Int. J. Dev. Biol. 67: 49 - 56 (2023)
The Dyslexia-associated gene KIAA0319L is involved in neuronal migration in the developing chick visual system
Open Access | Original Article | Published: 3 July 2023
Abstract
The gene KIAA0319-Like (KIAA0319L) is thought to confer susceptibility for developmental dyslexia. Dyslexia may be caused by alterations in neuronal migration, and in utero knockdown of KIAA0319L in rats indicated migration errors. However, studies carried out with KIAA0319L knockout mice did not reveal an altered neuronal migration phenotype. Gene knockout may activate compensatory mechanisms to buffer against genetic mutations during development. Here we assessed the role of KIAA0319L on migrating neurons in the chick developing tectum. Whole mount in situ hybridization was performed for KIAA0319L on embryonic day (E)3 – E5 chick embryos and in situ hybridization on sections was performed at later stages. The specificity and efficiency of engineered microRNA (miRNA) constructs targeting KIAA0319L for knocking down KIAA0319L were verified. miRNAs were electroporated into E5 chick optic tecta. Our studies demonstrate that KIAA0319L is expressed in the developing chick visual system, as well as in the otic vesicles. Knockdown of KIAA0319L in the optic tectum results in abnormal neuronal migration, strengthening the argument that KIAA0319L is involved in this developmental process.
Keywords
cell migration, brain development, KIAA0319L, optic tectum
Introduction
KIAA0319-like (KIAA0319L) has been identified as a susceptibility gene for developmental dyslexia (Couto et al., 2008). Dyslexia is one of the most common of the complex neurobehavioral disorders, affecting 5-10% of school aged children (Katusic et al., 2001). KIAA0319L has also been associated with language impairment and IQ in autism spectrum disorders (Wang et al., 2013; Eicher and Gruen 2015). KIAA0319L shares homology with KIAA0319, which is one of the four initial identified candidate dyslexia susceptibility genes: KIAA0319 (Cope et al., 2005), DYX1C1 (Taipale et al., 2003), ROBO1 (Hannula-Jouppi et al., 2005) and DCDC2 (Schumacher et al., 2006). In addition to dyslexia, variations in KIAA0319 are also associated with cortical thickness reductions in language areas of the brain in patients with frontotemporal dementia (Paternicó et al., 2016).
The protein product of the KIAA0319L gene (KIAA0319L; AU040320 in mice), which has been identified as the entry receptor for adeno-associated virus (Pillay et al., 2016), is predicted to be a single-pass transmembrane protein with an N-terminal cysteine rich domain and an extracellular region consisting of several PKD domains. PKD domains are involved in strong calcium independent homophilic interactions and intercellular adhesion (Ibraghimov-Beskrovnaya et al., 2000). Little is known regarding signaling mechanisms of KIAA0319L, though it has been shown to interact with Nogo receptor 1 (Poon et al., 2011). It was found that silencing of Kiaa0319l or Kiaa0319 impaired post-mitotic cortical neuron migration in rats (Paracchini et al., 2006; Platt et al., 2013; Peschansky et al., 2010; Szalkowski et al., 2012), however knockout mouse models showed no indication of abnormal cortical neuron migration (Martinez-Garay et al., 2017; Guidi et al., 2017). However, Kiaa0319 and Kiaa0319L knockout (KO) mice exhibited deficits in auditory processing (Guidi et al., 2017). Altered auditory processing has been reported in individuals with RD (Ramus 2006; Tallal et al., 1996; Strehlow et al., 2006; Kujala et al., 2001). To gain relevant insights on the role of KIAA0319L, we decided to study its role in the visual system.
For visual circuitry to function normally, the proper development of both the retina and retinorecipient targets in the brain are indispensable. The optic tectum is the major visual processing center in the non-mammalian vertebrate brain and is the primary synaptic target of retinal ganglion cell axons. In chick, the adult optic tectum consist of sixteen laminae: SO (stratum opticum – fibers of the optic tract), SGFS (stratum griseum et fibrosum superficiale, a, b, c, d, e, f, g, h, i, j), SGC (stratum griseum central – the principle efferent), SAC (stratum album central), SGP (stratum griseum periventriculare), SFP (stratum fibosum periventriculare) and the ependymal layer (LaVail and Cowan 1971; Mey and Thanos 2000).
The manner of postmitotic cell migration in the developing chick optic tectum differs from that seen in the cerebral cortex. In the cortex, an “inside-out” pattern of migration generally occurs where the earliest born neurons are found in the deepest cortical layers, while later born neurons are found in the more superficial layers (McConnell 1988; Jackson, Peduzzi, and Hickey 1989). The laminar structure of the chick optic tectum on the other hand is the result of three migration waves (LaVail and Cowan 1971). The first wave takes place between E4 and E6 and forms the inner laminae (SGC, SAC, SGP, and SFP), while the second wave takes place between E4 and E8 and forms the outer laminae (SGFS a–g); together, neurons from these first two waves are termed early migratory neurons. The third wave (E5-E9) splits the neuronal layers formed by the first and second waves in order to make up the middle lamina. These neurons are termed late migratory neurons and form laminae h–j of SGFS (LaVail and Cowan 1971; Sugiyama and Nakamura 2003).
Here we show that KIAA0319L is expressed in the developing chick visual and auditory systems during time points suggestive of roles both within and possibly outside of neuronal migration. Using silencing with specific miRNAs, we provide evidence that KIAA0319Lis involved in neuronal migration in the chick visual system development.
Results
KIAA0319-like is expressed in the visual system in developing chick embryos
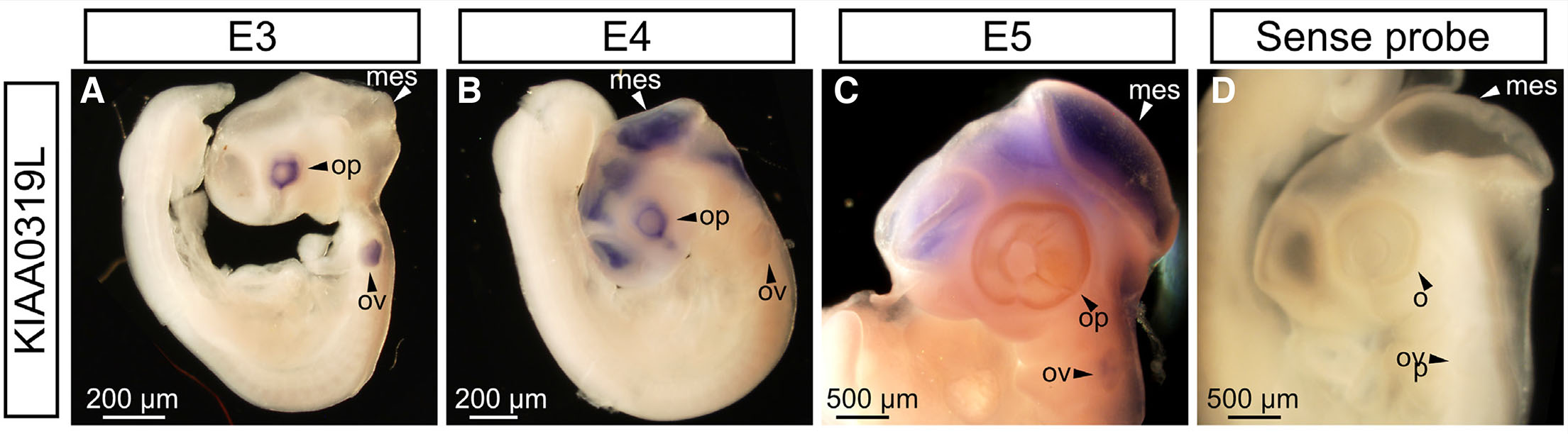
To explore KIAA0319L expression in the developing chick, we first performed in situ hybridization on whole mount embryos. At E3, KIAA0319L mRNA was observed predominantly in the optic cup as well the otic vesicles (Fig. 1A). This time point corresponds to the onset of neurogenesis in the developing chick eye and ear (Aburto et al., 2012; Doh et al., 2010). Sense probe controls did not show any signal, indicating that the anti-sense probe specifically detects KIAA0319L mRNA (Fig. 1D). By E4, KIAA0319L had commenced expression in the brain, with signal present in the myelencephalon, metencephalon, mesencephalon (optic tectum), diencephalon, telencephalon and the neural tube. This time point corresponds to the beginning of radial migration in the optic tectum (LaVail and Cowan 1971; Sugiyama and Nakamura 2003; Guidi et al., 2017). Expression in the developing eye remained strong at E4, whereas expression in the otic vesicles became reduced relative to the expression in the eye and brain (Fig. 1B). By E5, expression in the developing eye became reduced relative to expression levels in the brain, with the most prominent signal being in the developing optic tectum (Fig. 1C). The majority of retinal lamination and neuronal migration takes place following E4 (Doh et al., 2010), suggesting that KIAA0319L may be involved in non-migration functions in this developing tissue.
Fig. 1. KIAA0319 expression in early stage chick embryos.
(A) KIAA0319L is expressed in the optic cups and otic vesicles at E3. (B) At E4, KIAA0319L displays strong staining in the diencephalon, mesencephalon, telencephalon, metencephalon and optic vesicle. (C) At E5, signal persisted in the telencephalon, mesencephalon and metencephalon. Expression in the optic vesicle is diminished. (D) Control sense probes gave no visible signal. mes, mesencephalon; ov, otic vesicle; op, optic vesicle.
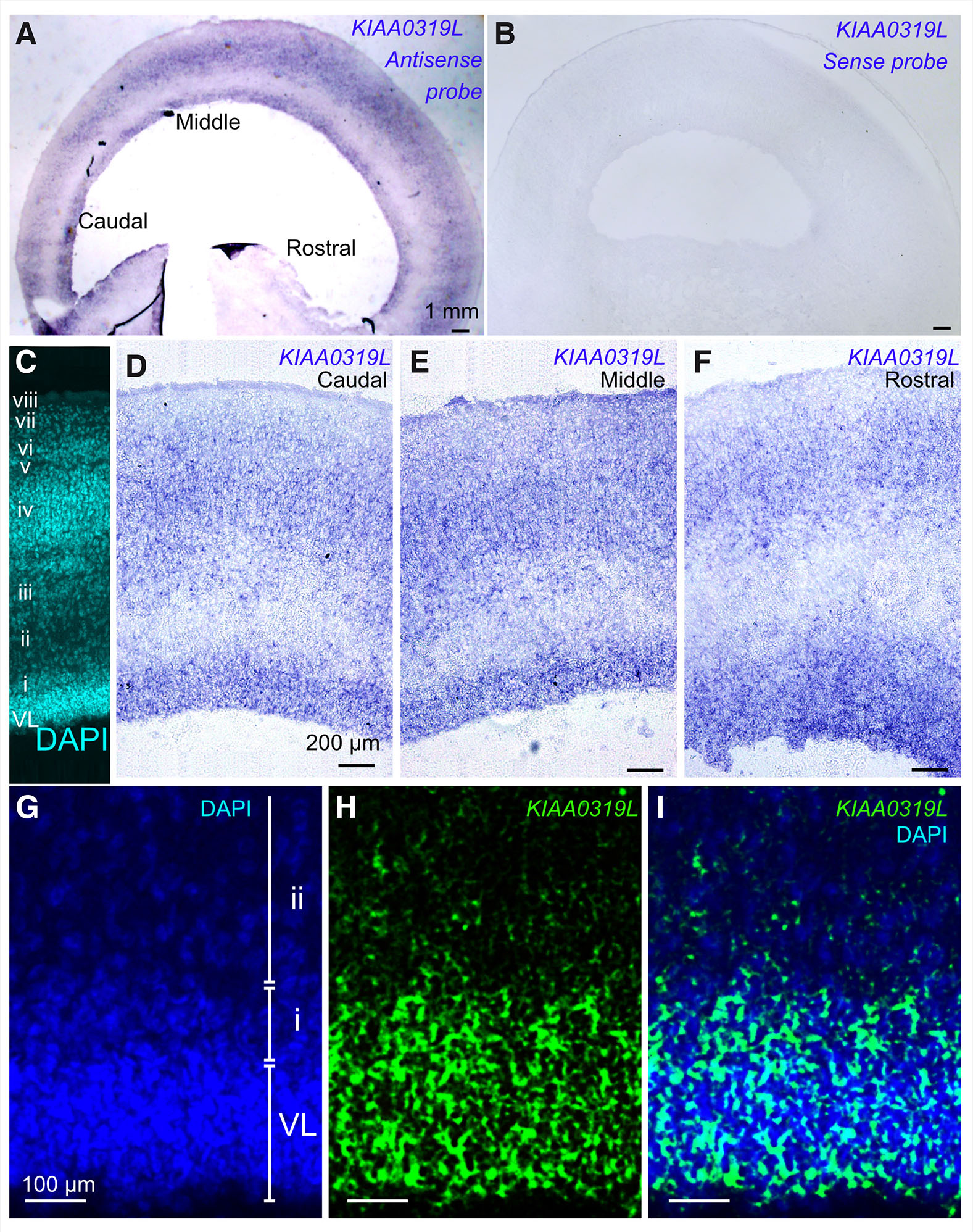
We next sought to analyze expression of KIAA0319L mRNA at later ages in the optic tectum in order to gain insight into what function this gene may play in visual system development. We carried out in situ hybridization on cryosections of the E9 optic tectum. KIAA0319L was expressed in deep and middle layers of the optic tectum uniformly along the rostro-caudal axis (Fig. 2 A-F). Within the middle layer, KIAA0319L was localized in the layer iii and layer iv (Fig. 2 C-F). To determine KIAA0319L mRNA localization in the deeper layers, we inverted the KIAA0310L in situ hybridization picture taken in bright field and merged it with a nuclear stain (DAPI) (Fig. 2 G-I). This analysis confirmed that KIAA0319L was expressed in the ventricular zone and the subventricular layer.
Fig. 2. KIAA0319L expression in the E9 chick optic tectum.
(A) KIAA0319L expression in the E9 chick optic tectum along the rostral-caudal axis. Relatively strong signal is seen in the ventricular layer and in the middle layers as indicated by in situ hybridization. (B) Sense control does not display any signal. (C) DAPI staining (cyan) displays layer structure of the E9 optic tectum. The ventricular layer (VL) and layers i to viii can be observed. (D-F) KIAA0319L expression as seen under higher magnification in the caudal, middle and rostral position in the optic tectum. KIAA0319L expression is observed in the deeper layer, layer iii and layer iv. (G-I) High power magnification of the deeper layer. The bright field picture is inverted and is given a pseudo-color (green: KIAA0319L) and merged to DAPI (blue). KIAA0319L expression overlaps with the ventricular layer as well as with layer i (sub-ventricular layer).
Specific and effective knock down of KIAA0319L
To investigate KIAA0319L in-vivo function during development, four synthetic miRNA constructs targeting KIAA0319L were generated based on modified primers according to Das et al.,(Das et al., 2006a). Given that the homologous gene KIAA0319 is also expressed in the chick visual system (Fig. 3B'), we sought to exclude the possibility that our miRNA constructs have non-specific effects by analyzing whether our miRNA constructs targeting KIAA0319L have any effect on KIAA0319 expression.
To assess the specificity and effectiveness of our synthetic miRNAs, miRNAs targeting KIAA0319L were electroporated to the right optic vesicle in ovo at E1.5. Optic vesicle electroporation enables quicker miRNA evaluation compared to optic tectum electroporation given KIAA0319L expression commences at an earlier time point in the optic vesicle (Fig. 1A). Since the miRNA expression vector contains an RFP expression cassette on the same vector, RFP expression levels indicate the relative strength of the miRNA expression level. Two days after electroporation, we selected embryos with high RFP expression levels in optic vesicle. Next, we carried out in situ hybridization against KIAA0319L or KIAA0319 on whole mount embryos.
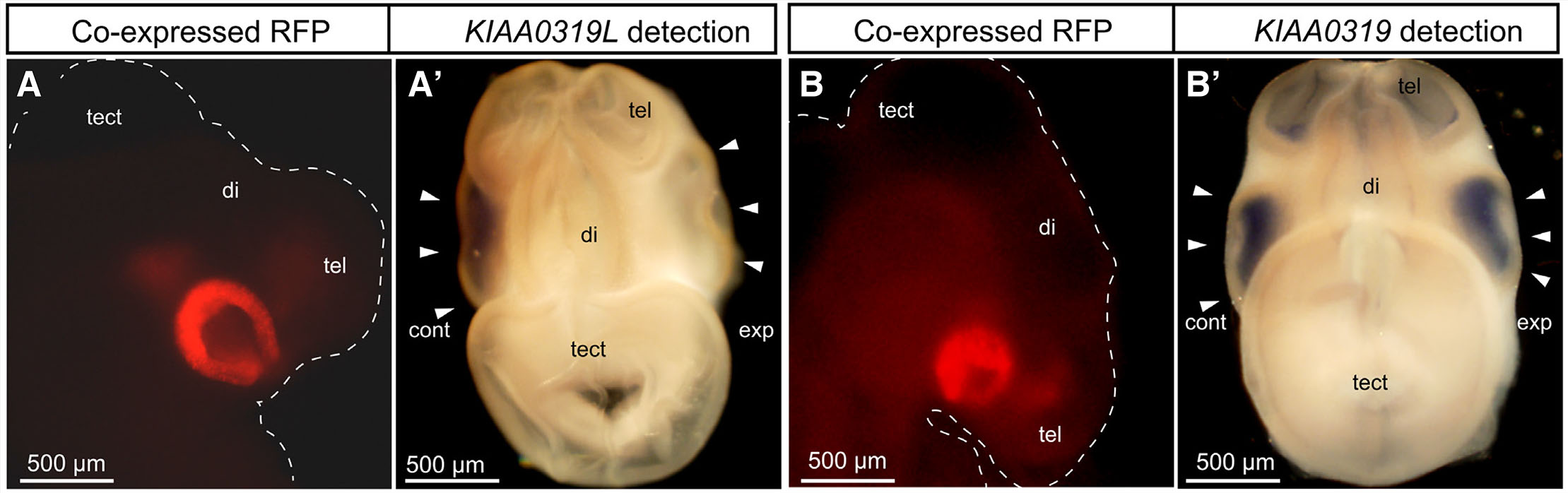
Among the miRNAs that we screened, two miRNA constructs, namely miKIAA0319L1 and miKIAA0319L2, exhibited effective knockdown of KIAA0319L expression (Fig. 3 A,A’). To confirm the specificity of KIAA0319L knockdown, we electroporated the KIAA0319L miRNAs and subsequently analyzed the effect of these miRNAs on the expression of KIAA00319, a homologous gene of KIAA0319L. Neither miKIAA0319L1 nor miKIAA0319L2 altered KIAA0319 expression when the transfected retina was compared to the control side, even when high levels of RFP expression were observed (Fig. 3 B,B’). These results indicate that these miRNA constructs specifically silence KIAA0319L gene expression. These experiments also confirm the specificity of the KIAA0319L probe used in our in situ hybridization analysis.
Fig. 3. Specificity and effectiveness of miKIAA0391L constructs.
(A) A lateral view of an E3.5 miKIAA0319L electroporated embryo. Red Fluorescent Protein (RFP) indicates successful electroporation. (A’) A dorsal view of the miKIAA0319L electroporated embryo. KIAA00319L expression is not detected in the right eye (arrowhead), while strong signal is observed in the control side eye, demonstrating effective knockdown of KIAA0319L in the right eye (arrowhead). (B) A lateral view of an E3.5 miKIAA0319L electroporated embryo. RFP indicates successful electroporation. (B’) A dorsal view of the miKIAA0319L electroporated embryo. KIAA0319 (a gene homologous to KIAA0319L), expression is detected in the miKIAA0319L transfected eye (arrowhead) at the same level of the control side eye (arrowhead), demonstrating specific knockdown of KIAA0319L. Abbreviations: cont, control side; di, diencephalon; exp, experimental side; tect, optic tectum; tel, telencephalon.
Silencing KIAA0319L affects cell migration during tectal layer formation
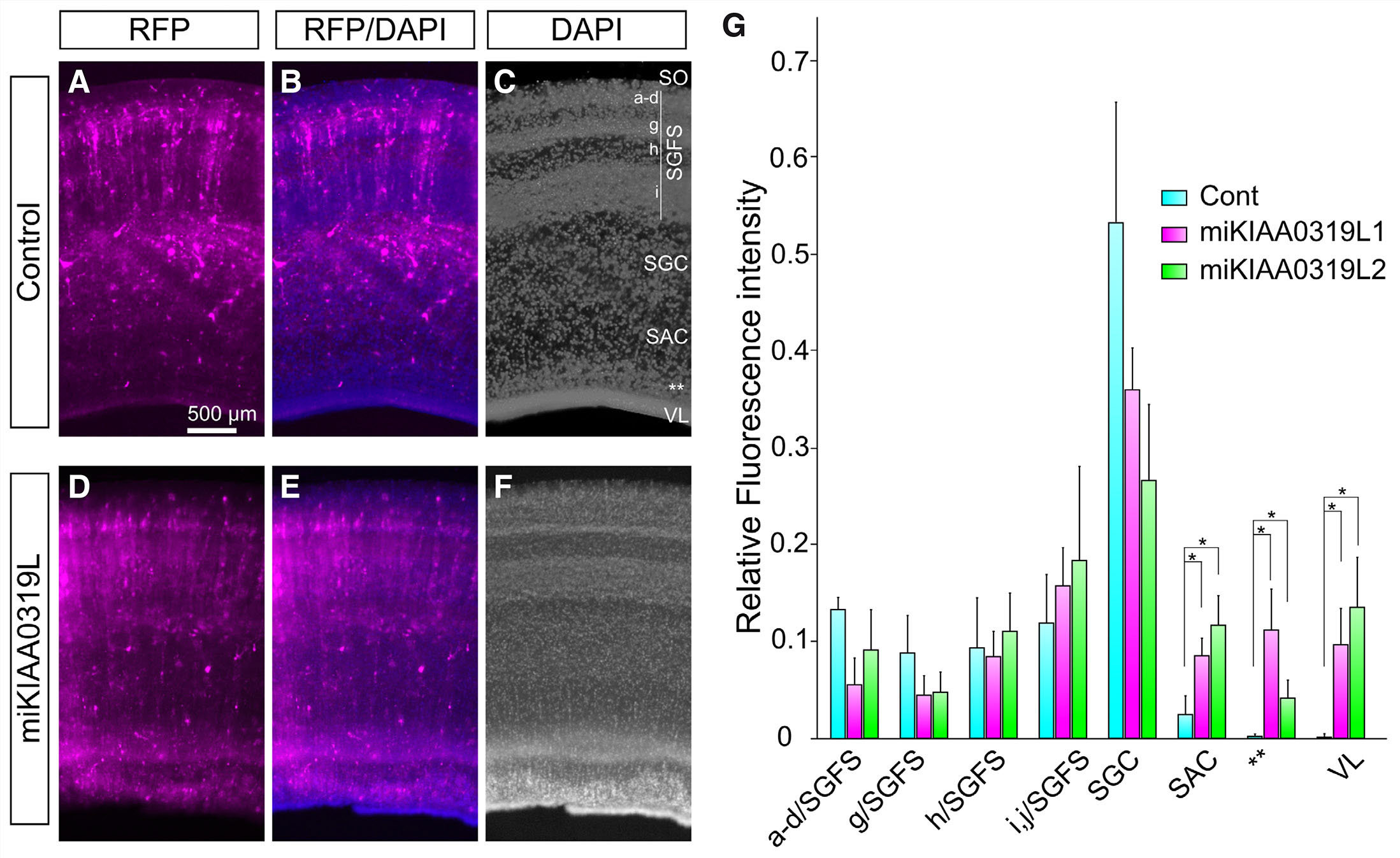
In order to study the role of KIAA0319L protein during chick visual system development, the two independent miRNAs targeting KIAA0319L were electroporated into the ventricular zone of the E5 optic tectum. Since the ventricular zone at the E5 optic tectum is a mixture of early and late migratory cells, miKIAA0319L can be applied to the both cell population by in ovo electroporation (Sugiyama and Nakamura 2003). Following electroporation, the presence of RFP is indicative of cells that have been successfully electroporated with the miRNA construct. Five days after electroporation (E12), the embryos were sacrificed and analyzed for the presence of RFP in each layer in of the optic tecta. In controls at E12, the majority of cells labeled at E5 with RFP migrated away from the VZ and were predominantly located in upper layers of the optic tectum (Fig. 4 A-C). In contrast, miRNA treated cells showed a more even distribution pattern at E12 with a significant amount of cells remaining near the VZ (Fig. 4 D-F).
Fig. 4. Location of E5 labeled cells at E12 in control and miRNA transfected optic tectum (OT).
in ovo electroporation was used to target miRNA constructs to the ventricular layer of the OT at E5. OT were harvested at E12 and the migratory pattern of electroporated cells was assessed. (A-C) Control miRNA-transfected OT at E5. Control miRNA was transfected at E5 and the distribution of the control miRNA-transfected cells indicated by red fluorescent protein (RFP) was observed at E12. (D-F) miKIAA0319L-transfected optic tectum at E12. miKIAA0319L was transfected at E5 and the miKIAA0319L-transfected cells indicated by RFP were observed at E12. (G) Quantification of RFP intensity in the each layer of the OT. RFP intensity was significantly increased in the SAC, subventricular layer (**), and ventricular layer. Abbreviations: RFP, red fluorescent protein; SAC, stratum album centrale; SGC, stratum griseum centrale; SGFS, stratum griseum et fibrosum superficiale; SO, stratum opticum; VL, ventricular layer; **, subventricular layer. * P<0.05.
We then carried out statistical analysis in each layer of the optic tectum recognized at this stage of development. The amount of miKIAA0319L1 or mi KIAA0319L2 transfected cells was significantly increased in the SAC, SVL (indicated by **) and VZ (Fig. 4 G, P<0.05) compared to control miRNA electroporated embryos. In controls, 2.6±1.7% of electroporated RFP was located in the SAC, whereas miKIAA0319L-1 and miKIAA0319L-2 had 8.6±1.7% and 11.8±2.9% respectively. In the control miRNA experiments, 0.2±0.2% of electroporated RFP was located in the SVL, whereas miKIAA0319L-1 and miKIAA0319L-2 had 11.2±4.2% and 4.3±1.7% respectively. With respect to the VL, control miRNA had 0.2±0.2% of electroporated RFP in this layer, whereas miKIAA0319L1 and miKIAA0319L2 had 9.6±3.8% and 13.7±5.4% respectively. In the upper layers, only miKIAA0319L1 showed significant decrease in the layer a-d/SGFS. In the control miRNA experiment, 13.4±0.2% of electroporated RFP was located in the a-d/SGFS, whereas miKIAA0319L-1 and miKIAA0319L-2 had 5.5±2.8% (P<0.05) and 9.1±3.9% (P>0.05) respectively. This quantitative analysis demonstrates that knockdown of KIAA0319L resulted in a significant increase in RFP localized in deeper layers of the optic tectum (SAC, SVZ and VZ). Together, these data suggest that KIAA0319L might be involved in cell migration to the upper layers and/or neuronal differentiation in the chick optic tectum.
Discussion
Developmental dyslexia is a disorder characterized by difficulties with reading, despite adequate intelligence and education. Here we investigated the expression profile and function of the dyslexia-associated gene KIAA0319L in the developing chick embryo. To do this, in situ hybridization analysis was performed on whole mount chick embryos aged E3 to E5, and on sections of the E9 optic tectum. Data from these experiments indicate that KIAA0319L is expressed at various time points during visual system and otic vesicle development. In the otic vesicle KIAA0319L is expressed at E3 and stops at E5. Interestingly, this period corresponds to the initiation of neurogenesis in this organ (Adam et al., 1998). Although, this study focuses on the role of KIAA0319L in the developing visual system, this expression pattern may provide some insights into why mice that have loss of KIAA0319L present a deficit in auditory processing (Guidi et al., 2017). In the visual system, we observed that KIAA0319L mRNA begins to be expressed in the optic tectum during a time point coinciding with the onset of radial post-mitotic neuronal migration. On the other hand, retinal expression of KIAA0319L mRNA coincides with the beginning of neurogenesis and subsequently diminishes during time points coinciding with laminar development, which suggests KIAA0319L participates in additional functions beyond neuronal migration. Within the developing E9 optic tectum, KIAA0319L is expressed by the cells in layer iii, layer iv, the SVZ and the VZ (3). Also, as indicated by the WISH experiments, the broad expression profile of KIAA0319L is similar to that of KIAA0319, suggesting that they may play similar or complementary functions during visual system development.
Between E5 and E12, cells primarily migrate in a radial direction away from the VZ. Therefore, to characterize the involvement of KIAA0319L in this process, undifferentiated cells lining the VZ of the E5 optic tectum were electroporated with miRNAs targeting KIAA0319L, and the distribution pattern of these electroporated cells was observed at E12. RFP positive cells at E12 were considered to be indicative of cells in which KIAA0319L had been knocked down. Significant difference were prominent in the deepest layers, with KIAA0319L silencing resulting in significant increase in RFP presence in VZ, SVZ and SAC, with a trend towards decreased RFP in the SGC as well as a trend towards decreased RFP in superficial layers of SGFS (a-g). This suggests that KIAA0319L is involved in waves I and II of tectal migration by early migratory neurons. We did not observe any significant changes in the middle layer cell numbers, indicating that KIAA0319L in not required for migration of late migratory neurons in wave III.
It has been shown that Grg4, a co-repressor of Engrailed, confers the late migratory neuron cell in the ventricular layer of the optic tectum. Overexpressing Grg4 changes alters the normal migratory destination of the neurons (middle layers; h, i, and j of the SGFS) (Sugiyama and Nakamura 2003). Moreover, overexpression of Engrailed-2 (En2) alters the normal destination of early migratory neurons from the superficial layers of a-g/SGFS to middle layers (h, I and j of SGFS)(Omi and Nakamura 2015). Grg4 and En2 are critical for post-mitotic cellular migration towards the middle layers (wave III)(Omi and Nakamura 2015), however the downstream genes that serve to regulate cellular migration at the cell surface have yet to be identified. Since the Grg4 and En2 are transcription repressors, one possibility is that KIAA0319L is downregulated by the Grg4/En2 complex in order to switch the default destination from the superficial layers to the middle layers.
With respect to developmental dyslexia, the suggestion that impaired neuronal migration may act as a cellular cause of dyslexia initially came from post-mortem identification of abnormalities in cortical neuron organization in language areas of the brain in individual with dyslexia (Galaburda 1992, 1994). This was followed by the identification of the aforementioned dyslexia susceptibility genes and subsequent RNAi experiments in the rat neocortex, which identified KIAA0319, KIAA0319L, DYX1C1, and DCDC2 as all playing roles in neuronal migration. However, genetic mouse models in which these genes were knocked out, alone (Martinez-Garay et al., 2017; Wang et al., 2011; Guidi et al., 2017; Rendall et al., 2017) or, in the case of KIAA0319 and KIAA0319L, in combination (Guidi et al., 2017), failed to reproduce the expected neuronal migration phenotypes.
It is not uncommon to have discrepancies between knockdown and knockout models, with potential contributing factors including i) interspecies differences, ii) differences in dynamics between RNAi techniques and Cre-recombinase, iii) off target effects of shRNA constructs or iv) compensation in genetic models. For example, it is possible that KIAA0319L knockout mice display genetic compensation through transcriptional adaptation, which has previously been observed in genetic models, but not following RNAi mediated knockdown. Indeed, in a mouse knockout model of Dcdc2, the Dcx gene, a member of the same gene family, functionally compensates for the loss of the Dcdc2 gene (Wang et al., 2011). While it still remains unclear why KIAA00319 and KIAA0319L knockout mice fail to present with altered neuronal migration phenotypes, our present findings add to this picture by indicating that KIAA0319L does play a role in neuronal migration/differentiation in the developing chick visual system.
Materials and Methods
Preparation of chick eggs
Fertilized eggs (White Leghorn) were obtained from a local supplier (Frey’s Hatchery, St. Jacobs, Ontario), stored at 4ºC for a maximum of 2 weeks. Eggs were incubated at 38 ˚C and 60% humidity.
KIAA0319L constructs
Reverse transcriptase polymerase chain reaction (RT-PCR) was performed to generate 500bp KIAA0319 fragment and 700bp KIAA0319L fragment and cloned into pGEM T-easy vector (Promega, Madison, Wisconsin). Antisense probes were linearized with KpnI and transcribed with Sp6 RNA Polymerase (Roche, Basel, Switzerland) while sense probes were linearized with HindIII and transcribed with T7 RNA polymerase (Roche).
miRNA hairpin sequences against KIAA0319L (target sequence of miKIAA0319L1;ATGTCAGCTGAACTGCTCTGA, miKIAA0391L2; TGTCAACGTTATTGTCAAAGA) was prepared and cloned into a miRNA operon expression cassette (MOEC) vector that contains the reporter gene RFP (Red Fluorescent Protein).
In situ Hybridization
In situ hybridization was performed as described previously (Banerjee et al., 2016). Briefly, for whole mount in situ hybridization, embryonic day 3 (E3), E4 and E5 chick embryos were fixed overnight in 4% paraformaldehyde. Embryos were processed as described previously. Developing reaction took place with 125 µg/ml of 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) and 250 µg/ml of 4-nitoro blue tetrazolium chloride (NBT) in NTMT (0.1M NaCl, 0.1M Tris pH9.5, 0.04 M MgCl2, 2 mM levamisole, 0.1% Tween20).
For in situ hybridization on section, E9 and E12 embryos were dissected and frozen in Tissue Tek OCT compound (Sakura, Torrance, California), then sectioned at 14 μm, then were processed described previously (Banerjee et al., 2016). Color development took place in NBT and BCIP in 0.1M Tris pH9.5, 0.1M NaCl, 50mM MgCl2, levamisole. Tissue sections were then stained with DAPI (0.1ug/mL).
miRNA constructs
miRNA hairpin sequences were prepared with PCR using modified primers from (Das et al., 2006b). The PCR product was then inserted into a miRNA operon expression cassette (MOEC) inside a vector that also contains red fluorescent protein (pRFPmiRNA). The pRFPmiRNA plasmids targeting KIAA0319L were sent for sequencing. Midipreps were performed on DNA so as to obtain the plasmids with a concentration of 1.5 μg/μl, which is ideal for electroporation.
In ovo electroporation
In ovo electroporation in optic vesicle at E1.5 (HH10-12)
The use of fertilized eggs is not subject to any ethic approval. Electroporation of miRNA plasmid vectors was carried out as we described previously with some minor modifications (Harada et al., 2019). Briefly, eggs were incubated for 36 hours to reach around HH stage 10 (Hamburger and Hamilton) in a well-humidified chamber at 38 ˚C. DNA plasmid solution was injected into the right optic vesicle and three times square pulses were applied with CUY21 (Genetronics, San Diego, CA) at 15V, three 50 ms pulses at 950 ms intervals. The eggs were then re-incubated at 37˚C for two days. Embryos were fixed in 4% PFA at E3.5.
In ovo electroporation in the optic tectum at E5 (HH 25-27)
Eggs were incubated for five days in a well-humidified chamber. DNA plasmid solution was injected into the middle part of the optic tectum. Electroporation was performed at 8V with three 50ms pulses with 50ms intervals (Banerjee et al., 2016). The embryos were resealed and incubated at 37˚C. The embryos were sacrificed at E12, fixed in 4% PFA, then 150 µm sections were prepared using a vibratome and stained with DAPI.
Acknowledgements
We thank Donald K. Johnston and The Krembil Foundation for their constant support and enthusiasm for vision research.
Declarations
Competing interests
The authors declare that they have no competing interests.
Author contributions
JC performed in situ hybridization and electroporation. HH did the immunostaining and blinded quantitative analyses. XC helped generating miRNAs. TW and CB, contributed to manuscript redaction. PPM designed the study and wrote the manuscript.
Funding
This work was supported by CIHR to PPM and the Vision Science Research Program to JC.
Availability of data and materials
All data and materials are available upon request.
References
Aburto M. R., Sánchez-Calderón H., Hurlé J. M., Varela-Nieto I., Magariños M. (2012). Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death & Disease 3: e394-e394.
Adam J., Myat A., Roux I. L., Eddison M., Henrique D., Ish-Horowicz D., Lewis J. (1998). Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development . Development 125: 4645-4654.
Banerjee P., Harada H., Tassew N. G., Charish J., Goldschneider D., Wallace V. A., Sugita S., Mehlen P., Monnier P. P. (2016). ϒ-secretase and LARG mediate distinct RGMa activities to control appropriate layer targeting within the optic tectum. Cell Death & Differentiation 23: 442-453.
Cope N., Harold D., Hill G., Moskvina V., Stevenson J., Holmans P., Owen M. J., O’Donovan M. C., Williams J. (2005). Strong Evidence That KIAA0319 on Chromosome 6p Is a Susceptibility Gene for Developmental Dyslexia. The American Journal of Human Genetics 76: 581-591.
Couto J. M., Gomez L., Wigg K., Cate-Carter T., Archibald J., Anderson B., Tannock R., Kerr E. N., Lovett M. W., Humphries T., Barr C. L. (2008). The KIAA0319-Like (KIAA0319L) Gene on Chromosome 1p34 as a Candidate for Reading Disabilities . Journal of Neurogenetics 22: 295-313.
Das R. M., Van Hateren N. J., Howell G. R., Farrell E. R., Bangs F. K., Porteous V. C., Manning E. M., McGrew M. J., Ohyama K., Sacco M. A., Halley P. A., Sang H. M., Storey K. G., Placzek M., Tickle C., Nair V. K., Wilson S. A. (2006). A robust system for RNA interference in the chicken using a modified microRNA operon. Developmental Biology 294: 554-563.
Doh S. T., Hao H., Loh S. C., Patel T., Tawil H. Y., Chen D. K., Pashkova A., Shen A., Wang H., Cai L. (2010). Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques. BMC Developmental Biology 10: 8.
Eicher J. D., Gruen J. R. (2015). Language Impairment and Dyslexia Genes Influence Language Skills in Children With Autism Spectrum Disorders. Autism Research 8: 229-234.
Galaburda A. M., (1992). Dyslexia. New England Journal of Medicine 327: 279; author reply 80-1-281.
Galaburda A. M. (1994). Developmental dyslexia and animal studies: at the interface between cognition and neurology. Cognition 50: 133-149.
Guidi L. G., Mattley J., Martinez-Garay I., Monaco A. P., Linden J. F., Velayos-Baeza A., Molnár Z. (2017). Knockout Mice for Dyslexia Susceptibility Gene Homologs KIAA0319 and KIAA0319L have Unaffected Neuronal Migration but Display Abnormal Auditory Processing. Cerebral Cortex 27: 5831-5845.
Hannula-Jouppi K., Kaminen-Ahola N., Taipale M., Eklund R., Nopola-Hemmi J., Kääriäinen H., Kere J. (2005). The Axon Guidance Receptor Gene ROBO1 Is a Candidate Gene for Developmental Dyslexia. PLoS Genetics 1: e50.
Harada H., Farhani N., Wang X.F., Sugita S., Charish J., Attisano L., Moran M., Cloutier J.F., Reber M., Bremner R., Monnier P. P. (2019). Extracellular phosphorylation drives the formation of neuronal circuitry. Nature Chemical Biology 15: 1035-1042.
Ibraghimov-Beskrovnaya O. (2000). Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Human Molecular Genetics 9: 1641-1649.
Jackson C.A., Peduzzi J.D., Hickey T.L. (1989). Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. The Journal of Neuroscience 9: 1242-1253.
Katusic S. K., Colligan R. C., Barbaresi W. J., Schaid D. J., Jacobsen S. J. (2001). Incidence of Reading Disability in a Population-Based Birth Cohort, 1976–1982, Rochester, Minn. Mayo Clinic Proceedings 76: 1081-1092.
Kujala T., Karma K., Ceponiene R., Belitz S., Turkkila P., Tervaniemi M., Näätänen R. (2001). Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proceedings of the National Academy of Sciences 98: 10509-10514.
LaVail J. H., Maxwell Cowan W. (1971). The development of the chick optic tectum. I. normal morphology and cytoarchitectonic development. Brain Research 28: 391-419.
Martinez-Garay I., Guidi L. G., Holloway Z. G., Bailey M. A. G., Lyngholm D., Schneider T., Donnison T., Butt S. J. B., Monaco A. P., Molnár Z., Velayos-Baeza A. (2017). Normal radial migration and lamination are maintained in dyslexia-susceptibility candidate gene homolog Kiaa0319 knockout mice. Brain Structure and Function 222: 1367-1384.
McConnell S.K. (1988). Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. The Journal of Neuroscience 8: 945-974.
Mey J., Thanos S. (2000). Development of the visual system of the chick. Brain Research Reviews 32: 343-379.
Omi M., Nakamura H. (2015). Engrailed and tectum development. Development, Growth & Differentiation 57: 135-145.
Paracchini S., Thomas A., Castro S., Lai C., Paramasivam M., Wang Y., Keating B. J., Taylor J. M., Hacking D. F., Scerri T., Francks C., Richardson A. J., Wade-Martins R., Stein J. F., Knight J. C., Copp A. J., LoTurco J., Monaco A. P. (2006). The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319 , a novel gene involved in neuronal migration. Human Molecular Genetics 15: 1659-1666.
Paternicó D., Manes M., Premi E., Cosseddu M., Gazzina S., Alberici A., Archetti S., Bonomi E., Cotelli M. S., Cotelli M., Turla M., Micheli A., Gasparotti R., Padovani A., Borroni B. (2016). Frontotemporal dementia and language networks: cortical thickness reduction is driven by dyslexia susceptibility genes. Scientific Reports 6: 30848.
Peschansky V. J., Burbridge T. J., Volz A. J., Fiondella C., Wissner-Gross Z., Galaburda A. M., Turco J. J. L., Rosen G. D. (2010). The Effect of Variation in Expression of the Candidate Dyslexia Susceptibility Gene Homolog Kiaa0319 on Neuronal Migration and Dendritic Morphology in the Rat. Cerebral Cortex 20: 884-897.
Pillay S., Meyer N. L., Puschnik A. S., Davulcu O., Diep J., Ishikawa Y., Jae L. T., Wosen J. E., Nagamine C. M., Chapman M. S., Carette J. E. (2016). An essential receptor for adeno-associated virus infection. Nature 530: 108-112.
Platt M.P., Adler W.T., Mehlhorn A.J., Johnson G.C., Wright K.A., Choi R.T., Tsang W.H., Poon M.W., Yeung S.Y., Waye M.M.Y., Galaburda A.M., Rosen G.D. (2013). Embryonic disruption of the candidate dyslexia susceptibility gene homolog Kiaa0319-like results in neuronal migration disorders. Neuroscience 248: 585-593.
Poon M.W., Tsang W.H., Chan S.O., Li H.M., Ng H.K., Waye M. M.Y. (2011). Dyslexia-Associated Kiaa0319-Like Protein Interacts with Axon Guidance Receptor Nogo Receptor 1. Cellular and Molecular Neurobiology 31: 27-35.
Ramus F. (2006). Genes, brain, and cognition: A roadmap for the cognitive scientist. Cognition 101: 247-269.
Rendall A. R., Tarkar A., Contreras-Mora H. M., LoTurco J. J., Fitch R. H. (2017). Deficits in learning and memory in mice with a mutation of the candidate dyslexia susceptibility gene Dyx1c1. Brain and Language 172: 30-38.
Schumacher J., Anthoni H., Dahdouh F., König I. R., Hillmer A. M., Kluck N., Manthey M., Plume E., Warnke A., Remschmidt H., Hülsmann J., Cichon S., Lindgren C. M., Propping P., Zucchelli M., Ziegler A., Peyrard-Janvid M., Schulte-Körne G., Nöthen M. M., Kere J. (2006). Strong Genetic Evidence of DCDC2 as a Susceptibility Gene for Dyslexia. The American Journal of Human Genetics 78: 52-62.
Strehlow U., Haffner J., Bischof J., Gratzka V., Parzer P., Resch F. (2006). Does successful training of temporal processing of sound and phoneme stimuli improve reading and spelling?. European Child & Adolescent Psychiatry 15: 19-29.
Sugiyama S., Nakamura H. (2003). The role of Grg4 in tectal laminar formation . Development 130: 451-462.
Szalkowski C. E., Fiondella C. G., Galaburda A. M., Rosen G. D., LoTurco J. J., Fitch R. H. (2012). Neocortical disruption and behavioral impairments in rats following in utero RNAi of candidate dyslexia risk gene Kiaa0319 . International Journal of Developmental Neuroscience 30: 293-302.
Taipale M., Kaminen N., Nopola-Hemmi J., Haltia T., Myllyluoma B., Lyytinen H., Muller K., Kaaranen M., Lindsberg P. J., Hannula-Jouppi K., Kere J. (2003). A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proceedings of the National Academy of Sciences 100: 11553-11558.
Tallal P., Miller S. L., Bedi G., Byma G., Wang X., Nagarajan S. S., Schreiner C., Jenkins W. M., Merzenich M. M. (1996). Language Comprehension in Language-Learning Impaired Children Improved with Acoustically Modified Speech. Science 271: 81-84.
Wang H. Z., Qin H.D., Guo W., Samuels J., Shugart Y. Y. (2013). New insights into the genetic mechanism of IQ in autism spectrum disorders. Frontiers in Genetics 4: 195.
Wang Y., Yin X., Rosen G., Gabel L., Guadiana S. M., Sarkisian M. R., Galaburda A. M., LoTurco J. J. (2011). Dcdc2 knockout mice display exacerbated developmental disruptions following knockdown of doublecortin. Neuroscience 190: 398-408.