Int. J. Dev. Biol. 68: 47 - 53 (2024)
Coenocystic oogenesis - modification of or deviation from the germ cell cyst paradigm?
Open Access | Essay | Published: 9 July 2024
Abstract
Invertebrate and vertebrate species have many unusual cellular structures, such as long- or short-lived cell-in-cell structures and coenocytes. Coenocytes (often incorrectly described as syncytia) are multinuclear cells derived, unlike syncytia, not from the fusion of multiple cells but from multiple nuclear divisions without cytokinesis. An example of a somatic coenocyte is the coenocytic blastoderm in Drosophila. An astonishing property of coenocytes is the ability to differentiate the nuclei sharing a common cytoplasm into different subpopulations with different fate trajectories. An example of a germline coenocyte is the oogenic precursor of appendicularian tunicates, which shares many features with the somatic coenocyte of Drosophila. The germline coenocyte (coenocyst) is quite an unexpected structure because in most animals, including Drosophila, Xenopus, and mice, oogenesis proceeds within a group (cyst, nest) of sibling cells (cystocytes) connected by the intercellular bridges (ring canals, RCs) derived from multiple divisions with incomplete cytokinesis of a progenitor cell called the cystoblast. Here, I discuss the differences and similarities between cystocyte-based and coenocyst-based oogenesis, and the resemblance of coenocystic oogenesis to coenocytic somatic blastoderm in Drosophila. I also describe cell-in-cell structures that although not mechanistically, cytologically, or molecularly connected to somatic or germline coenocytes, are both unorthodox and intriguing cytological phenomena rarely covered by scientific literature.
Keywords
Coenocyte, syncytium, oogenesis, germ cell cyst, coenocyst, tunicate, insect, Xenopus, mouse
Introduction
Cell and organismal biology are full of wonders, surprises, and departures from the norm. Although we pretend to have a deep knowledge of how cells and organisms are built and function, every day we discover astonishing unorthodox phenomena that were previously undescribed or recorded a long time ago and forgotten. One such example is the existence of cell-in-cell structures (sometimes described as an emperipolesis) in which intact, viable, non-apoptotic cells live and function inside the cytoplasm of other cells. Cell-in-cell structures consisting of lymphocytes inside intestinal epithelial cells were first described in 1864 by the German pathologist and bacteriologist Karl Joseph Eberth, followed, in 1904, by the discovery of tumor cells inside other tumor cells by the German physician and father of oncology Ernst von Leyden (Overholtzer and Brugge, 2008). The cell-in-cell structure can be heterotypic when the internalized cell and the host are of different types or homotypic when both cells are of the same type. Overholtzer and Brugge (2008) list over 100 currently known examples of heterotypic and homotypic cell-in-cell structures. Some of the cell-in-cell structures are short-lived, such as in the case of leukocytes traversing through endothelial cells and released (Fig. 1) or T cells engulfed by melanoma cells and digested to provide nutrients (Cernuda-Morollón and Ridley, 2006; Engelhardt and Wolburg, 2004; Lugini et al., 2006). Other cell-in-cell structures are long-lived, with the host cells serving as the protectors and nursing hubs of internalized cells (Fig. 1). For example, astrocytes promote the survival and proliferation of internalized oligodendrocytes (Nishie et al., 2006; Wu and Raine,1992), thymic nurse cells facilitate the maturation of internalized thymocytes, Kupffer cells in the human fetal liver promote the differentiation of internalized erythroblasts, and follicular dendritic cells facilitate the maturation of internalized B cells (Lee et al., 1999; Pezzano et al., 1995; Tsunoda et al., 2000). However, it remains unknown why only certain types of cells are internalized, and how exactly they escape lysosomal degradation within the host cells.
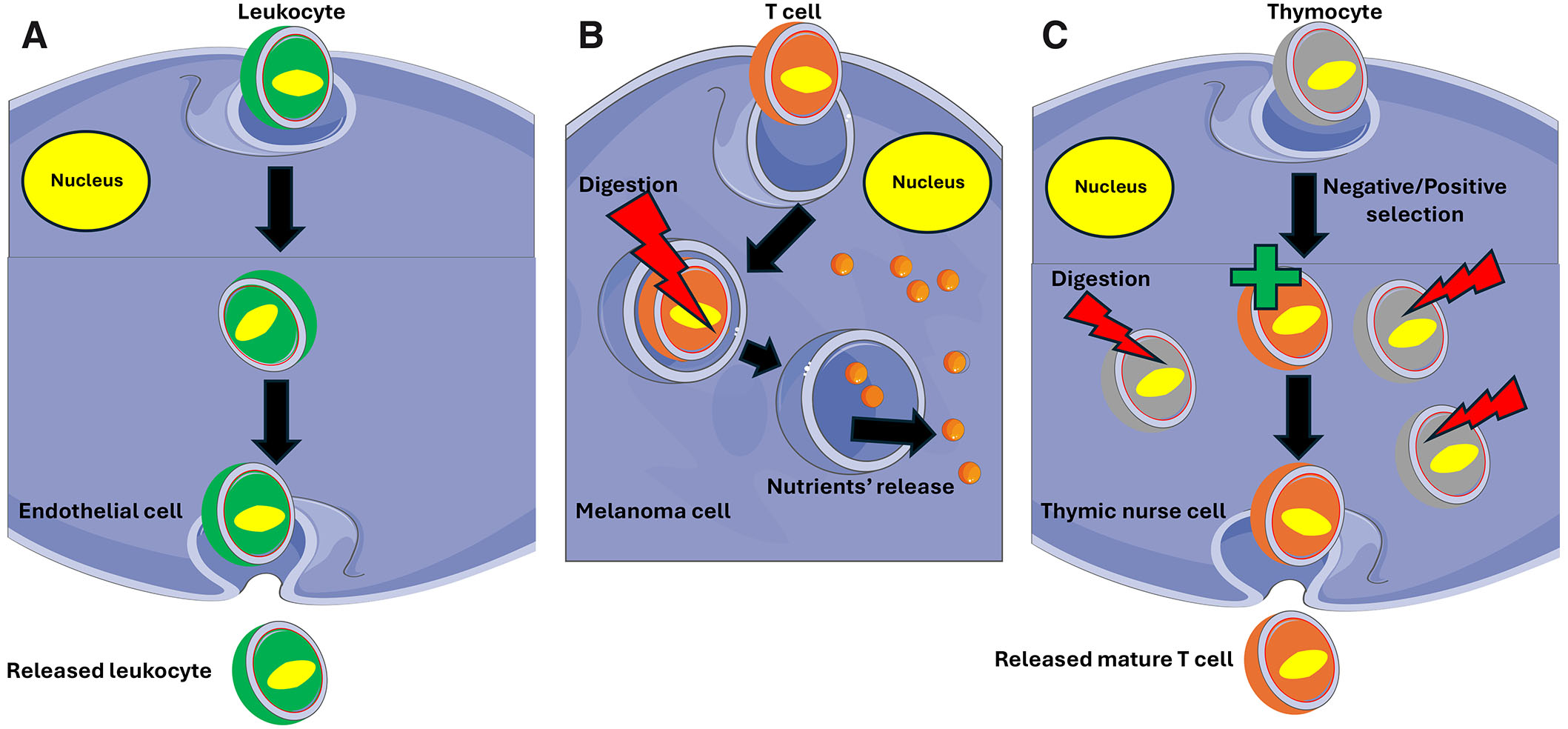
Fig. 1. Cell-in-cell structures.
Cell-in-cell structures can be short-lived (A, B) or longer-lasting (C). (A) Leukocyte passage through the endothelium when the engulfed leukocyte remains in the endothelial cell for a short time before being released on the other side of the endothelial barrier. (B) A T cell ingested by a melanoma cell. After a short time, the T cell is digested through the lysosome pathway, and released nutrients nourish the host cell. (C) A longer-lasting cell-in-cell structure when thymocytes are engulfed by the thymic nurse cell that not only nourishes them, but also performs negative/positive selection releasing mature, antigen-specific, self-tolerant T cells. Thymic nurse cells are epithelial cells that express MHC Class I and MHC Class II antigens. The interaction of these antigens with the developing thymocytes determines whether the thymocyte undergoes positive or negative selection. Thymocytes expressing T cell receptors (TCR) with affinity to MHC class I and II molecules are positively selected while those expressing potentially harmful TCR are deactivated and destroyed (negative selection) by the lysosomal pathway.
Another unorthodox phenomenon, unrelated to the cell-in-cell structures but equally fascinating, is coenocytosis. Coenocytes are multinuclear cells, which, in contrast to syncytia that derive from the fusion of many cells, result from repetitive nuclear divisions without cytokinesis (Daubenmire, 1936). Although coenocytosis is quite common in algae, fungi, and plants, it is anatomically restricted in animals (Ali et al., 2023; Ondracka et al., 2018; Płachno et al., 2024).
An invertebrate example of a coenocyte is the multinuclear blastoderm (usually incorrectly called a syncytium) of the insect's early embryos (Gilbert, 2000; Tram 2002). In many insects, including Drosophila, the interior of a fully-grown egg is occupied by the yolk, and the yolk-free cytoplasm forms a cortical rim at the egg periphery. After fertilization, the centrally located zygotic nucleus divides eight times (each nuclear division lasts around 8 minutes), resulting in 256 nuclei that subsequently migrate to the yolk-free cortical cytoplasm when they undergo four more divisions at a slower rate (Fig. 2A). The cortical cytoplasm contains a network of actomyosin whose contraction during each cell cycle creates a cytoplasmic flow that uniformly distributes nuclei along the cortex (Fig. 2B). Once in the cortex, each nucleus generates an actin-related protein 2/3 complex (Arp2/3)-based actin cap. Subsequently, the caps form cupolas, which surround individual nuclei at the cortex (Tam and Harris, 2024). The uniform spacing of the nuclei and proper alignment of mitotic spindles at the cortex is achieved through repulsive interactions of microtubules associated with each nucleus. The resulting coenocyte is called a syncytial blastoderm. Eventually, the coenocytic blastoderm cellularizes, through the invaginations of the egg cell membrane, forming a blastodermal epithelium consisting of ~ 6000 mononuclear cells (Mazumdar and Mazumdar, 2002), while several nuclei that had migrated to the posterior pole of the egg form the pole cells, which are the precursors of future gametes. The germ cell fate of pole cells is determined by the subpopulation of maternal RNAs and proteins present within the pole plasm (germ plasm) containing germinal granules (nuage) located at the posterior pole of the egg (Fig. 2) (Kloc et al., 2013; Tam and Harris, 2024; Trcek and Lehmann, 2019).
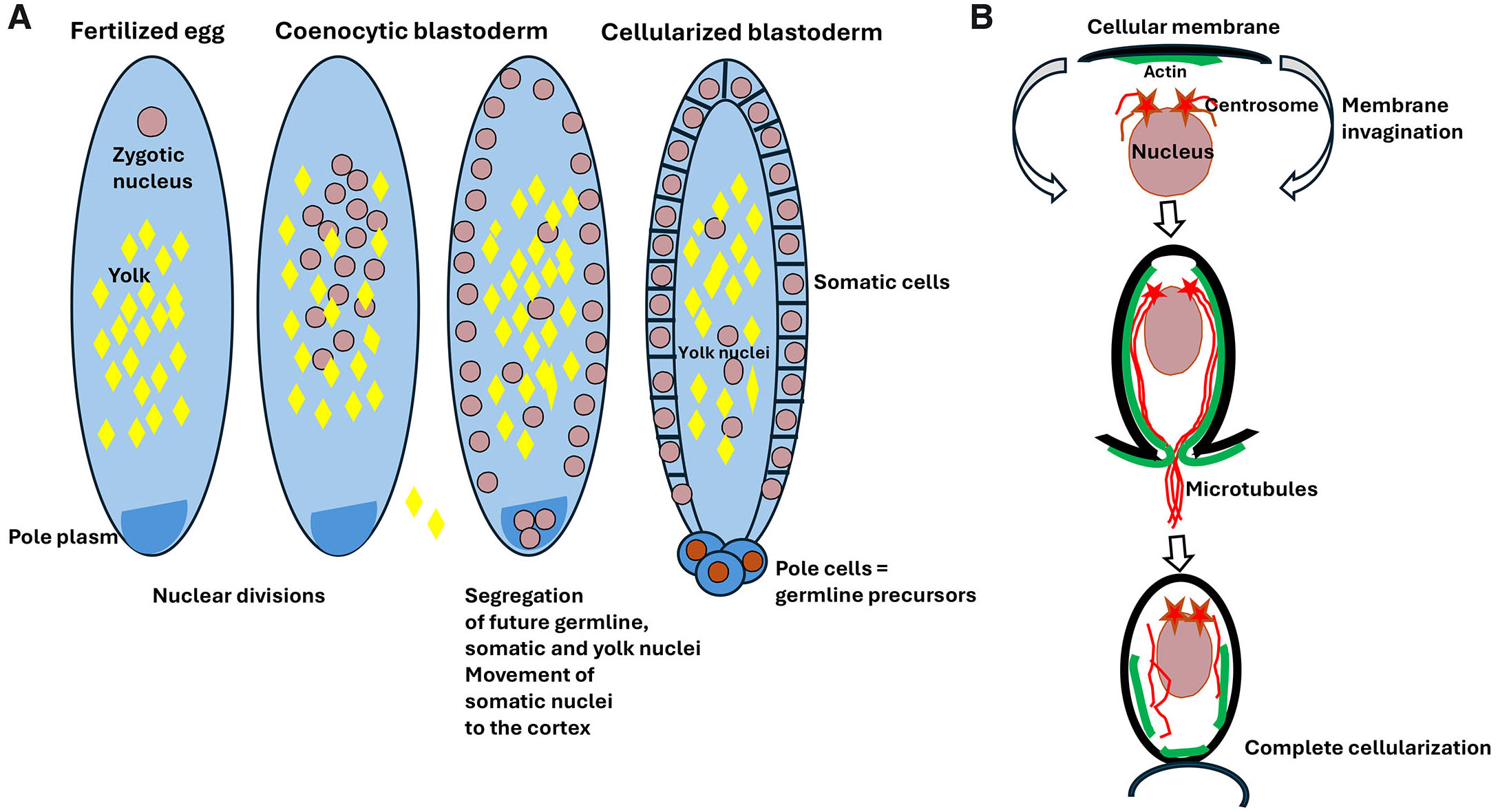
Fig. 2. Drosophila coenocytic (syncytial) blastoderm.
(A) After egg fertilization, the zygotic nucleus divides producing a population of nuclei within the common egg cytoplasm forming the coenocytic (syncytial) blastoderm. In the next step, some of the nuclei migrate to the egg posterior pole where the pole plasm containing germ cell determinants is located. These nuclei will eventually form the pole cells, which are the precursor of future germline. Most somatic nuclei move to the cell cortex where they eventually cellularize. Some of the somatic nuclei remain in the yolky center part of the egg as the yolk nuclei, which become polyploid, but their exact function remains unknown. (B) Blastoderm cellularization starts from the nucleus moving to the cortical, yolkless layer of cytoplasm. Once there, nucleus and its associated centrosomes induce the formation of under-membrane actin cup, which is followed by the elongation of aster microtubules, invagination of cellular membrane, and redistribution of actin along the membrane. Eventually, the cellular membrane completely encloses the nucleus and separates cell at the blastoderm surface.
Oogenesis within the germ cell cyst
In most invertebrates and vertebrates, oocytes develop within a group, called a germline cyst (nest) of sibling cells. The cyst progenitor cell, the cystoblast, divides several times with incomplete cytokinesis, which creates a nest of daughter cells (cystocytes) interconnected by cytoplasmic bridges called ring canals (RC) (Gerhold et al., 2022). At the end of division, the contractile ring does not fully close, resulting in a connection between the cytoplasm of daughter cells. Such an arrested contractile ring undergoes a maturation process to form a stable intercellular bridge (RC). Maturation of RCs is a multistep process in which an arrested cleavage furrow transforms into a stable intercellular bridge by assembling filamentous actin, membrane-skeletal adducin hu-li tai shao (Hts), and kelch proteins (Fig. 3D). Drosophila Kelch (KEL), in combination with Cullin-3 (Cullin-RING ubiquitin E3 ligase), facilitates crosslinking of RC actin filaments and organizing their inner rim cytoskeleton (Hudson and Cooley, 2010; Kelso et al., 2002; Matsuoka et al., 2000; Robinson et al., 1997). The size of RCs is regulated by the Misshapen (Msn) kinase. Msn depletion increases while over-expression of Msn decreases RC diameter (Kline et al., 2018). All these components of RCs are present in Drosophila, and Kelch and Hts were also found in Xenopus (Kloc et al., 2004a), but further studies are needed to confirm if these components are universal for different animal taxa and species. The RCs of Xenopus and many insects, including Drosophila, are penetrated by the fusome, a cytoskeletal structure that contains microtubules, alpha-spectrin, adducin-like, and ER proteins, and regulates cystocyte divisions and communication (Barr et al., 2024; Kloc et al., 2004a; Lin et al.1994; Spradling et al., 2022; St Johnston, 2023).
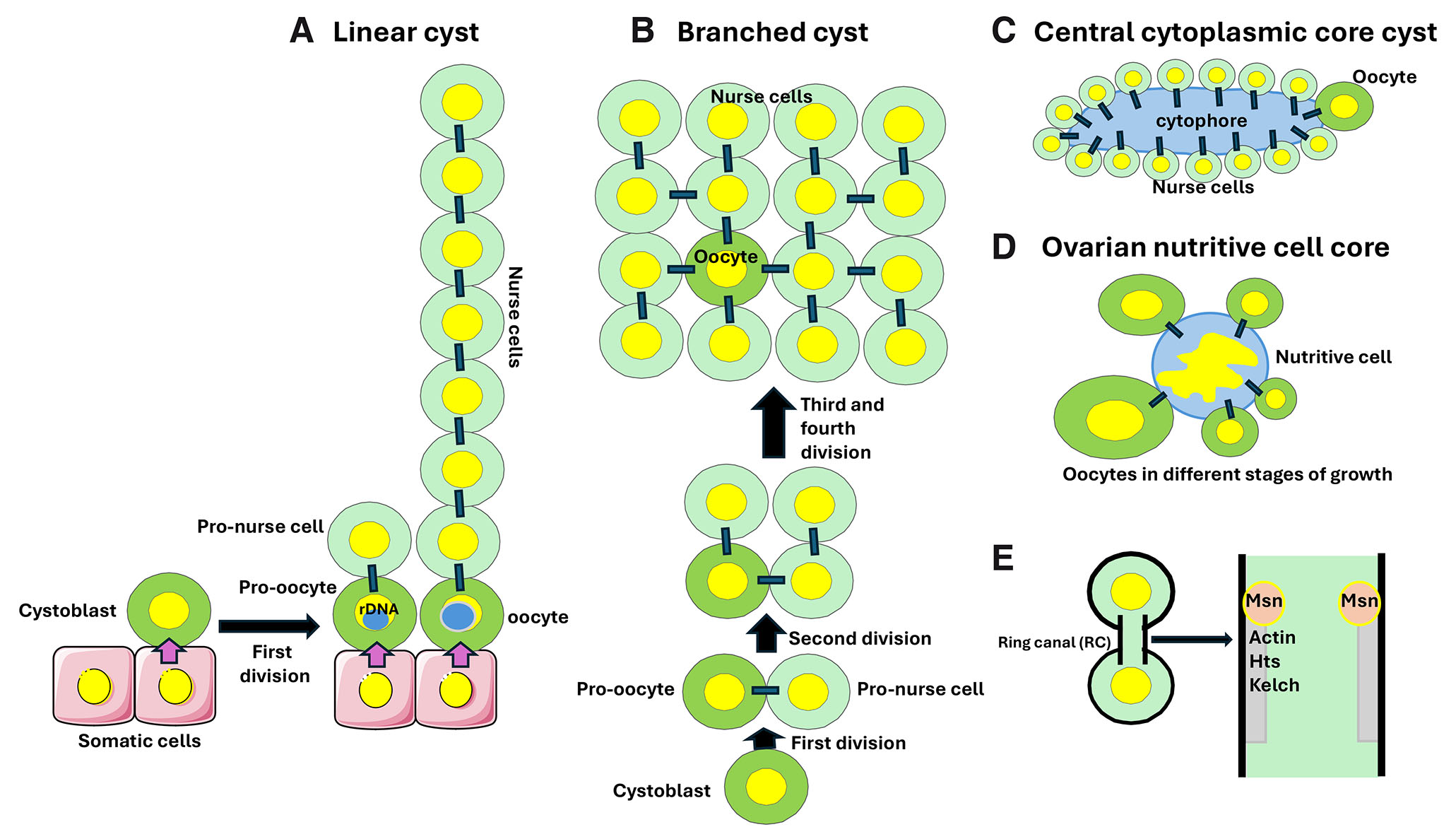
Fig. 3. Germline cysts.
(A) The linear cysts form during development of the telotrophic ovaries in the rove beetle Creophilus maxillosus. The first division of the cystoblast produces two cystocytes connected by a ring canal (RC; marked by a black rectangle): the pro-oocyte, always remaining in contact with the somatic cell, and the pro-nurse cell. Next, several synchronous divisions produce a chain of cells connected by RCs. In each chain of sibling cells, the pro-oocyte is always located at the top of somatic cells. The, so far, unidentified signal emanating from somatic cells induces ribosomal DNA amplification, resulting in a large rDNA body (marked by a blue sphere) in the pro-oocyte nucleus. The pro-oocyte becomes the oocyte and the remaining cystocytes undergo endoreplication and become the nurse cells. At each division, the rDNA body always segregates to the pro-oocyte. For a detailed description of Creophilus oogenesis see Kloc (2019). (B) The branched cystocyst in Drosophila. In the 16-cell cyst, only the two oldest cystocytes have four RCs each. One of these cystocytes becomes the oocyte, while the remaining cystocytes endoreplicate and become nurse cells. (C) In the annelid worm Enchytraeus albidus, a female germline cyst contains 15 nurse cells and one oocyte, all connected by individual RCs to the anuclear island of cytoplasm, the cytophore. (D) In some arachnids, the germline cyst contains the growing oocytes connected by the individual RCs to a centrally located large nurse cell with a large and branched nucleus. (E) The RCs are stabilized intercellular bridges derived from incomplete cytokinesis of cystocytes. Drosophila RCs contain various proteins such as actin, Hts, and Kelch, and their size is regulated by the Msn kinase.
Germline cysts are either branched (e.g., Drosophila, Xenopus, mouse) or linear (e.g., some beetles, some annelids) (Fig. 3 A,B). The branched cyst of Drosophila and Xenopus consists of 16 cystocytes with 15 intercellular bridges: eight cystocytes have one intercellular bridge, four have two, two have three, and the two oldest cystocytes have four bridges (Kloc et al., 2004a, 2004b, 2013; Spradling et al., 2022). In some animals, such as Xenopus, after the cessation of cystocyte divisions, all cystocytes become oocytes. In Drosophila and the rove beetle Creophilus maxillosus, only one cystocyte becomes the oocyte, and the rest become nurse cells, which feed the oocyte (Kloc, 2019; Kloc and Matuszewski, 1977; Spradling et al.2022). In Drosophila, two of sixteen cystocytes that contain four RCs become the pro-oocytes. Initially, both pro-oocytes seem to be identical as both enter meiotic prophase. Eventually, only one pro-oocyte becomes the oocyte, while the other will exit meiosis, and like the remaining 14 cystocytes, will endoreplicate DNA and become a nurse cell (González-Reyes et al., 1977).
There are two main models of how oocyte fate is established in Drosophila (reviewed in Hinnant et al., 2020). One model posits that in 16-cell cysts, two cells with four RCs are equivalent pro-oocytes. Indeed, they both enter meiosis and form synaptonemal complexes. This model predicts that out of two pro-oocytes, the oocyte is selected randomly through the accumulation of certain proteins and RNAs. Another model posits that the oocyte’s fate is established much earlier i.e., during the first, asymmetric division of the cystoblast when one daughter cell inherits more fusomal material than another. The asymmetrical inheritance of the fusome is repeated at each subsequent cystocyte division, resulting in only one cystocyte with four RCs, which has much more fusomal material than other cystocytes. This cystocyte (pro-oocyte) will accumulate Bicaudal-D (Bic-D), Egalitarian (Egl), Cup (eIF4E binding protein), and Orb (mRNA binding protein) proteins, and oskar mRNA (González-Reyes et al., 1977; reviewed in Hinnant et al., 2020).
However, some basal hexapods (springtails, proturans, and campodeid diplurans) have linear cysts, which lack fusomes (reviewed by Stys and Bilinski, 1990). Recent studies indicate that the determination of oocyte fate also depends on the microtubule polymerase Mini spindles (Msps) and dynein. Msps mRNA concentrates in the oocyte by dynein-dependent transport along microtubules, and after its translation, the Msps protein induces microtubule polymerization in the oocyte and growth of microtubules from the oocyte through the RCs into nurse cells, promoting dynein-dependent nurse cell-to-oocyte transport. These studies also showed that Msps knockdown inhibits oocyte growth and causes a gradual loss of oocyte fate (Lu et al., 2023). In the rove beetle Creophilus maxillosus only one cystocyte, the most basally located and in contact with somatic cells, will amplify ribosomal DNA in its nucleus and become an oocyte. (Fig. 3A) (Kloc, 2019). This indicates that an as-yet-unidentified signal emanating from the somatic cells imposes oocyte fate on the apposing cystocyte. In mice, after cessation of cystocyte divisions, the cyst fragments into about 4–6 smaller derivative cysts, in which only one cystocyte per cyst becomes the oocyte with the remaining cystocytes become the nurse cells (Kloc et al., 2008; Spradling, 2022).
The architecture of the linear and branched cysts may be more complicated than it seems. For example, in Creophilus and some beetles, pro-oocytes are probably connected by cytoplasmic bridges for a short period of time (Buning, 1993; Kloc, 2019). Eventually, the RCs between pro-oocytes may degrade separating cysts into linear chains. This would indicate that the linear cysts derive from the initially branched cysts. Similar fragmentation of branched cysts into linear chains was found in mice (Spradling, 2024) and the polychaetae worm Ophryotrocha labronica (Brubacher and Huebner, 2011).
Other modifications of germline cysts occur in some nematodes and annelids, where the cytoplasmic bridges connect individual cystocytes to a nutritive cytoplasmic core called the cytophore (Foor, 1967; Hall et al., 1999; Hirsh et al., 1976; Rudel et al., 2005; Świątek and Urbisz, 2019). The cytophore is anuclear and forms from cytoplasm extruded by cystocytes. In the annelid white worm Enchytraeus albidus, a female germline cyst contains 15 nurse cells and one oocyte, all connected to a cytophore by individual cytoplasmic bridges (Fig. 3C) (Urbisz et al., 2017). Another modification occurs in mites, where oocytes in different stages of growth are arranged around and connected by cytoplasmic bridges to a large central nutritive cell that is a modified germ line cell (Fig. 3D); (Witalinski, 2014).
All this information suggests that the germline cyst is a conserved feature of invertebrate and vertebrate oogenesis. However, while germline cysts are conserved among male invertebrates and vertebrates, for female cysts the evolutionary pattern is considerably more complex (Brubacher, 2024; Eckelbarger and Hodgson, 2021). Additionally, in flatworms, molluscs, echinoderms, and some insects, female germline cysts appear to be absent (Brubacher. 2024). Interestingly, some species of tunicates have very atypical oogenesis that seems not to conform to the germline cyst paradigm.
Coenocystic oogenesis in Appendicularia
Appendicularia (larvaceans), such as Oikopleura dioica, are free-swimming, solitary, pelagic tunicates widely distributed in the oceans. Adult O. dioica resembles a tadpole with a distinct trunk and long tail. O. dioica is a popular model organism in developmental biology because it is transparent, has a typical chordate body plan, has a very short (several days) generation time, a short (4-6.5 days) life span, produces many (~400) eggs, is built of only 550 cells at hatching, and because its miniature (70 Mb) genome (consisting of 15,000 genes), the smallest genome ever found in a chordate, has been sequenced (Seo et al., 2001).
Studies by Ganot et al. (2007a, 2007b), showed that in O. dioica a whole germline consists of a single multinuclear cell, the coenocyst. The coenocyst forms through the proliferation of nuclei within a common cytoplasm between days 1-3 of development. At day 3, the nuclei segregate (at a 1:1 ratio) into two subpopulations with distinct fates. The smaller, 3μm diameter nuclei enter meiotic prophase and become oocyte nuclei, while the other nuclei will endoreplicate (reaching 200C DNA content and ~20μm diameter) and become nurse cell nuclei. During nuclear proliferation and differentiation, the coenocyst is positive for germline marker Vasa protein, and both subpopulations of nuclei are associated with nuage (germinal granules) that contains Vasa proteins and RNAs. During all this time, the coenocyst cytoplasm contains an open network of actin filaments and plasma membranes. Between days 4-5.5, some of the meiotic nuclei become selected toward the oocyte fate. They become enclosed by the plasma membrane, forming pro-oocytes which are connected to the common cytoplasm by the RCs. Around day 5.5 the pro-oocytes grow by engulfing common cytoplasm through the RCs. The non-selected meiotic nuclei and nurse cell nuclei serve as a nutrition source for growing oocytes (Fig. 4). Around day 6 of female development, the coenocyst partitions into individual oocytes while the non-selected meiotic nuclei and nurse nuclei degenerate. Thus, in the coenocystic oogenesis of O. dioica, the meiotic nuclei share a common cytoplasm with the endoreplicating nuclei. Such coenocystic oogenesis seems to be also a common feature for other appendicularian species.
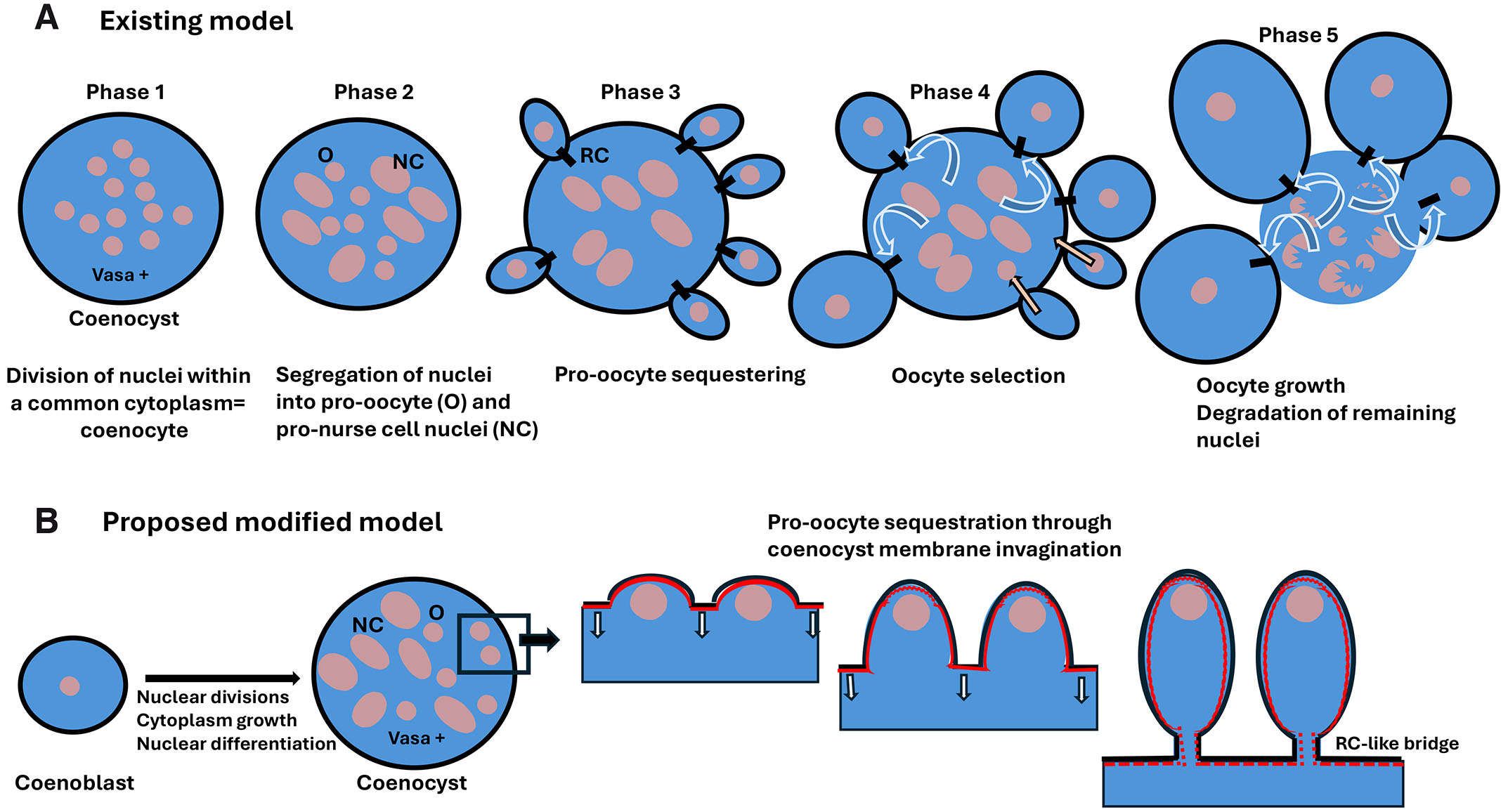
Fig. 4. Coenocystic oogenesis in appendicularian tunicates.
(A) Existing model of appendicularian oogenesis according to Ganot et al. (2007a, 2007b). Phase 1. The coenocyst, which is positive for germline marker Vasa protein, contains many nuclei embedded in a common cytoplasm. It originates from nuclear divisions without cytokinesis. Phase 2. Nuclei segregate and differentiate into two distinct subpopulations: smaller pro-oocyte nuclei and larger (endoreplicating) pro-nurse cell nuclei. Phase 3. Pro-oocytes become separated from the common cytoplasm but remain connected to it by ring canals (RCs). Phase 4. Some of the pro-oocytes are selected as the future oocytes. They grow using nutrients from a common cytoplasm and become the oocytes, while the nuclei of non-selected pro-oocytes return to the common cytoplasm and eventually, like the pro-nurse nuclei, endoreplicate their DNA. Phase 5. Oocytes connected to the coenocyst by RCs grow further, while the remaining nuclei degrade. (B) Proposed modified model. The coenocyst progenitor (an equivalent of the cystoblast), the coenoblast, undergoes nuclear divisions without cytokinesis, producing a multinuclear coenocyst. After nuclear segregation into pro-oocytes and pro-nurse cell fate, the pro-oocytes become sequestered by the invagination of coenocyst membrane underlined by actin (red) in the process reminiscent of cellularization of coenocytic blastoderm in Drosophila. However, in contrast to the Drosophila blastoderm, the cellularization of pro-oocytes is incomplete and leaves RC-like intercellular connections between the pro-oocyte and coenocyst cytoplasm. These RC-like bridges serve to transport nutrients from the common cytoplasm to the growing oocytes.
Ganot et al. (2007a, 2007b, 2008) proposed a quintaphasic model of coenocystic oogenesis. In the first phase, there are several nuclear divisions within the common cytoplasm which is positive for the germline marker Vasa protein. In the second phase, nuclei differentiate into two distinct subpopulations: one entering meiosis (prospective oocytes) and another endoreplicating (prospective nurse cells). In phase 3 the meiotic nuclei become sequestered within small pro-oocytes connected to the common cytoplasm by RCs. In phase 4 some “selected” pro-oocytes grow more than others by engulfing the coenocyst cytoplasm, while the unselected meiotic nuclei move back to the coenocyst cytoplasm toward the nurse cell nuclei. In phase 5, selected oocytes continue to grow into eggs and the remaining nuclei become degraded (Fig. 4).
This model leaves some unanswered questions, such as: 1) The authors of the Appendicularia paper correctly stated the coenocyst must be a germline cell because it contains a germline marker Vasa protein, but what is the identity of coenocyst precursor? 2) How the pro-oocytes can be connected to the coenocyst by the RCs if the RCs are stabilized intercellular bridges derived from incomplete cytokinesis, but there are no cytokineses in the Appendicularia coenocyst?
Based on similarities between Drosophila coenocytic (syncytial) blastoderm and coenocystic oogenesis in Appendicularia, I propose the following revised model of coenocystic oogenesis (Fig. 4). In this model, the precursor cell of the coenocyte is the cystoblast-like cell, which I named the coenoblast. The coenoblast undergoes a series of nuclear divisions resulting in daughter nuclei embedded in a common cytoplasm. After differentiation of the nuclei into pro-oocyte and pro-nurse cell fate (as proposed in the original model), the pro-oocytes undergo a cellularization process very similar to the cellularization of the Drosophila blastoderm. However, in contrast to Drosophila, the cellularization is incomplete resulting in the intercellular bridges connecting pro-oocyte cytoplasm to coenocyst cytoplasm. In my opinion, these intercellular bridges should not be called ring canals (RCs) but rather “RC-like” because they do not form by incomplete cytokinesis but by incomplete cellularization (which are structurally and mechanistically different processes), thus they probably have different molecular and structural characteristics. Also, like in the coenocytic blastoderm of Drosophila, the appendicularian coenocyst differentiates nuclei into different fates (somatic, pole cell, and yolk nuclei in Drosophila, and pro-oocyte and pro-nurse nuclei in Appendicularia). Although further ultrastructural and molecular studies of appendicularian oogenesis are needed, if the revised model of coenocystic oogenesis is correct, then it would be the first example of a common mechanism implemented by the somatic and germline coenocytic structures to sequester and separate cells of different fate from a common cytoplasm.
Acknowledgements
Drawings used to make figures were from the Servier Medical ART: SMART, smart.servier.com.
Abbreviations
Msp, microtubule polymerase Mini spindle ; RC, ring canal ;Declarations
Conflicts of interest
The authors declare no conflict of interest.
Funding
Support by the J.C. Walter Jr. Transplant Center fund at the Houston Methodist Hospital Foundation, and William Stamps Farish Fund.
References
Ali M. F., Shin J. M., Fatema U., Kurihara D., Berger F., Yuan L., Kawashima T. (2023). Cellular dynamics of coenocytic endosperm development in Arabidopsis thaliana. Nature Plants 9: 330-342.
Barr J., Diegmiller R., Colonnetta M. M., Ke W., Imran Alsous J., Stern T., Shvartsman S. Y., Schedl P. (2024). To be or not to be: orb , the fusome and oocyte specification in Drosophila . GENETICS 226: iyae020.
Brubacher J. L. (2024). Female Germline Cysts in Animals: Evolution and Function. In Syncytia: Origin, Structure, and Functions. (Ed. Kloc Malgorzata, Uosef Ahmed) Springer International Publishing, Cham.
Brubacher J. L., Huebner E. (2011). Evolution and development of polarized germ cell cysts: New insights from a polychaete worm, Ophryotrocha labronica. Developmental Biology 357: 96-107.
Buning J. (1993). Germ cell cluster formation in insect ovaries. International Journal of Insect Morphology and Embryology 22: 237-253.
Cernuda-Morollón E., Ridley A. J. (2006). Rho GTPases and Leukocyte Adhesion Receptor Expression and Function in Endothelial Cells. Circulation Research 98: 757-767.
Daubenmire R. F. (1936). The Use of the Terms Coenocyte and Syncytium in Biology. Science 84: 533-533.
Eckelbarger K. J., Hodgson A. N. (2021). Invertebrate oogenesis – a review and synthesis: comparative ovarian morphology, accessory cell function and the origins of yolk precursors. Invertebrate Reproduction & Development 65: 71-140.
Engelhardt B., Wolburg H. (2004). Mini‐review: Transendothelial migration of leukocytes: through the front door or around the side of the house?. European Journal of Immunology 34: 2955-2963.
Foor W. E. (1967). Ultrastructural Aspects of Oocyte Development and Shell Formation in Ascaris lumbricoides. The Journal of Parasitology 53: 1245.
Ganot P., Kallesøe T., Thompson E. M. (2007a). The cytoskeleton organizes germ nuclei with divergent fates and asynchronous cycles in a common cytoplasm during oogenesis in the chordate Oikopleura. Developmental Biology 302: 577-590.
Ganot P., Bouquet J.M., Kallesøe T., Thompson E. M. (2007b). The Oikopleura coenocyst, a unique chordate germ cell permitting rapid, extensive modulation of oocyte production. Developmental Biology 302: 591-600.
Ganot P., Moosmann-Schulmeister A., Thompson E. M. (2008). Oocyte selection is concurrent with meiosis resumption in the coenocystic oogenesis of Oikopleura. Developmental Biology 324: 266-276.
Gerhold A. R., Labbé J.C., Singh R. (2022). Uncoupling cell division and cytokinesis during germline development in metazoans. Frontiers in Cell and Developmental Biology 10: 1001689.
Gilbert S.F. (2000). Early Drosophila Development. In Developmental Biology. Sinauer Associates, Sunderland (MA).
González-Reyes A., Elliott H., Johnston D. S. (1997). Oocyte determination and the origin of polarity in Drosophila: the role of the spindle genes . Development 124: 4927-4937.
Hall D. H., Winfrey V. P., Blaeuer G., Hoffman L. H., Furuta T., Rose K. L., Hobert O., Greenstein D. (1999). Ultrastructural Features of the Adult Hermaphrodite Gonad of Caenorhabditis elegans: Relations between the Germ Line and Soma. Developmental Biology 212: 101-123.
Hinnant T. D., Merkle J. A., Ables E. T. (2020). Coordinating Proliferation, Polarity, and Cell Fate in the Drosophila Female Germline. Frontiers in Cell and Developmental Biology 8: 19.
Hirsh D., Oppenheim D., Klass M. (1976). Development of the reproductive system of Caenorhabditis elegans. Developmental Biology 49: 200-219.
Hudson A. M., Cooley L. (2010). Drosophila Kelch functions with Cullin-3 to organize the ring canal actin cytoskeleton . Journal of Cell Biology 188: 29-37.
Kelso R. J., Hudson A. M., Cooley L. (2002). Drosophila Kelch regulates actin organization via Src64-dependent tyrosine phosphorylation . The Journal of Cell Biology 156: 703-713.
Kline A., Curry T., Lewellyn L. (2018). The Misshapen kinase regulates the size and stability of the germline ring canals in the Drosophila egg chamber. Developmental Biology 440: 99-112.
Kloc M. (2019). The Rove Beetle Creophilus maxillosus as a Model System to Study Asymmetric Division, Oocyte Specification, and the Germ-Somatic Cell Signaling. In Evo-Devo: Non-model Species in Cell and Developmental Biology. (Ed. Tworzydlo Waclaw, Bilinski Szczepan M.) Springer International Publishing, Cham.
Kloc M., Bilinski S., Dougherty M. T., Brey E. M., Etkin L. D. (2004a). Formation, architecture and polarity of female germline cyst in Xenopus. Developmental Biology 266: 43-61.
Kloc M., Bilinski S., Etkin L. D. (2004b). The Balbiani Body and Germ Cell Determinants: 150 Years Later. In . Elsevier.
Kloc M., Ghobrial R. M., Kubiak J. Z., (2013). Recent advances in the understanding of germ cell-specific organelles and their functions. In Recent Advances in Germ Cells Research. Nova Science Publishers.
Kloc M., Jaglarz M., Dougherty M., Stewart M., Nelthemaat L., Bilinski S. (2008). Mouse early oocytes are transiently polar: Three-dimensional and ultrastructural analysis. Experimental Cell Research 314: 3245-3254.
Kloc M., Matuszewski B. (1977). Extrachromosomal DNA and the origin of oocytes in the telotrophic-meroistic ovary ofCreophilus maxillosus (L.) (Staphylinidae, Coleoptera-Polyphaga). Wilhelm Roux's Archives of Developmental Biology 183: 351-368.
Lee W. B., Erm S. K., Kim K. Y., Becker R. P. (1999). Emperipolesis of erythroblasts within Kupffer cells during hepatic hemopoiesis in human fetus. The Anatomical Record 256: 158-164.
Lin H., Yue L., Spradling A. C. (1994). The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation . Development 120: 947-956.
Lu W., Lakonishok M., Gelfand V. I. (2023). The dynamic duo of microtubule polymerase Mini spindles/XMAP215 and cytoplasmic dynein is essential for maintaining Drosophila oocyte fate . Proceedings of the National Academy of Sciences 120: e2303376120.
Lugini L., Matarrese P., Tinari A., Lozupone F., Federici C., Iessi E., Gentile M., Luciani F., Parmiani G., Rivoltini L., Malorni W., Fais S. (2006). Cannibalism of Live Lymphocytes by Human Metastatic but Not Primary Melanoma Cells. Cancer Research 66: 3629-3638.
Matsuoka Y., Li X., Bennett V. (2000). Adducin: structure, function and regulation. Cellular and Molecular Life Sciences 57: 884-895.
Mazumdar A., Mazumdar M. (2002). How one becomes many: Blastoderm cellularization in Drosophila melanogaster . BioEssays 24: 1012-1022.
Nishie M., Mori F., Houzen H., Yamaguchi J., Jensen P. H., Wakabayashi K. (2006). Oligodendrocytes within astrocytes (“emperipolesis”) in the cerebral white matter in hepatic and hypoglycemic encephalopathy. Neuropathology 26: 62-65.
Ondracka A., Dudin O., Ruiz-Trillo I. (2018). Decoupling of Nuclear Division Cycles and Cell Size during the Coenocytic Growth of the Ichthyosporean Sphaeroforma arctica. Current Biology 28: 1964-1969.e2.
Overholtzer M., Brugge J. S. (2008). The cell biology of cell-in-cell structures. Nature Reviews Molecular Cell Biology 9: 796-809.
Pezzano M., Li Y., Philp D., Omene C., Cantey M., Saunders G., Guyden J. C., (1995). Thymic nurse cell rescue of early CD4+CD8+ thymocytes from apoptosis. Cellular and molecular biology (Noisy-le-Grand, France) 41: 1099-1111.
Płachno B. J., Kapusta M., Świątek P. (2024). Syncytia in Utricularia: Origin and Structure. In Syncytia: Origin, Structure, and Functions. (Ed. Kloc Malgorzata, Uosef Ahmed) Springer International Publishing, Cham.
Robinson D. N., Smith-Leiker T. A., Sokol N. S., Hudson A. M., Cooley L. (1997). Formation of the Drosophila Ovarian Ring Canal Inner Rim Depends on cheerio . Genetics 145: 1063-1072.
Rudel D., Riebesell M., Sommer R. J. (2005). Gonadogenesis in Pristionchus pacificus and organ evolution: development, adult morphology and cell–cell interactions in the hermaphrodite gonad. Developmental Biology 277: 200-221.
Seo H.C., Kube M., Edvardsen R. B., Jensen M. F., Beck A., Spriet E., Gorsky G., Thompson E. M., Lehrach H., Reinhardt R., Chourrout D. (2001). Miniature Genome in the Marine Chordate Oikopleura dioica . Science 294: 2506-2506.
Spradling A. C., Niu W., Yin Q., Pathak M., Maurya B. (2022). Conservation of oocyte development in germline cysts from Drosophila to mouse. eLife 11: e83230.
Spradling A. C. (2024). The Ancient Origin and Function of Germline Cysts. In Syncytia: Origin, Structure, and Functions. (Ed. Kloc Malgorzata, Uosef Ahmed) Springer International Publishing, Cham.
St Johnston D. (2023). Polarity and axis formation in the Drosophila female germ line. In Cell Polarity in Development and Disease. Elsevier.
Štys P., Biliński S (1990). Ovariole types and the phylogeny of Hexapods. Biological Reviews 65: 401-429.
Świątek P., Urbisz A. Z. (2019). Architecture and Life History of Female Germ-Line Cysts in Clitellate Annelids. In Evo-Devo: Non-model Species in Cell and Developmental Biology. (Ed. Tworzydlo Waclaw, Bilinski Szczepan M.) Springer International Publishing, Cham.
Tam R., Harris T. J. C. (2024). Reshaping the Syncytial Drosophila Embryo with Cortical Actin Networks: Four Main Steps of Early Development. In Syncytia: Origin, Structure, and Functions. (Ed. Kloc Malgorzata, Uosef Ahmed) Springer International Publishing, Cham.
Tram U., Riggs B., Sullivan W., (2002). Cleavage and Gastrulation in Drosophila Embryos. In . John Wiley & Sons, Ltd.
Trcek T., Lehmann R. (2019). Germ granules in Drosophila . Traffic 20: 650-660.
Tsunoda R., Heinen E., Sugai N. (2000). Follicular dendritic cells in vitro modulate the expression of Fas and Bcl-2 on germinal center B cells. Cell and Tissue Research 299: 395-402.
Urbisz A. Z., Chajec Ł, Brąszewska-Zalewska A., Kubrakiewicz J., Świątek P. (2017). Ovaries of the white worm ( Enchytraeus albidus , Annelida, Clitellata) are composed of 16-celled meroistic germ-line cysts. Developmental Biology 426: 28-42.
Witaliński W. (2014). Gonads and gametogenesis in astigmatic mites (Acariformes: Astigmata). Arthropod Structure & Development 43: 323-340.
Wu E., Raine C. S., (1992). Multiple sclerosis. Interactions between oligodendrocytes and hypertrophic astrocytes and their occurrence in other, nondemyelinating conditions. Laboratory investigation; a journal of technical methods and pathology 67: 88-99.