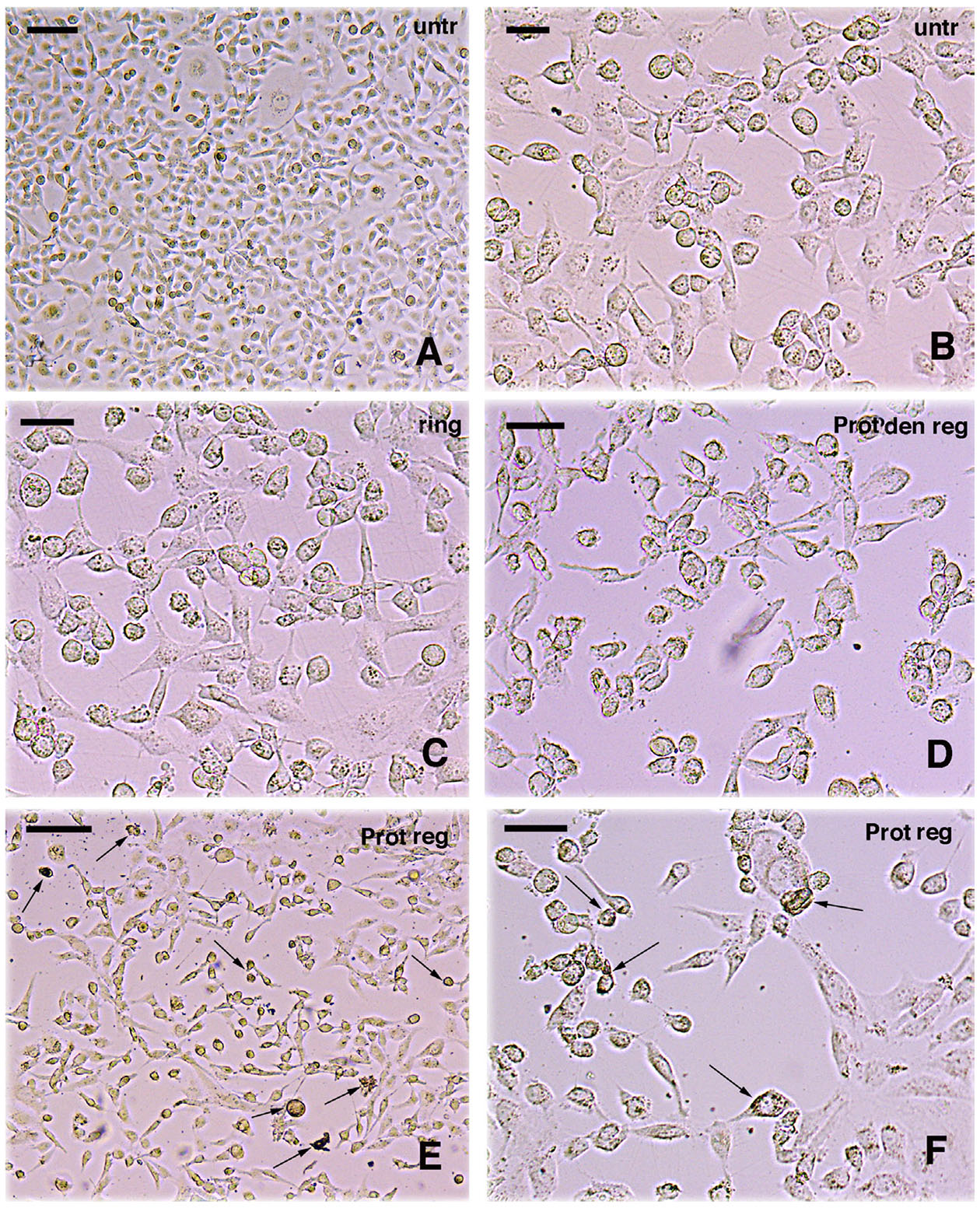
Fig. 4. Effect of protein treatment on cells in vitro.
(A) Low magnification of untreated cells (untr); bar, 20 µm. (B) Higher magnification of untreated cells; bar, 10 µm. (C) Cells only treated with Ringer (ring); bar, 10 µm. (D) Cells treated with denatured proteins from regenerating tail tissues (Prot den reg); bar, 10 µm. (E) Low magnification of cells (numerous are degenerating, some indicated by arrows) treated with proteins extracted from regenerating tail tissues (Prot reg); bar, 20 µm. (F) Higher view of cells treated with proteins extracted from regenerating tail tissues. Some degenerating and granular cells are indicated by arrows; bar, 10 µm. (See description in the text).
The observations on cell cultures after RNA delivery through liposomes, showed normal cytological aspects in untreated controls at 3 days (Fig. 5A), and also in other controls where the cultured cells were treated with empty (no RNA) liposomes (Fig. 5B). Also, cancer cells treated with RNA extracted from the stump (normal) tail, and incorporated into liposomes, did not show relevant aspects of cell degeneration after 3 days from the administration, indicatively resembling untreated cultures (Fig. 5C). In contrast, a dramatic aspect, with reduction of cells numbers and increase of cell debris, was instead observed at 2 and at 3 days post-treatment in the culture treated with liposomes containing RNA derived from regenerating tissues (Fig. 5D). At higher magnification most of these cells appeared detached from the substrate and flat-attached cells were almost absent, and cells were devoid of cell elongations, shrunken, and numerous cells appeared degenerated and/or mixed to cell debris (Fig. 5 E,F).
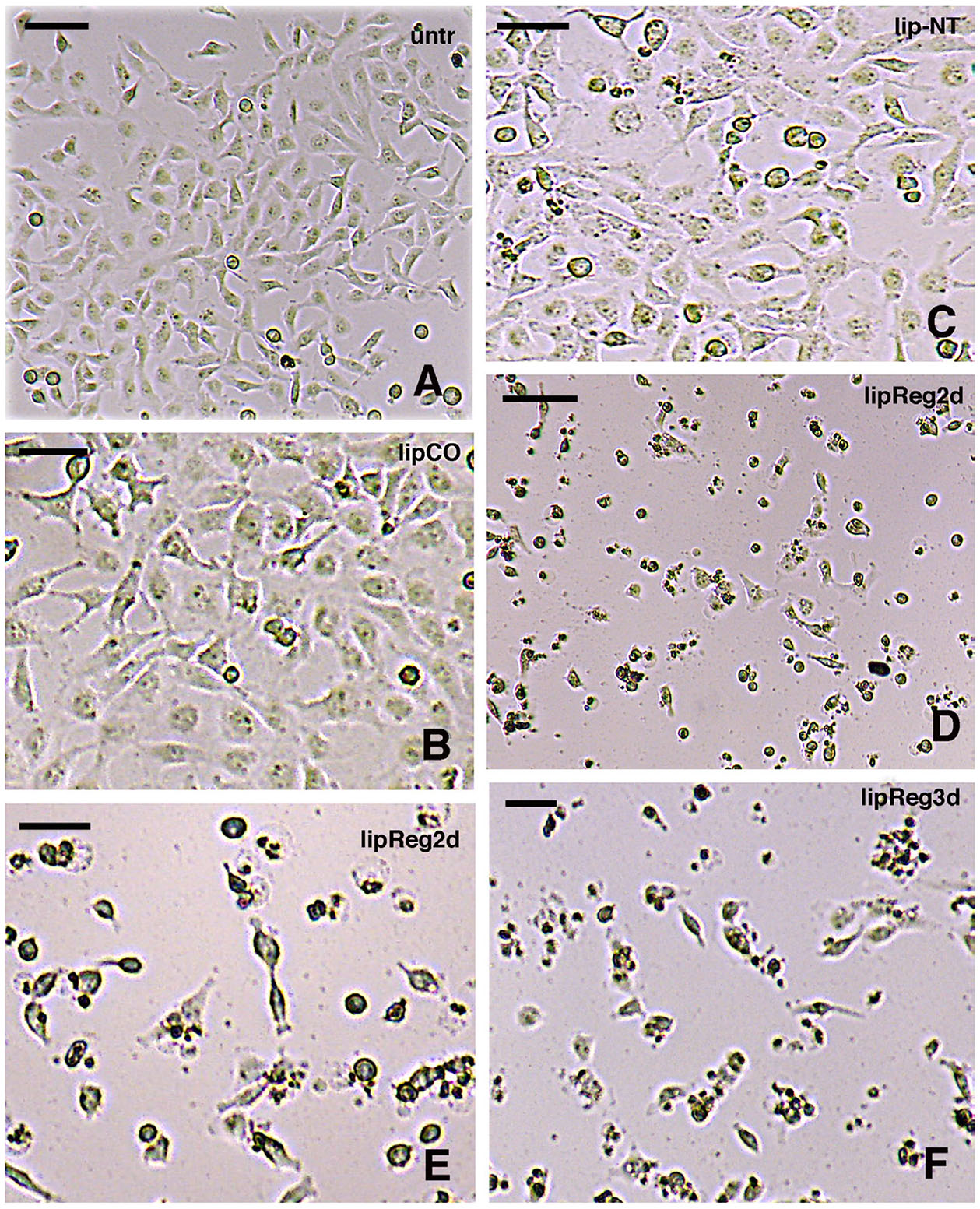
Fig. 5. Effect of RNA-liposome treatments on cells in vitro.
(A) Low magnification of untreated cells (unt); bar, 20 µm. (B) Higher magnification of cells treated with empty liposomes as controls (lipCO), bar, 10 µm. (C) Cells treated with RNA-liposome extracted from normal (stump) tissues (lip-NT), bar, 10 µm. (D) Low magnification showing cells (numerous are degenerating) treated with RNA-liposomes from regenerating tail tissues, after 2 days in culture (lipReg2d), bar, 20 µm. (E) Higher magnification of cells treated with RNA-liposomes from regenerating tail tissues, after 2 days (lipReg2d); bar, 10 µm. (F) Cells treated with RNA-liposome from regenerating tissues after 3 days (lipReg3d); bar, 10 µm. (see description in the text).
The qualitative observations of cells sectioned from resin-embedded cultures, confirmed the cytological alterations previously reported between untreated cells versus those treated with proteins or with RNA-liposomes at high concentrations (Fig. 6). The histological examination of normal versus treated cancer cells also evidenced a cytotoxic action after separate protein or separated RNA treatments. Untreated, control cancer cells appeared prevalently bi- or multi-polar, stainable (basophilic), their nucleus and nucleolus were evident, and the membrane was intact (Fig. 6 A,B). In contrast, cancer cells that were treated with extracted, untreated proteins (not denatured) from regenerating tissues contained numerous granules and their cytoplasm was paler, and more poorly stainable, than untreated controls (Fig. 6 C-E). Nuclei of these cells were often not seen, and also the cell membrane or cell profile evidenced some interruptions. Similar aspects were also detected in cells treated with RNA-liposome at high concentration, isolated from regenerating tail, featuring numerous cytoplasmic granules, lack of nuclei and evidencing some discontinuity in their cell membranes (Fig. 6 F-H). Cell fragments of small dimension were present among the treated cells, suggesting cell fragmentation.
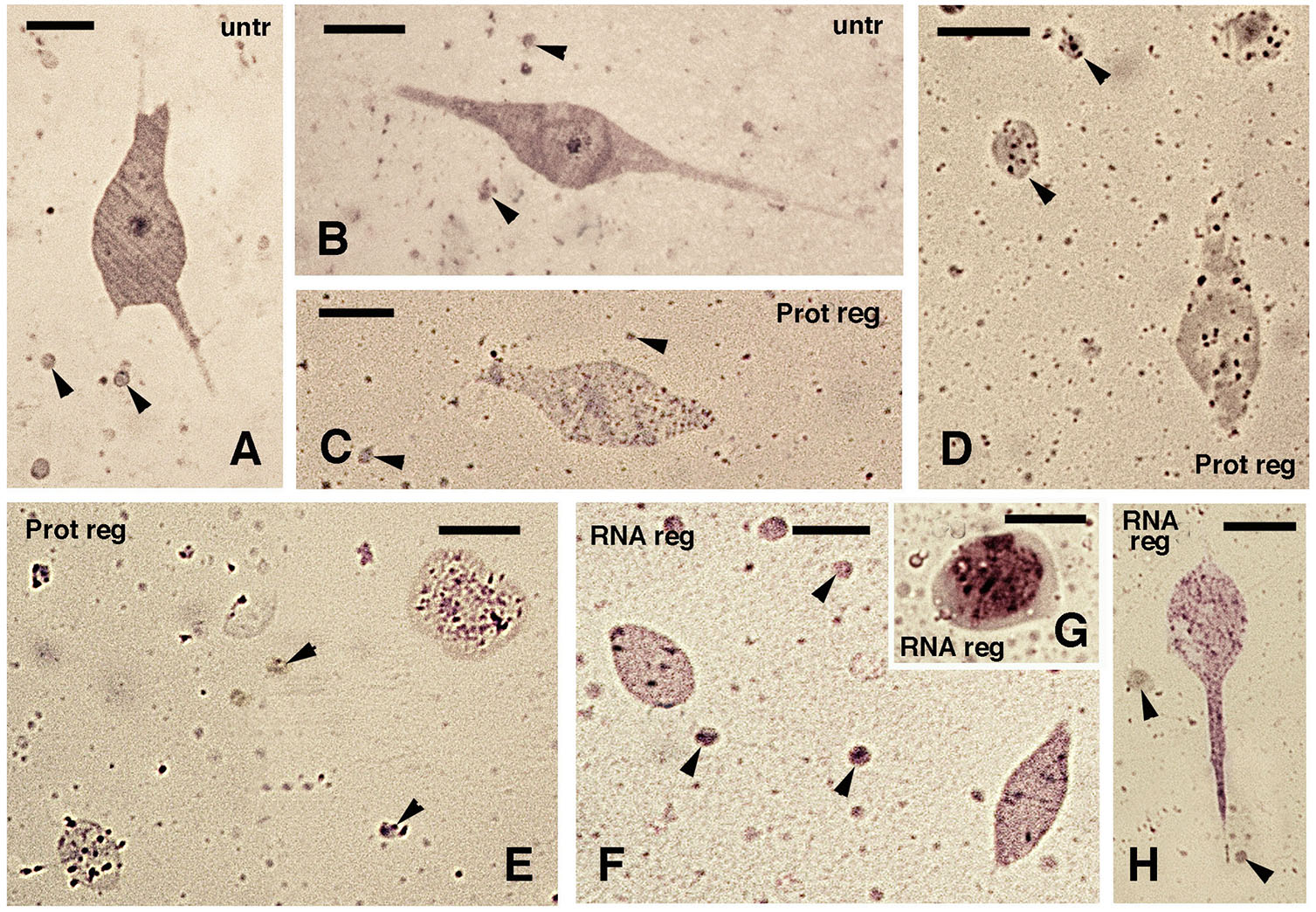
Fig. 6. Effects of protein treatment and RNA-liposome treatment at high concentrations.
Cells were subsequently embedded in Epon resin. (A,B) Untreated culture controls. (C-E) After protein treatment (Pot reg) and (F-H) after RNA-liposome treatment at high concentrations (Rreg). (A) Multipolar cell. Arrowheads indicate cross-sectioned cell elongations; bar, 5 µm. (B) Bipolar cell. Arrowheads indicate cross-sectioned cell elongations; bar, 5 µm. (C) Degenerating cells and cross-sectioned cell elongations (arrowheads) that contain cytoplasmic granules; bar, 5 µm. (D) Granulated cells and cell elongations (arrowheads); bar, 5 µm. (E) Other granulated cells, likely in cross-section, surrounded by cell elongations (arrowheads); bar, 5 µm. (F) sections of granulated cells of bipolar shape and cell elongations (arrowheads); bar, 5 µm. (G) Detail of roundish cell filled with granules. (H) A bipolar cell with granulations and close to sectioned cell elongations (arrowheads); bar, 5 µm. (see description in the text).
Discussion
Effects of extracted proteins and RNAs on cancer cells
The reason to initiate the present research derived from previous studies (summarized in Alibardi, 2023), indicating that the lizard blastema might produce inhibitory molecules capable to regulate cell proliferation. Therefore, it was of interest to check if molecules present in the lizard blastema were somehow also effective on cancer cells. A previous study, conducted until 4 days post-treatment on cancer cells (breast and prostate) In vitro with high concentration (200-400 µg/ml) of a physiological (Ringer) tissue solution derived only from regenerating tail tissues but not from normal tissues, evidenced an inhibition of about 30-35% of the total vitality of cancer cells (Greco et al., 2023). Therefore, it was considered that while cancer cells with no treatment maintain for days their vitality and proliferation, the addition of cellular extracts from regenerating tissues only, negatively influenced the growth of these cancer cells In vitro. Previous experiments on two types of aggressive cancer cell lines cultivated In vitro showed a similar inhibition, suggesting that cancer cells of different histological origin are affected from molecules present in the regenerating tail tissues (Greco et al., 2023). Therefore, the present and more focused study specifically tested the cellular response of breast cancer cells to the exposure of separated total RNA or separated proteins, extracted and isolated from normal and regenerating tail tissues. The present study confirmed that only molecules isolated from regenerating tail tissues but not from normal tissues significantly inhibit human cancer cells. In a previous study, using normal tails from gecko lizards, an inhibitory effect was also detected from normal tail tissues, only versus In vitro cultivated cancer cells but not effecting the vitality of normal cells (Jeong et al., 2012). Although in our study, no effect was obtained using extracted proteins or RNAs from normal tail tissues, it is also possible that yielding a higher quantity of proteins or RNAs from normal tissues some inhibition could be detected. However, our main goal was to see a possible anti-cancer effect under our conditions of low proteins/RNA concentrations.
With respect to the previous study that utilized Ringer extracts (Greco et al., 2023), where smaller thermic resistant molecules such as amino acids, peptides, nucleotide bases, vitamins, non-protein hormones, ions etc. were likely present, the present experiments have indicated that isolated proteins or isolated RNAs are specific sources of the inhibitory effect. Lowering cell vitality and increasing cell degeneration was limited to 3 days of exposition to protein extracts or to RNAs administered via liposomes. This result suggests that the damage and inhibition of vitality and growth in cancer cells could be higher after longer exposure to RNA or to proteins derived from the regenerating tissues.
The histological examination of normal versus treated cancer cells also evidences a cytotoxic action after separate protein or separated RNA treatments. The elimination of the inhibitory action of isolated proteins after boiling or protease treatment (Fig. 2A), indicates that the proteins deactivated from the treatments, effect cancer growth. Although the present study reveals that RNA alone is also or even prevalently involved in the cytotoxic action (Fig. 2 B,C), it remains to be specifically identified whether RNA acts directly on cancer cells or RNA codes for tumor suppressor proteins after it enters, though liposome, into cancer cells. It is known that the regenerating tail contains a number of tumor suppressors, such as Arhgap28, Rb, APC, etc. (Vitulo et al., 2017; Degan et al., 2021; Degan and Alibardi, 2023). The other possibility is that the inhibitory effect derives from some of the ncRNAs previously detected in the regenerating tissues of lizard (Vitulo et al., 2017; Alibardi, 2019).
Due to the shortage of available material for more experiments tagging specifically RNAs in the extracted tissue solutions (e.g. RNAse treatments), the present study cannot specifically indicate which type of RNA is responsible for the observed inhibition. Despite this missing information, we can however hypothesize that the inhibitory effect largely derives from ncRNAs, based on the following reasoning: a) transcriptome data (Vitulo et al., 2017) indicate these ncRNAs are exclusive of the regenerating tail but are absent in the scarring limb. These studies have suggested that (a) SNORD26, SNORD87, U8, SNORD50 and SNORD15 may be candidates as tumor suppressors in the blastema of the lizard P. muralis; b) ncRNAs are among the most highly expressed transcripts in the regenerating tail, higher than most coding mRNAs (Vitulo et al., 2017; Degan and Alibardi, 2021) and c) if mRNAs are also involved in the inhibition effect, the latter however derive from the protein coded from these mRNAs, such as Arhgap28, APC, Rb, Ephrin etc. (Vitulo et al., 2017; Alibardi, 2019, 2021, 2022). More detailed experiments in the future could aim to identify specific inhibitory ncRNAs or proteins on breast or other types of cancer cells.
Cancer inhibition and regeneration
Cell proliferation and differentiation are very active during development, regeneration and cancer. Although tumors are also present among amphibians (Asashima et al., 1987), the regenerating organs of amphibians are resistant to tumor transformation, likely because of their effective regulatory regenerative mechanisms that impede cell deregulation (Brockes, 1998; Fior, 2014; Boilly et al., 2017; Sarig and Tzahor, 2017; Wong and Whited, 2020). It is reasonable to assume that these regulatory mechanisms have evolved from long time in amphibians, especially urodeles (Asashima et al., 1987; Anver, 1992; Oviedo and Beane, 2009; Fior, 2014), but also in lizards. Paleontological studies have indeed indicated that broad abilities to regenerated organs was already present in ancient amphibians of the Carboniferous Period, at least 320 million years ago, and also in ancestors of lizards of the Permian and Triassic, about 250 years ago (Bellairs and Bryant, 1985; Fröbisch et al., 2014; LeBlanc et al., 2017).
The study on lizard regeneration further indicates that also blastema cells of this amniote are capable to resist neoplastic transformation, perhaps using cell control mechanisms similar to those of amphibians. Although the details on the control mechanisms remain unknown in both urodeles and lizards, in the latter a balanced role of oncogenes and tumor suppressors has been hypothesized, an equilibrium that impedes tumor transformation but instead allows for a regulated regeneration of the tail (Alibardi, 2019, 2023). How amphibians and lizards evolved this cancer-resistance is a subject worthy to be investigated in the future in order to discover regulative processes useful for human cancer studies or even therapies. While the cellular localization of tumor suppressor proteins in the lizard blastema is partially known (Alibardi, 2021, 2022), no information on the specific cells or tissues expressing ncRNAs are presently available, a subject for next research using lizards as experimental models. The present study was limited to test only cancer cells but we do not know whether lizard proteins or RNAs from the normal or regenerating tail can also affect normal human cells in vitro, a limitation of the present study.
In conclusion, at the present stage, we can hypothesize that RNA alone, or specific proteins present in the regenerating tail tissue of the lizard P. muralis, are capable of damaging and inhibiting the proliferation of human breast cancer cells in vitro. Further experiments, including also tests on other types of cancer cells, should be conducted in order to specifically identify the types of RNAs or proteins involved in the inhibition that seems to regulate tail regeneration in lizards.
Materials and Methods
Lizard tail collection
In the present study, we utilized eleven samples that comprised two normal tail samples and three regenerating tails of 3-4 mm in length for protein testing, three normal tail samples and three regenerating tails of about 3 mm in length for RNA testing. The tissues derived from adult individuals of the wall lizard (Podarcis muralis), kept in cages at 27-30°C and fed with mealworms alternated with maggots, as previously reported (Alibardi, 2022; Greco et al., 2023). Experiments were performed under the Italian regulations for the care and use of animals for experimentation (art. 5DL 116/92). Autotomy (a voluntary self-amputation triggered after grabbing the tail, see Quattrini, 1954) was induced by twisting the tail at about 1/3rd proximal, stimulating the animal to release it. The more proximal part of the tail, 3-5 mm in length, was utilized as tissue control and is indicated as "stump or normal" tail tissues (ST in the following text). After a regenerating tail cone (RT in the following text) of 3-4 mm in length was formed, it was collected following the induction of hypothermia to obtain numbed animals, and the lizards were later released in the wild.
Tissues from the normal or from the regenerating tail were reduced in size by cutting the tissues with a razor blade and immediately collected in TRIzol reagent (#15596-026, ThermoFisher Scientific) for RNA isolation (see below) or in Ringer solution (NaCl 4.5 mM, NaHCO3 2.5 mM, KCl 100 mM, NaH2PO4 0.5 mM, CaCl2 1.44 mM, Glucose 16.8 mM) for protein extraction (see below) and stored at -80°C until use.
Protein extraction
Protein extraction was performed as described in a previous work (Greco, 2023). The normal or regenerating tail tissues were collected in a sterile Ringer solution, and they were homogenized to obtain a protein extract. To avoid possible influences on the following In vitro tests, no protease inhibitors were added to the samples. After centrifugation, the supernatant solution containing the proteins was collected while the pellet was discharged. Tail extracts were sonicated for 30 seconds, centrifuged for 30 min at 12000g at 4 °C, and the supernatant was filtered through a 0.22 µm filter. Protein concentration was measured by PierceTM BCA Protein Assay kit (#23227, ThermoFisher Scientific, Rockford, USA).
Protein denaturation by boiling and enzymatic digestion
To discriminate the role of proteins in these studies, the protein extracts were degraded in two different ways, and considered as negative controls. One method consisted in the physical inactivation (denaturation) of proteins by boiling the protein extracts for 10 min at 100 °C. Another method consisted in the enzymatic digestion of the protein extracts using proteinase K (PK) (100 µg/ml), by incubating the protein extracts at 37 °C for 1 h with the enzyme. PK digestion was terminated by the addition of cOmplete™ Mini Protease Inhibitor Cocktails (#11836153001, Roche) at a final concentration of 1 µM.
RNA isolation and transfection
To promote total RNA extraction from normal or regenerating tail tissue, the samples were mechanically homogenized by the use of Benchmark D1000 Homogenizer (Benchmark, Sayreville, USA). The RNA was extracted from the homogenates by TRIzol™ reagent (#15596-026, ThermoFisher Scientific, Rockford, USA), an RNA extraction kit designed to isolate high-quality total RNA and to maintain its integrity, according to the manufacturer’s instructions. The RNA samples obtained through this procedure, and their purity, were quantified using a NanoDrop™ One/OneC Microvolume UV-Vis Spectrophotometer (ThermoFisher Scientific, Rockford, USA). To avoid RNA degradation after delivery of RNA in the cell culture medium for testing its effect on cancer cells, the extracted RNA was delivered by Lipofectamine RNA iMAX Transfection Reagent (liposomes, #13778150, ThermoFisher Scientific, Rockford, USA) following the manufacturer’s protocol.
Cell cultures
MDA-MB 231, triple-negative breast cancer cell line (ATCC), were cultured in Dulbecco's Modified Eagle Medium-High Glucose (DMEM-HG, Gibco, ThermoFisher Scientific, Rockford, USA), completed with 10% fetal bovine serum (FBS), 2 mM L-glutamine, penicillin (100 U/mL), and streptomycin (100 µg/mL). Cells were maintained in a humidified 95% air 5% CO2 incubator at 37 °C.
Cell viability assay
CyQUANT XTT cell viability assay (#X12223, ThermoFisher Scientific) was used to evaluate cell viability after lizard tail extracts treatment, as previously described (Greco et al., 2023). Briefly, 5 * 103 MDA-MB 231 cells/well were plated in 96-well plates. Cells were then treated with different concentrations of normal or regenerating tail extracts of proteins (400 µg/mL) or RNAs extracts (at a final concentration of 5 pmol and 2 pmol) in triplicate. These concentrations were chosen to maximize the use of the total quantity of extracted Proteins or of RNAs from the available samples. Ringer solution and RNA transfection reagent (empty liposomes) were used as a negative control. At 24, 48, and 72 hours, the proliferation rate was tested by adding 70 µL of working solution to each well of 96 well-plate containing 100 µL of the medium. After 4 hours of incubation, the absorbance of the plate was read at 450 nm (XTT specific absorbance) and 660 nm (used to eliminate background signal for all non-specific absorbance).
In vitro images after cell treatment
When cells reached 80% of confluence, they were detached and dissociated by Trypsin-EDTA solution and were plated on a Nitrocellulose membrane with a pore size of 3 µm (Millipore, Milan, Italy) that was pre-coated with 0.2 mg/mL of Matrigel (BD Biosciences, Milan, Italy). After 24 hours, cells were treated with RNAs (15 pmol and 6.25 pmol) or proteins (400 µg/mL) and incubated in a humidified atmosphere at 37 °C containing 5% CO2. After 3 days of incubation, control cells, RNA and proteins treated-cells were photographed with an inverted microscope at 10x and 20x using a digital camera (Leica DMil Camera MC170 S40, Wetzlar, Germany). Then, the membranes with the attached cancer cells were fixed for one hour at room temperature in Karnowsky fixative containing 2.5% glutaraldehyde in phosphate buffer. The fixed material was dehydrated in ethanol and embedded in Epon resin for the following cytological study under light microscopy.
From the resin block, sections of 1-2 µm in thickness were obtained using an ultramicrotome (NOVA, LKB), and they were collected on glass slides, stained with 1% toluidine blue or with Giemsa stain, and observed with a light microscope at 20x or 40x. Tissues from two regenerating tail cones of 3-4 mm in length, collected, fixed, and prepared in previous studies (Alibardi, 2022), were also used for determining the histological composition of the regenerating tails at similar stages as those utilized in the experimental study. These sections were stained with 1% toluidine blue, Hematoxylin-Eosin, or using the nuclear blue fluorophore DAPI, and then observed under an epifluorescent microscope. Images were photographed using a digital camera (Leica DMil Camera MC170 S40, Wetzlar, Germany or a Toupcam, Labwaretools, version 3.7, Treviso, Italy), and selected images were composed into figures making use of Adobe Photoshop, version. 8.0.
Statistics
Comparisons were made by using ANOVA on repeated measures and p < 0.05 was considered as statistically significant. A repeated measures ANOVA was employed to account for both the day and concentration factors. Normality of the data was assessed by evaluating their distribution, which appeared as consistent as a normal. All data are represented as mean ± standard deviation. All the statistical analyses were performed with RStudio, the open-source software of R language.
Acknowledgements
The study was conducted with grant ONIS_SID19_01-BIRD 2019 (M.O), and by Comparative Histolab Padova (L.A.).
Declarations
Conflicts of interest
The authors declare no conflict of interest.
References
Alibardi L. (2014). Histochemical, Biochemical and Cell Biological aspects of tail regeneration in lizard, an amniote model for studies on tissue regeneration. Progress in Histochemistry and Cytochemistry 48: 143-244.
Alibardi L. (2017). Review: Biological and Molecular Differences between Tail Regeneration and Limb Scarring in Lizard: An Inspiring Model Addressing Limb Regeneration in Amniotes. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 328: 493-514.
Alibardi L. (2019). Review: The Regenerating Tail Blastema of Lizards as a Model to Study Organ Regeneration and Tumor Growth Regulation in Amniotes. The Anatomical Record 302: 1469-1490.
Alibardi L. (2021). Review: Regeneration of the tail in lizards appears regulated by a balanced expression of oncogenes and tumor suppressors. Annals of Anatomy - Anatomischer Anzeiger 239: 151824.
Alibardi L. (2022). Immunolocalization of tumor suppressors arhgap28 and retinoblastoma in the lizard Podarcis muralis suggests that they contribute to the regulated regeneration of the tail . Journal of Morphology 283: 973-986.
Alibardi L. (2023). The regenerating tail of lizard transits through a tumour‐like stage represented by the regenerative blastema. Acta Zoologica 104: 489-496.
Anver M. R., (1992). Amphibian tumors: a comparison of anurans and urodeles. In vivo (Athens, Greece) 6: 435-438.
Asashima M., Oinuma T., Meyer-Rochow V. B., (1987). Tumors in amphibia. Zoological Science 4: 411-425.
Bellairs A. D. 'A., Bryant S. V., (1985). Biology of the Reptilia. (Ed. Gans C., Billet F., Maderson P. F. A., ) John Wiley & Sons, NY, USA.
Boilly B., Faulkner S., Jobling P., Hondermarck H. (2017). Nerve Dependence: From Regeneration to Cancer. Cancer Cell 31: 342-354.
Brockes J. (1998). Regeneration and cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1377: M1-M11.
Degan M., Alibardi L. (2023). SnoRNAs may accelerate protein synthesis for the rapid growth of the regenerating tail blastema in the lizard Podarcis muralis . Acta Zoologica 105: 355-365.
Degan M., Dalla Valle L., Alibardi L. (2021). Gene expression in regenerating and scarring tails of lizard evidences three main key genes (wnt2b, egfl6, and arhgap28) activated during the regulated process of tail regeneration. Protoplasma 258: 3-17.
Fior J. (2014). Salamander Regeneration as a Model for Developing Novel Regenerative and Anticancer Therapies. Journal of Cancer 5: 715-719.
Fisher R. E., Geiger L. A., Stroik L. K., Hutchins E. D., George R. M., Denardo D. F., Kusumi K., Rawls J. A., Wilson‐Rawls J. (2012). A Histological Comparison of the Original and Regenerated Tail in the Green Anole, Anolis carolinensis . The Anatomical Record 295: 1609-1619.
Fröbisch N. B., Bickelmann C., Witzmann F. (2014). Early evolution of limb regeneration in tetrapods: evidence from a 300-million-year-old amphibian. Proceedings of the Royal Society B: Biological Sciences 281: 20141550.
Gilbert E. A. B., Delorme S. L., Vickaryous M. K. (2015). The regeneration blastema of lizards: an amniote model for the study of appendage replacement. Regeneration 2: 45-53.
Greco N., Onisto M., Alibardi L. (2023). Protein extracts from regenerating lizard tail show an inhibitory effect on human cancer cells cultivated in-vitro. Annals of Anatomy - Anatomischer Anzeiger 250: 152115.
Hutchins E. D., Markov G. J., Eckalbar W. L., George R. M., King J. M., Tokuyama M. A., Geiger L. A., Emmert N., Ammar M. J., Allen A. N., Siniard A. L., Corneveaux J. J., Fisher R. E., Wade J., DeNardo D. F., Rawls J. A., Huentelman M. J., Wilson-Rawls J., Kusumi K. (2014). Transcriptomic Analysis of Tail Regeneration in the Lizard Anolis carolinensis Reveals Activation of Conserved Vertebrate Developmental and Repair Mechanisms. PLoS ONE 9: e105004.
Hutchins E. D., Eckalbar W. L., Wolter J. M., Mangone M., Kusumi K. (2016). Differential expression of conserved and novel microRNAs during tail regeneration in the lizard Anolis carolinensis. BMC Genomics 17: 339.
Jacyniak K., McDonald R. P., Vickaryous M. K. (2017). Tail regeneration and other phenomena of wound healing and tissue restoration in lizards. Journal of Experimental Biology 220: 2858-2869.
Jeong A.J., Chung C.N., Kim H.J., Bae K. S., Choi S., Jun W. J., Shim S. I., Kang T.H., Leem S.H., Chung J. W. (2012). Gecko Proteins Exert Anti-Tumor Effect against Cervical Cancer Cells Via PI3-Kinase/Akt Pathway . The Korean Journal of Physiology & Pharmacology 16: 361.
LeBlanc A. R. H., MacDougall M. J., Haridy Y., Scott D., Reisz R. R. (2017). Caudal autotomy as anti-predatory behaviour in Palaeozoic reptiles. Scientific Reports 8: 3328.
Liu Y., Zhou Q., Wang Y., Luo L., Yang J., Yang L., Liu M., Li Y., Qian T., Zheng Y., Li M., Li J., Gu Y., Han Z., Xu M., Wang Y., Zhu C., Yu B., Yang Y., Ding F., Jiang J., Yang H., Gu X. (2015). Gekko japonicus genome reveals evolution of adhesive toe pads and tail regeneration. Nature Communications 6: 10033.
Londono R., Wenzhong W., Wang B., Tuan R. S., Lozito T. P. (2017). Cartilage and Muscle Cell Fate and Origins during Lizard Tail Regeneration. Frontiers in Bioengineering and Biotechnology 5: 70.
Lozito T. P., Tuan R. S. (2016). Lizard tail regeneration as an instructive model of enhanced healing capabilities in an adult amniote. Connective Tissue Research 58: 145-154.
Mannoor K., Liao J., Jiang F. (2012). Small nucleolar RNAs in cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1826: 121-128.
Murawala H., Ranadive I., Patel S., Desai I., Balakrishnan S. (2018). Protein expression pattern and analysis of differentially expressed peptides during various stages of tail regeneration in Hemidactylus flaviviridis. Mechanisms of Development 150: 1-9.
Nagumantri S. P., Banu S., Idris M. M. (2021). Transcriptomic and proteomic analysis of Hemidactylus frenatus during initial stages of tail regeneration. Scientific Reports 11: 3675.
Oviedo N. J., Beane W. S. (2009). Regeneration: The origin of cancer or a possible cure?. Seminars in Cell & Developmental Biology 20: 557-564.
Patel S., Ranadive I., Buch P., Khaire K., Balakrishnan S. (2022). De Novo Transcriptome Sequencing and Analysis of Differential Gene Expression among Various Stages of Tail Regeneration in Hemidactylus flaviviridis. Journal of Developmental Biology 10: 24.
Prehn R. (1997). Regeneration versus neoplastic growth. Carcinogenesis 18: 1439-1444.
Quattrini D., (1954). Piano di autotomia e rigenerazione della coda nei Sauri. Archivio Italiano di Anatomia ed Embriologia 59: 225-282.
Radha G., Lopus M. (2021). The spontaneous remission of cancer: Current insights and therapeutic significance. Translational Oncology 14: 101166.
Ranadive I., Patel S., Buch P., Uggini G., Desai I., Balakrishnan S. (2018). Inherent variations in the cellular events at the site of amputation orchestrate scar‐free wound healing in the tail and scarred wound healing in the limb of lizard Hemidactylus flaviviridis . Wound Repair and Regeneration 26: 366-380.
Sarig R., Tzahor E. (2017). The cancer paradigms of mammalian regeneration: can mammals regenerate as amphibians?. Carcinogenesis 38: 359-366.
Smetana K., Dvořánková B., Lacina L. (2013). Phylogeny, Regeneration, Ageing and Cancer: Role of Microenvironment and Possibility of Its Therapeutic Manipulation. Folia Biologica 59: 207-216.
Vitulo N., Dalla Valle L., Skobo T., Valle G., Alibardi L. (2017). Transcriptome analysis of the regenerating tail vs. the scarring limb in lizard reveals pathways leading to successful vs. unsuccessful organ regeneration in amniotes. Developmental Dynamics 246: 116-134.
Wong A. Y., Whited J. L. (2020). Parallels between wound healing, epimorphic regeneration and solid tumors. Development 147: dev181636.
Xu C., Hutchins E. D., Tokuyama M. A., Wilson-Rawls J., Kusumi K. (2020). Transcriptional analysis of scar-free wound healing during early stages of tail regeneration in the green anole lizard, Anolis carolinensis. Journal of Immunology and Regenerative Medicine 7: 100025.