Int. J. Dev. Biol. 69: 1 - 9 (2025)
Three Decades of the Spanish Society for Developmental Biology (SEBD): Insights and Emerging Perspectives from the 18th Spanish Society for Developmental Biology Meeting (SEBD 2024)
Open Access | Meeting Report | Published: 15 April 2025
Abstract
The Spanish Society for Developmental Biology (SEBD) organized its 18th meeting in October 2024 (hereafter SEBD2024), coinciding with the society’s 30th anniversary and serving as the stage for its celebrations. This article provides an overview of the event, including the speakers, scientific sessions and the different activities related to the anniversary.
Keywords
Developmental Biology, Virtual Meeting, Spanish Society for Developmental Biology, SEBD, Scientific Society, Meeting, Morphogenesis, Organogenesis, Neurodevelopment, Genomics, Cell Biology, Evo-devo, Disease
Introduction
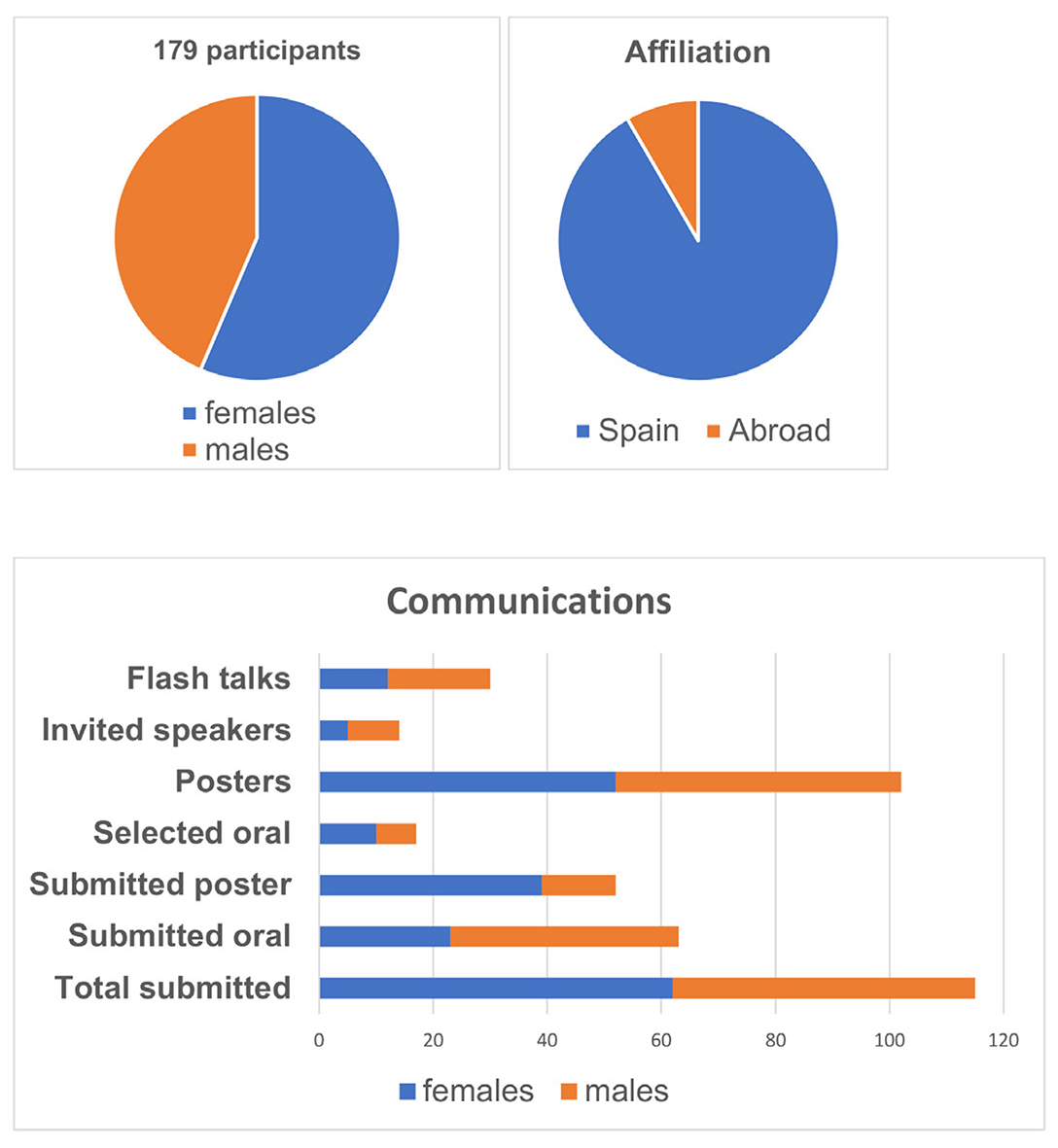
From October 16th to 18th, 2024, the Spanish developmental biologist community gathered in Sant Feliu de Guíxols, Girona, for a unique opportunity to explore and experience the latest research carried out in Spain and abroad. Organized by Sofia J. Araújo (Universitat de Barcelona), Elisa Martí (CSIC) and Cristina Pujades (Universitat Pompeu Fabra), the meeting brought together 179 participants and received 115 scientific abstracts. Attendees ranged from undergraduate students to senior researchers, with a broad participation of Ph.D. students (Fig. 1). The event fostered a warm, joyful and collaborative atmosphere, sparking engaging discussions both during the scientific sessions and social breaks (Fig. 2).
Fig. 1. Proportion of abstracts submitted according to gender and academic afilliation.
The meeting gathered about 180 registrants and received 132 Abstracts. It included 9 invited oral presentations (6 females), 16 short presentations selected from Abstracts (10 females), 31 Flash Talk presentations (13 females) and 115 poster presentations. Registrants ranged from undergraduate to senior researchers, with a broad participation of Ph.D. students.
Opening developmental dynamics lecture
The scientific sessions of the meeting kicked off with a plenary talk by Paul Trainor (Stowers Institute for Medical Research, USA), sponsored by the journal Developmental Dynamics. He presented a novel model for Treacher-Collins Syndrome (TCS), a human craniofacial disorder. Using Tcof1 mouse mutant models, which closely replicate the defects observed in patients, his research highlights the critical role of embryonic neural crest cells in the pathogenesis of TCS. Transcriptomic analyses revealed the upregulation of genes linked to the cell cycle, apoptosis, and p53 signaling, driven primarily by disruptions in ribosome biogenesis. These findings emphasize the conservation of these mechanisms across vertebrate models, including humans, mice, and zebrafish, providing valuable insights into the molecular mechanisms of TCS (Falcon et al., 2022). It was an excellent opening lecture, with a lively follow-up discussion.
Organogenesis
The first session focused on organogenesis using various animal models and both experimental and theoretical approaches. James Sharpe from EMBL Barcelona showcased the evolution of mathematical models used to explain limb growth. He illustrated how the integration of experimental data from numerous mouse samples with increasingly complex models can provide a framework to identify the dominant variables driving the dynamic progression of limb shape during development (Dalmasso et al., 2022). Starting with simple gene networks, the recent models now include more parameters, providing a more detailed and accurate description of the process.
Marta Llimargas from IBMB-CSIC presented compelling data on using the tracheal system of Drosophila melanogaster to understand the morphogenesis of tubular organs. She emphasized the importance of the extracellular matrix in guiding morphogenesis and the role of specific proteins in establishing epithelial tube polarity, demonstrating how proteins from adherens junctions (AJs) interact to influence membrane trafficking crucial for organ development (Letizia et al., 2019). Marta also showed that Wengen, a tumor necrosis factor receptor (TNFR), represses the tracheal terminal cell program by regulating the MAPK pathway downstream of Breathless, an FGFR, through an unconventional mechanism involving their colocalization and complex formation in intracellular vesicles (Letizia et al., 2023).
Marian Ros from IBBTEC-CSIC-University of Cantabria revisited limb development, sharing recent findings on Lmx1b's role in establishing dorso-ventral polarity. She highlighted that two enhancers of this gene have distinct functions in setting up this polarity axis at specific times and locations (Haro et al., 2021). Elements in the LARM1 region exhibited both enhancer and silencer activities in dorsal and ventral regions, revealing the importance of regional functionalization within the same regulatory region.
Finally, Clara Gordillo (Marie Breau lab) from Sorbonne Université-INSERM discussed recent advances in the understanding of how epithelial tissues generate openings in the surface of animals. Using live imaging of the developing zebrafish nostril and various genetic and mechanical manipulations, she provided evidence on the role of mechanical forces in specific cells of the rosette forming the orifice, which is crucial for the correct morphogenesis of the structure (Baraban et al., 2023).
Stem and progenitor cells
This session began with a talk by Meritxell Rovira from the IDIBELL in Barcelona. Meritxell’s laboratory focuses on pancreas regeneration, particularly pancreatic progenitors and their niche, aiming to determine whether facultative progenitors exist in the adult mammalian pancreas. Her talk raised the question of pancreatic duct cellular diversity, as these ducts form a complex network that facilitates secretion yet remain poorly understood. Using single-cell RNA sequencing, organoid cultures, and injury models, her team identified 15 distinct ductal populations in the mouse pancreas, including Wnt-responsive, ciliated, and Flrt3+ cells. These populations exhibit high organoid formation capacity and endocrine differentiation potential. Notably, Wnt-responsive and epithelial-to-mesenchymal transition markers are enriched in chronic pancreatitis and tumors. These findings reveal previously unrecognized ductal populations involved in regeneration and disease, emphasizing the need for lineage-tracing models to further investigate their in vivo functions. Rovira highlighted that organoid differentiation efficiency into the endocrine lineage remains low, and there is still no reliable method to predict which organoids will successfully differentiate. Her team observed distinct morphological changes when organoids begin to differentiate. Therefore, an imaging-based screening approach would be highly valuable in identifying organoids with high differentiation potential and in exploring the molecular, chromatin, and signaling mechanisms that drive this process.
This session was followed by a short talk by Salvador Herrera (Centro Andaluz de Biología del Desarrollo (CABD), CSIC-UPO-JdA, Sevilla) about a stem cell activation state that couples spermatogenesis with social interactions in male animals. Reproduction is fundamental to animals, requiring the coordination of social behavior, physiology, and gamete production for success. How social cues are perceived, and influence physiology and gametogenesis remain an open question. While extensive research has explored this coordination in females across species, its role in males is less understood. Using the Drosophila male as a model, Herrera showed that the mere presence of potential mating partners activates testis stem cell populations, enhancing spermatogenesis. This response relies on pheromonal communication, independent of direct interactions or mating. At the molecular level this is coordinated through TNF-α/Eiger and JNK pathways, as well as changes in calcium dynamics in cyst stem cells. Together they drive increased germline stem cell proliferation (Martin-Diaz and Herrera, 2024).
The session continued with a shift in topic, moving from long-range signaling to DNA damage control. Juan Garrido (Acaimo González lab), also from CABD-CSIC in Seville, gave a compelling talk about genome integrity control and cell survival in the female germline stem cell niche of Drosophila. In this context, proper gamete formation involves the mitosis of germline stem cells followed by meiosis, a process that induces DNA double-strand breaks (DSBs) to facilitate meiotic recombination. Juan's research explored how germline stem cells survive upon high incidence of DSBs while preserving genome integrity, identifying Bruno, a germline translational repressor, as a key player in this process. Using Bruno full and heterozygous knockouts (KOs) combined with a p53 reporter, which is activated downstream of persistent DSB damage, they found that depletion of just one copy of Bruno results in increased p53 activation in the germline, while complete Bruno depletion leads to p53 activation in early oogenesis. This indicates that Bruno dosage is crucial for apoptotic regulation. Further experiments using mei-W68 mutants, which prevent meiotic DSBs, revealed that Bruno’s role in blocking DSB induction is not restricted to meiosis but extends to genome integrity control in both female and male germline stem cells. This suggests a possible link between Bruno and the prevention of replication stress in these cells. Overall, Juan's findings shed light on a critical developmental process—how genome integrity is maintained in the germline and how a balance between DNA repair and cell death is achieved in this cell population.
The session concluded with a talk by Miguel Manzanares from CBSO Madrid, a long-standing member of the developmental biology community. Miguel presented the latest advances on understanding the impact of chromatin architecture on early mouse embryo development. Early embryonic stages are characterized by a relative lack of 3D chromatin structure, which in mammals becomes established after the first cell divisions, coinciding with the loss of totipotency. His research investigated whether this acquisition of chromatin structure is necessary for proper embryo development using both genetic (maternal-zygotic deletion of the chromatin insulator protein CTCF) and drug-based approaches (curaxin). A challenging Hi-C analysis of single blastocyst embryos revealed that while CTCF is required for maintaining 3D genome structure at early developmental stages in mice, the transcriptomic changes observed in mutant embryos cannot be solely explained by the loss of 3D chromatin architecture. Instead, CTCF appears to regulate gene expression through local binding (Andreu et al., 2022). Next, Miguel demonstrated how curaxin treatment mimics the effect of CTCF depletion in mouse embryos and embryonic stem cells. Their results show that curaxin-treated mouse embryonic stem cells undergo a "reprogramming" towards a totipotent-like state resembling the two-cell stage embryo, similar to what was previously described for CTCF knockdown (Nora et al., 2017), including strong induction of the totipotency marker Zscan4. These findings highlight a promising avenue for using curaxin to disrupt 3D chromatin structure in both mouse embryonic stem cells and early embryos. Finally, Miguel presented their latest projects on how DNA replication impacts embryonic progression and the establishment of the first lineages.
Following this session, the first day of the meeting ended with flash talks selected from the submitted posters, providing a sneak peek into the research projects.
Development journal lecture
In her keynote presentation at the SEBD meeting, sponsored by the journal Development, Isabel Fariñas, a leading expert in the field of adult neural stem cells, provided a comprehensive overview of the state of the art in this area of research. Many groundbreaking contributions to the field have emerged from her laboratory at the University of Valencia (Blasco-Chamarro and Fariñas, 2023). She also presented novel findings that emphasized the critical role of interactions between neural stem cells and their extracellular niche, with a particular focus on the importance of extracellular matrix (ECM)-associated proteins in regulating stem cell quiescence. At the conclusion of her presentation, the organizers had the privilege of announcing the extraordinary news that Isabel Fariñas had been awarded this year's Ramón y Cajal National Science Prize by the Spanish Ministry of Science. The announcement was met with heartfelt applause and an emotional ovation, celebrating her remarkable achievement.
Morphogenesis
With Isabel Fariñas’ plenary talk ending on such a high note, the bar for the speakers of the Morphogenesis session had been suddenly set at an incredible height. Not only did they rise to the challenge but also presented us with one of the most vibrant sessions of the meeting. Divided into two parts, this session featured eight talks covering a wide range of questions and systems in which modern developmental biologists are approaching the emergence of form during development and regeneration with the help of the latest genetic and imaging techniques, plus growing support from physics and math.
Morphogenesis I
Fernando Casares, from the Andalusian Center of Developmental Biology (CABD), and former SEBD president, opened the session. Fernando told us about gill regeneration in the mayfly Cloeon (Martin-Blanco et al., 2024). Fernando explained us how after amputation, regenerating gills grow faster than during normal development, revealing the intriguing capability of regenerating organs to sense not only the spatial proportions they need to achieve, but also the temporal urge to recover functional capabilities. Regenerating gills show increased proliferation rates and cell area compared to developing gills, which correlates to higher levels of cell cycle-related genes and growth promoting genes (i.e. Insulin, Wnt, Activin and TOR pathways) revealed by comparative transcriptomic analysis. These transcriptomic analyses also revealed protein neddylation and other proteostatic processes to be important during mayfly gill regeneration, novel molecules not previously described for insect appendage regeneration. Finally, they proved that the role of some of these novel genes is conserved during Drosophila wing regeneration, supporting the idea of a widespread function of these pathways in insect regeneration. Overall, Fernando presented a novel model of insect regeneration, the mayfly Cloeon, from a molecular and functional perspectives, reporting novel regenerative paradigms and molecular players
Next, Yanín Guerra (Elisabeth Fischer-Friedrich´s lab), from the Technical University of Dresden, Germany, presented her work on how the shape of epithelia is influenced by the basement membrane (BM), the collagen IV-rich planar matrix polymer underlying animal epithelia (Pastor-Pareja, 2020). For this, she uses genetic and optogenetic manipulations, pharmacological drugs, osmotic and enzymatic treatments, and atomic force microscopy (AFM) to measure tension changes in the BM of the Drosophila imaginal wing disc, directly accessible to the AFM cantilever. The main conclusion is that the BM membrane is the main determinant of tension on the basal side of the epithelium. While morphogenetic force on the apical side is well known to rely on actomyosin contractility at the level of the zonula adherens, basal force generation, according to her results, works quite differently. This is despite recent emphasis on basal acto-myosin networks contracting in a way analogous to apical ones. Karla Yanín’s work, however, shows that acto-myosin contraction on the lateral side, shortening cells apico-basally, generates a basal expanding force that is opposed by the limited elasticity of the BM. Accordingly, she proposes an updated mathematical framework to understand epithelial shape in which the interplay of apical contractility on one side and basement membrane elasticity/lateral contractility on the other dictates epithelial height and formation of concave or convex folds (Guerra Santillán et al., 2024).
Also using the Drosophila wing disc, José Esteban Collado (Florenci Serras lab), from the University of Barcelona, told us about his PhD work studying early signals during regeneration. This damaging system is controlled by temperature and entirely transgenic, combining the LexA/LexAop and GAL4/UAS dual expression systems (Santabárbara-Ruiz et al., 2015). Taking advantage of the high throughput and reproducibility of this system, José demonstrated the critical role of reactive oxygen species (ROS) from damaged cells in activating the JNK and p38 MAPK cascades, master orchestrators of the regenerative response. Importantly, he showed evidence that common upstream MAPKKK Ask1 is not only activated by ROS (Santabárbara-Ruiz et al., 2019), but also modulated by the nutrient-sensing Akt pathway so that positive nutritional conditions potentiate the p38 regenerative response whereas restrictive conditions favor partially pro-apoptotic JNK (Esteban-Collado et al., 2021). Finally, in addition to activating Ask1, ROS has an even earlier, more upstream role in triggering p38 and JNK signaling, in this case through the Drosophila TNF receptor Wengen and TNF receptor-associated TRAF1, apparently through direct ROS action and in a way that surprisingly is ligand-independent (Esteban-Collado et al., 2024).
To close this Morphogenesis I session, we had Manohara Mahadeva, from the Institute of Animal Reproduction and Food Research Polish Academy of Sciences, presenting his work on the relevance of membrane potential for somite embryo clock formation and growth (Mahadeva et al., 2025). Manohara studies somitogenesis in chick embryos and reported that membrane potential is different between somites with different onsets of differentiation and expression of specific transcription factors, although they are morphologically identical. Interestingly, induced depolarization of forming somites accelerates somite periodicity and growth, while hyperpolarization goes the opposite way. With this work, Manohara and colleagues, are the first to show that the periodicity of somite formation and somite growth, which depend on different cellular processes (migration vs. proliferation), can be collectively modified just by perturbing membrane potential of somite-forming cells (Mahadeva et al., 2025).
30-year anniversary celebration
This year we celebrated the 30th anniversary of the Spanish Society for Developmental Biology (SEBD), marking three decades of commitment to advancing our understanding of the mechanisms that drive the development of living organisms (Palmeirim and Aréchaga, 2009). Founded by Antonio García-Bellido, Juan Aréchaga and other colleagues in 1994, the SEBD has grown into a supportive and collaborative community, bringing together experienced researchers and a growing number of young scientists contributing fresh perspectives to the field. Developmental Biology’s integrative approach has been crucial for understanding diverse processes, from regeneration to evolution. The involvement of new talent continues to enrich our Society, ensuring that Spanish science remains active and innovative in this area of research.
To mark this milestone, we set aside time during our annual meeting to recognize the contributions of individuals who have shaped the SEBD over the years. Awards were presented to former SEBD presidents and board members in acknowledgment of their service, and an honorary membership and prize was given to Angela Nieto (Instituto de Neurociencias, Alicante) as a special recognition to her dedication to SEBD. Dr. Nieto also gave a short talk on the history of the Society, providing a fitting reflection on three decades of progress and collaboration (Fig. 3).
Fig. 3. An overview of the 30-year anniversary celebrations.
Award ceremony to past SEBD board members and an honorary membership and prize awarded to Angela Nieto. From left to right, our president, Eloisa Herrera with the different awardees: Top row: Eloisa (in black) with Angela Nieto and with Sergio Casas; Middle row: Eloisa with Isabel Fariñas, Rosa Barrio and Sergio Casas; with James Sharpe, Victor Borrell and Berta Alsina; Bottom row: current and past board members; Eloisa with Fernando Casares and Miguel Torres.
Morphogenesis II
Next, we moved on to the second part of the morphogenesis scientific session. It started with a talk by former president of SEBD, Miguel Torres, from the Spanish National Centre for Cardiovascular Research (CNIC), on the specification and differentiation of cardiovascular lineages in the mouse embryo. Previous clonal analyses have suggested that the specification of early cell lineages in the mouse epiblast initiates shortly before gastrulation. However, single-cell and spatial transcriptomics have not been able to identify cell lineage-specific expression profiles. Thus, Miguel Torres and collaborators set out to search differences in epigenetic modifications. By performing single-cell epigenomics (snATAC-seq) of pre-gastrula mouse embryos, they found the earliest epigenetic signatures of mesodermal/endothelial, endodermal and ectodermal cell lineages. They focused on the endothelial cell lineage and using a tour-de-force combination of clonal analysis, live imaging and quantitative analysis, they show that the early epigenetic priming of endothelial precursors remains phenotypically silent during gastrulation, making them indistinguishable in behavior from other mesodermal cells. These primed endothelial cells showed the first signs of differentiation after their ingression through the primitive streak and during their migration towards the cardiogenic region, which manifest in specific migratory features and increased affinity for the endoderm (Sendra et al., 2024).
Miguel’s talk was followed by two of abstract-selected talks. The first one, by María Jesús Gómez-Lamarca, from the Seville University and Institute of Biomedicine of Seville (IBiS, Sevilla), described CartoCell, a deep-learning based image analysis method to quantify cell shape in three dimensions (Andrés-San Román et al., 2023) and showed how it can be applied to study the cellularization process during early stages of Drosophila embryogenesis. This was followed by a talk by Guillermo Serrano-Najera (Ben Steventon lab) from the University of Cambridge, in which he described his work combining kinematic analysis and computational models to understand avian gastrulation based on attractors and repellents, large-scale tissue flows that arise from the coordinated activity of thousands of cells (Serrano Nájera et al., 2024).
The last talk of the session was presented by Jerome Solon (Instituto Biofisika, Leiola), who shared recent results on the biomechanics of wound healing in Drosophila embryos. Through a combination of live imaging, genetic perturbation and biophysical modelling, he showed data suggesting that tissue stiffness is an important parameter controlling the rate of closure of epithelial wounds. He further showed that upon wound generation, neighboring cells to the wound exhibit an astonishing compaction of the chromatin, revealing new levels of mechanosensitive mechanisms of wound healing regulation.
ISDB-C&D lecture
Sponsored by the ISDB, Nadia Mercader (University of Bern, Switzerland) presented her research on the processes underlying zebrafish heart regeneration. She highlighted that myocardial regeneration is initiated by the deposition of fibrotic tissue, which later regresses as newly formed cardiomyocytes replace the lost myocardium. Her talk explored the origin and fate of fibroblasts, emphasizing their crucial role in supporting cardiomyocyte proliferation during regeneration (Sánchez-Iranzo et al., 2018). Additionally, she discussed how metabolic adaptations in cardiomyocytes, particularly the assembly of the mitochondrial respiratory chain, influence their regenerative capacity, shedding light on key mechanisms driving heart repair (García-Poyatos et al., 2024).
Neurodevelopment I
In this afternoon session, we entered in the mysteries of mammalian brain development and the contribution of progenitors’ subtypes to the regional patterning and expansion of the brain. We started by a talk from Victor Borrell that focused on the expansion of the mammalian cortex. He summarized the state-of-the-art in the field and described the molecular hallmarks (genetic and epigenetic) and the anatomical contribution from multiple subtypes of Radial Glia cells (RGCs) in ferrets. Notably, the presence of two identities of RGC, amplificative and neurogenic, defines the presence of gyrus and sulcus (Del-Valle-Anton et al., 2024).
Next, we followed the session with short talks. Laia Caudet (Andreas Sagner lab) from the FAU Erlangen in Germany showed to us the contribution of sequential expression of Pou3f1 and Pou2f2 to the generation of a gene regulatory network that determines the spatio-temporal development of spinal motor neurons. This sequential transcriptional control determines the segregation of long-range projection neurons in the spinal cord. Diego García, junior Group Leader at IBIS, Seville, showed us how to make a “newron”, Diego tackled the ontogeny of the olfactory bulb (OB) neuronal population and the neuron identity derived from the intrinsically controlled regional-associated origin from interneuron progenitors at the ventricular and subventricular zones in mouse forebrain. The last talk of this session was given by Alfonso Aguilera (Marta Nieto lab), from CNB-CSIC at Madrid, who explained us how long-ranged transcallosal connectivity in cortical upper layers is determined by the GABAergic interneurons. Alfonso described the presence of SST and PV motifs in cortical pyramidal excitatory neurons necessary for the retraction of transcallosal L2/L3 neurons. Moreover, the persistence of L4 transcallosal projections is determined by the temporal accuracy in the PV interneurons activation, extending for the first two postnatal weeks (Bragg-Gonzalo et al., 2024).
Altogether, this session helped us to understand the relevance of the spatio-temporal control of molecular and cellular activation in the mammalian brain development.
Neurodevelopment II
Session two began with an invited talk by Berta Alsina (University of Pompeu Fabra, Barcelona), who discussed the cellular dynamics underlying neuroblast delamination, migration, and coalescence during the development of the inner ear ganglion in zebrafish (Bañón and Alsina, 2023; Nomdedeu-Sancho and Alsina, 2023). She captivated the audience with live imaging of pioneer axons extended by ganglions, illustrating how these axons find their target cells. Her research uncovered the critical role of chemokines as guidance cues in axonal projection.
Following this, three selected talks based on submitted abstracts were presented. The first, by Véronica Murcia (Eloisa Herrera lab) from the Institute of Neurosciences, Alicante, explored how distinct axonal translatomes play a crucial role in axon guidance in both contralateral and ipsilateral retinal ganglion cell axons, offering a look at the mechanisms driving axon guidance during CNS development. The second selected talk, by Axelle Wilmerding (Elisa Marti lab) from the IBMB-CSIC, Barcelona, used the developing chick spinal cord as a model to describe, in great live-cellular detail, the mechanisms of apical constriction during primary neurogenesis. She focused on the dynamic remodeling of the ciliary rootlet in coordination with the cytoskeleton. The third talk, presented by Matthias Blanc (Cristina Pujades lab) from the University of Pompeu Fabra, Barcelona, introduced an exceptional digital 3D atlas to monitor changes in the growth of the neuronal differentiation domain in the zebrafish hindbrain over time (Blanc et al., 2022). His work examined how proneural genes shape neuronal diversity, revealing asynchronous production, distinct neurogenin1 contributions, and proneural dependencies, with calcium imaging linking activity to neuronal origins and birthdates.
EvoDevo and genomics
The last session of the meeting was devoted to EvoDevo and Genomics, two fundamental aspects of Developmental Biology that help unraveling how the evolution of the mechanisms of development and genome regulation promote biodiversity and adaptations. Ozren Bogdanovic presented data from his lab in CABD (Sevilla) highlighting how conservation and divergence of DNA methylation in different genomic compartments between vertebrate and non-vertebrate animals appears as a central aspect to understand the evolution of developmental gene regulation. Recent findings point to mechanisms of active 5mC removal from regulatory regions is a common feature of deuterostome embryogenesis suggestive of an unexpected deep conservation of a major gene-regulatory module (Angeloni et al., 2024). Juan Pascual Anaya (UMA, Málaga) presented how the whole genome sequencing of a hagfish specie reveals that the second round of genome duplication suffered during early vertebrate evolution occurred in the gnathostome lineage after its split from the cyclostomes, which in turn might have suffered extra rounds of whole genome duplication (Yu et al., 2024). This is a key finding that illuminates the evolution of the vertebrate genetic toolkit and how may correlate with the evolution of developmental adaptations of vertebrates. Evo-Devo comparison of rhythmic yolk contractions (a.k.a. Tsunamis), led to Cielo Lucia Centola (Juan Ramón Martinez Lab) from the CABD, Sevilla, to discover that block of the “tsunamis” can lead to reversible developmental arrest and how these rhythmic yolk contractions are crucial for the correct progression of gastrulation in different teleost species. Finally, Marta Iglesias (Arnau Sebé-Pedrós lab), from the CRG, Barcelona, showed how data of single cell transcriptomic in the cnidarian Nematostella vectensis has helped to decode cell-type gene regulation, revealing how cis-regulatory sequence similarities among cell types reflect known ontogenetic relationships among them, and thus, can be used to study unexplored cell developmental or evolutionary origins.
Flash-talks
Flash talks have become a widely utilized format in scientific conferences, providing an efficient means for researchers to present the core aspects of their work in a concise and impactful manner. These short presentations are designed to highlight key findings or methodologies, acting as a primer to encourage more detailed discussions during subsequent sessions.
On both evenings of the meeting, we held flash-talk sessions featuring presentations selected from submitted abstracts (Fig. 2). Poster presenters were allotted two minutes to deliver a broad overview of their research, summarizing their main findings and the significance of their work. This format served to stimulate interest and direct attendees to their posters for further discussion. The selected talks encompassed topics from all scientific sessions of the meeting, offering participants a representative snapshot of the research presented. Importantly, this format provided an excellent platform for fostering discussion and gave early-career researchers the opportunity to present their data to a broad audience, promoting visibility and engagement within the scientific community.
Prizes and closing session
During the closing session of the meeting, prizes were announced, all evaluated by designated scientific committees. The SEBD board announced the winner of the “José Luis Gómez-Skarmeta Award to Scientific excellence in Developmental Biology” for young PIs. Darío Lupiañez (CABD, Sevilla) won this prize for his contributions to genome regulation in development and gave a prize talk on the past and present work from his research laboratory.
Also announced by the board were the “SEBD Awards for scientific excellence in doctoral theses” for the year’s 2021, 2022 and 2023, given to Verónica Company Devesa (Instituto de Neurociencias (IN) de Alicante), Lorena Bragg Gonzalo (Centro Nacional de Biotecnología, CNB-CSIC) and Lucía del Valle Anton (Instituto de Neurociencias (IN) de Alicante), respectively, for their outstanding Ph.D. work. All candidates were given the opportunity to give a short talk on their thesis work.
This was followed by the best talk and poster prizes awarded by the Developmental Dynamics. The best talk selected by abstract was given ex-aqueo to Clara Gordillo (Sorbonne Université), who presented her results on the epithelial remodeling driving the expansion of the zebrafish nostril, and Cielo Centola (CABD-CSIC), who presented her results on the species-specific rhythmic yolk contractions (aka tsunami) and their role in teleost gastrulation. The poster prizes went to Miquel Sendra (CNIC) for his poster on the epigenetic priming of cardiac endothelium in the mammalian epiblast; Adrián Holguín (IDIBELL-UB) for his poster on the identity of Peribiliary Glands (PBG) and Pancreatic Ductal Glands (PDG); and Yara el Majzoub (IBMB-CSIC) for her poster on human spinal cord organoids.
The winner of the Photography Competition sponsored by Zeiss was also awarded. In the photo SciArt competition, there were many beautiful images from different developmental biology models and methods. The winning image, a confocal image of retinal axons named “Solar storm” was authored by Veronica Murcia Belmonte (Instituto de Neurociencias (IN) de Alicante) (Fig. 4).
At the end of the meeting, Luisma Escudero, received this year’s SEBD Science Communication prize and entertained the audience with his outreach videos and stories plus the presentation of his latest book on morphogenesis (Ulldemolins and Escudero, 2023).
The closing ceremony finished on a happy note, with the offering of a gift to Sofia Araújo (Genetics-UB) for her work in the organizing committee (Fig. 5).
Acknowledgements
We would like to express our deepest gratitude to the Spanish Society for Developmental Biology (SEBD) for fostering a vibrant and supportive community over the past three decades. We also extend our heartfelt thanks to our respective institutions and research funders for their unwavering support and contributions to our work. We would like to thank the sponsors of the 18th Spanish Society for Developmental Biology Meeting: the Developmental Studies Hybridoma Bank (DSHB), the Company of Biologists, Development, ISDB-Cells and Development, eLife and Developmental Dynamics. Our gratitude also goes to Zeiss for sponsoring the photography prize. We are immensely grateful to our keynote and invited speakers for sharing their invaluable insights and expertise. Their contributions have greatly enhanced the quality and impact of the meeting. Finally, we thank all the participants of the meeting. Everyone’s enthusiasm, engagement, and contributions have made this event a resounding success.
References
Andrés-San Román J. A., Gordillo-Vázquez C., Franco-Barranco D., Morato L., Fernández-Espartero C. H., Baonza G., Tagua A., Vicente-Munuera P., Palacios A. M., Gavilán M. P., Martín-Belmonte F., Annese V., Gómez-Gálvez P., Arganda-Carreras I., Escudero L. M. (2023). CartoCell, a high-content pipeline for 3D image analysis, unveils cell morphology patterns in epithelia. Cell Reports Methods 3: 100597.
Andreu M. J., Alvarez-Franco A., Portela M., Gimenez-Llorente D., Cuadrado A., Badia-Careaga C., Tiana M., Losada A., Manzanares M. (2022). Establishment of 3D chromatin structure after fertilization and the metabolic switch at the morula-to-blastocyst transition require CTCF. Cell Reports 41: 111501.
Angeloni A., Fissette S., Kaya D., Hammond J. M., Gamaarachchi H., Deveson I. W., Klose R. J., Li W., Zhang X., Bogdanovic O. (2024). Extensive DNA methylome rearrangement during early lamprey embryogenesis. Nature Communications 15: 1977.
Bañón A., Alsina B. (2023). Pioneer statoacoustic neurons guide neuroblast behaviour during otic ganglion assembly. Development 150: dev201824.
Baraban M., Gordillo Pi C., Bonnet I., Gilles J.F., Lejeune C., Cabrera M., Tep F., Breau M. A. (2023). Actomyosin contractility in olfactory placode neurons opens the skin epithelium to form the zebrafish nostril. Developmental Cell 58: 361-375.e5.
Blanc M., Dalmasso G., Udina F., Pujades C. (2022). A dynamic and expandable digital 3D-atlas maker for monitoring the temporal changes in tissue growth during hindbrain morphogenesis. eLife 11: e78300.
Blasco-Chamarro L., Fariñas I. (2023). Fine-tuned Rest: Unveiling the Regulatory Landscape of Adult Quiescent Neural Stem Cells. Neuroscience 525: 26-37.
Bragg-Gonzalo L., Aguilera A., González-Arias C., De León Reyes N. S., Sánchez-Cruz A., Carballeira P., Leroy F., Perea G., Nieto M. (2024). Early cortical GABAergic interneurons determine the projection patterns of L4 excitatory neurons. Science Advances 10: eadj9911.
Dalmasso G., Musy M., Niksic M., Robert-Moreno A., Badía-Careaga C., Sanz-Ezquerro J. J., Sharpe J. (2022). 4D reconstruction of murine developmental trajectories using spherical harmonics. Developmental Cell 57: 2140-2150.e5.
Del-Valle-Anton L., Amin S., Cimino D., Neuhaus F., Dvoretskova E., Fernández V., Babal Y. K., Garcia-Frigola C., Prieto-Colomina A., Murcia-Ramón R., Nomura Y., Cárdenas A., Feng C., Moreno-Bravo J. A., Götz M., Mayer C., Borrell V. (2024). Multiple parallel cell lineages in the developing mammalian cerebral cortex. Science Advances 10: eadn9998.
Esteban-Collado J., Corominas M., Serras F. (2021). Nutrition and PI3K/Akt signaling are required for p38-dependent regeneration. Development 148: dev197087.
Esteban-Collado J., Fernández-Mañas M., Fernández-Moreno M., Maeso I., Corominas M., Serras F. (2024). Reactive oxygen species activate the Drosophila TNF receptor Wengen for damage-induced regeneration. The EMBO Journal 43: 3604-3626.
Falcon K. T., Watt K. E. N., Dash S., Zhao R., Sakai D., Moore E. L., Fitriasari S., Childers M., Sardiu M. E., Swanson S., Tsuchiya D., Unruh J., Bugarinovic G., Li L., Shiang R., Achilleos A., Dixon J., Dixon M. J., Trainor P. A. (2022). Dynamic regulation and requirement for ribosomal RNA transcription during mammalian development. Proceedings of the National Academy of Sciences 119: e2116974119.
García-Poyatos C., Arora P., Calvo E., Marques I. J., Kirschke N., Galardi-Castilla M., Lembke C., Meer M., Fernández-Montes P., Ernst A., Haberthür D., Hlushchuk R., Vázquez J., Vermathen P., Enríquez J. A., Mercader N. (2024). Cox7a1 controls skeletal muscle physiology and heart regeneration through complex IV dimerization. Developmental Cell 59: 1824-1841.e10.
Guerra Santillán K. Y., Dahmann C., Fischer-Friedrich E. (2024). Elastic Contractile Stress in the Basement Membrane Generates Basal Tension in Epithelia. PRX Life 2: 013004.
Haro E., Petit F., Pira C. U., Spady C. D., Lucas-Toca S., Yorozuya L. I., Gray A. L., Escande F., Jourdain A.S., Nguyen A., Fellmann F., Good J.M., Francannet C., Manouvrier-Hanu S., Ros M. A., Oberg K. C. (2021). Identification of limb-specific Lmx1b auto-regulatory modules with Nail-patella syndrome pathogenicity. Nature Communications 12: 5533.
Letizia A., Espinàs M. L., Giannios P., Llimargas M. (2023). The TNFR Wengen regulates the FGF pathway by an unconventional mechanism. Nature Communications 14: 5874.
Letizia A., He D.Q., Astigarraga S., Colombelli J., Hatini V., Llimargas M., Treisman J. E. (2019). Sidekick Is a Key Component of Tricellular Adherens Junctions that Acts to Resolve Cell Rearrangements. Developmental Cell 50: 313-326.e5.
Mahadeva M., Niestępski S., Kowacz M. (2025). Modifying membrane potential synchronously controls the somite's formation periodicity and growth. Developmental Biology 517: 317-326.
Martin-Blanco C. A., Navarro P., Esteban-Collado J., Serras F., Almudi I., Casares F. (2024). Gill regeneration in the mayfly Cloeon uncovers new molecular pathways in insect regeneration. Open Biology 14: 240118.
Martin-Diaz J., Herrera S. C. (2024). A stem cell activation state coupling spermatogenesis with social interactions in Drosophila males. Cell Reports 43: 114647.
Nomdedeu-Sancho G., Alsina B. (2023). Wiring the senses: Factors that regulate peripheral axon pathfinding in sensory systems. Developmental Dynamics 252: 81-103.
Nora E. P., Goloborodko A., Valton A.L., Gibcus J. H., Uebersohn A., Abdennur N., Dekker J., Mirny L. A., Bruneau B. G. (2017). Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell 169: 930-944.e22.
Palmeirim I., Arechaga J. (2009). A small great history of the sister Societiesof Developmental Biology in Spain and Portugal. The International Journal of Developmental Biology 53: 1261-1268.
Pastor-Pareja J. C. (2020). Atypical basement membranes and basement membrane diversity – what is normal anyway?. Journal of Cell Science 133: jcs241794.
Sánchez-Iranzo H., Galardi-Castilla M., Sanz-Morejón A., González-Rosa J. M., Costa R., Ernst A., Sainz de Aja J., Langa X., Mercader N. (2018). Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proceedings of the National Academy of Sciences 115: 4188-4193.
Santabárbara-Ruiz P., Esteban-Collado J., Pérez L., Viola G., Abril J. F., Milán M., Corominas M., Serras F. (2019). Ask1 and Akt act synergistically to promote ROS-dependent regeneration in Drosophila. PLOS Genetics 15: e1007926.
Santabárbara-Ruiz P., López-Santillán M., Martínez-Rodríguez I., Binagui-Casas A., Pérez L., Milán M., Corominas M., Serras F. (2015). ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration. PLOS Genetics 11: e1005595.
Sendra M., McDole K., Jimenez-Carretero D., Hourcade J. D. D., Temiño S., Raiola M., Guignard L., Keller P. J., Sánchez-Cabo F., Domínguez J. N., et al. (2024). Epigenetic priming of embryonic lineages in the mammalian epiblast. bioRxiv Preprint: 2024.01.11.575188.
Serrano Nájera G., Plum A. M., Steventon B., Weijer C. J., Serra M., (2024). Control of Modular Tissue Flows Shaping the Embryo in Avian Gastrulation. bioRxiv Preprint: 2024.07.04.601785.
Ulldemolins R. G., Escudero L. M., (2023). Papá, ¿cómo se enroscan las caracolas?. Editorial Crítica.
Yu D., Ren Y., Uesaka M., Beavan A. J. S., Muffato M., Shen J., Li Y., Sato I., Wan W., Clark J. W., Keating J. N., Carlisle E. M., Dearden R. P., Giles S., Randle E., Sansom R. S., Feuda R., Fleming J. F., Sugahara F., Cummins C., Patricio M., Akanni W., D’Aniello S., Bertolucci C., Irie N., Alev C., Sheng G., de Mendoza A., Maeso I., Irimia M., Fromm B., Peterson K. J., Das S., Hirano M., Rast J. P., Cooper M. D., Paps J., Pisani D., Kuratani S., Martin F. J., Wang W., Donoghue P. C. J., Zhang Y. E., Pascual-Anaya J. (2024). Hagfish genome elucidates vertebrate whole-genome duplication events and their evolutionary consequences. Nature Ecology & Evolution 8: 519-535.