Int. J. Dev. Biol. 66: 137 - 154 (2022)
Special Issue: Developmental Biology in Greece
Development of growth factor-incorporating liposomes for integration into scaffolds as a method to improve tissue regeneration
Open Access | Review | Published: 9 August 2021
Abstract
This review is an update with regard to the efforts to develop liposomal carriers for growth factor delivery. It is well known that growth factors have the potential to enhance/accelerate tissue regeneration; however, their poor stability, which results in rapid loss of their activity, together with their rapid clearance from defected tissues (when applied as free molecules) is a serious drawback for their use; their highly hydrophilic nature and low capability to permeate through biological barriers (cell membranes) are additional factors that limit their applicability. In recent years, the advantages of liposomal drug delivery systems have motivated efforts to deliver growth factors (GFs) in liposomal form. Herein, after briefly introducing the basic structural characteristics of liposome types and their advantages when used as drug carriers, as well as the basic problems encountered when GFs are applied for tissue regeneration, we focus on recent reports on the development and potential regenerative effects of liposomal GFs, towards defects of various tissues. The methodologies used for incorporation, attachment or immobilization of liposomal GFs in order to sustain their retention at the defected tissues are also highlighted.
Keywords
liposomes, growth factors, delivery, tissue, regeneration, defects, neurodegeneration, liver, disease, targeting, gene therapy
LIPs as carriers of active substances
Liposomes (LIPs) are defined as round vesicular structures constructed mainly by phospholipids and cholesterol (Chol) (Fig. 1). They may contain one (unilamellar vesicles [ULV]) or more (oligolamellar [OLV] or multilamellar vesicles [MLV]) lipid bi-layer(s) or lamella, and an aqueous core (aqueous compartments are also formed between the lamella of OLVs and MLVs) (Antimisiaris et al., 2021; Gregoriadis, 2016).
Fig. 1. Structure, advantages and disadvantages of LIPs as drug carriers.
Figure is republished from Antimisiaris et al., 2021, after permission provided by Elsevier and Copyright Clearance Center.
LIPs are highly biocompatible and completely biodegradable. Another important advantage of liposomes when considered as potential carriers for drugs is that their structural characteristics (size, structure, membrane rigidity, charge, surface properties), as well as the technology used for their manufacturing, can be modified or selected in order to adjust to the features of the substance intended for delivery (physicochemical properties, stability, and delivery problems) and the specific therapeutic application (route of administration, drug dosage, biological barriers encountered, need for sustained drug concentrations, etc.) (Antimisiaris et al., 2021; Gregoriadis, 2016; Antimisiaris et al., 2008).
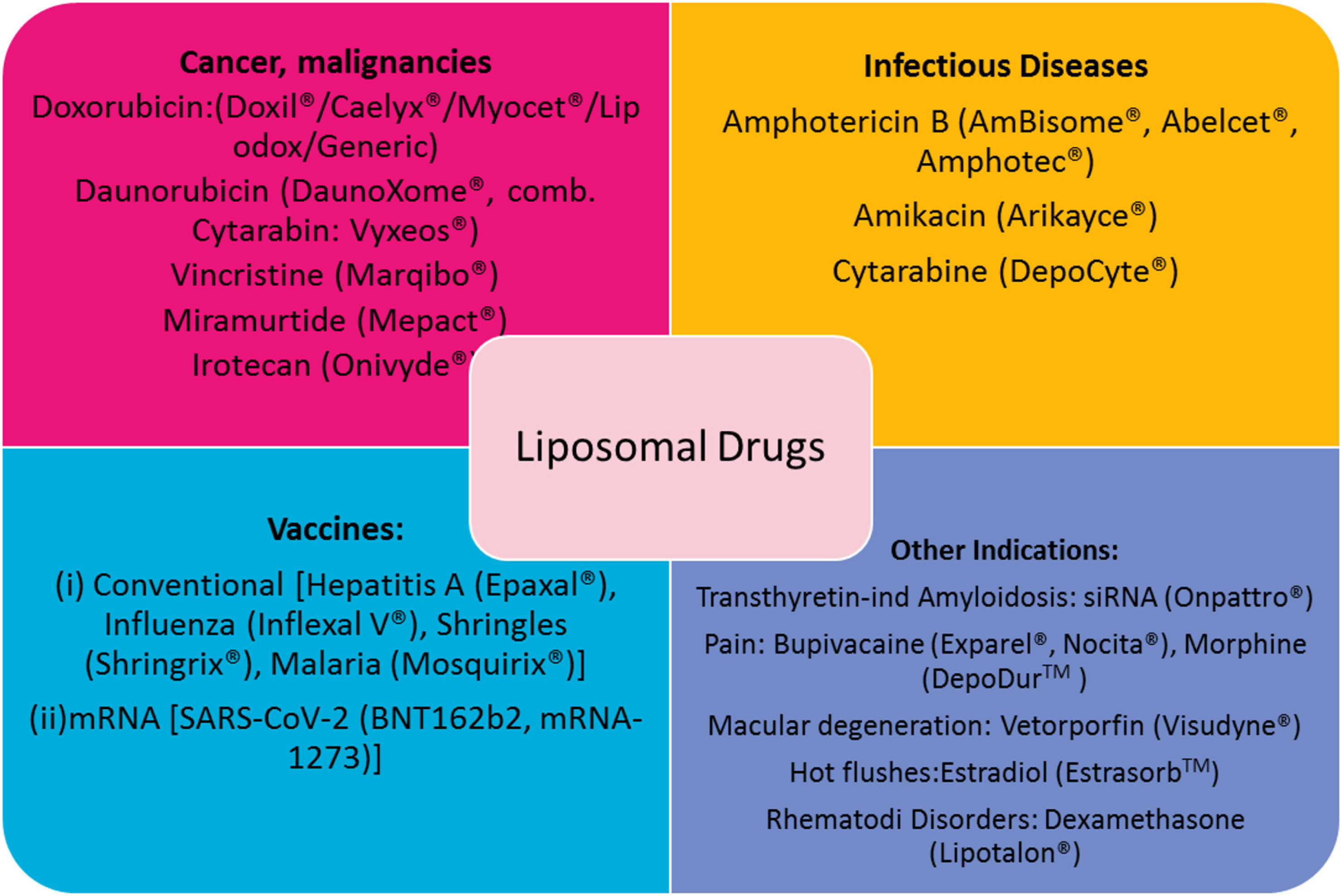
LIPs were first discovered in the mid-60s by Dr. Alec Bangham, a biologist who used them as models of cell membranes to study the effects of anesthetics on membranes. However, it was not until the early 1970s that the potential advantages of LIPs as vesicular carriers for drug delivery were noticed by Brenda Ryman and her colleague Gregory Gregoriadis . Since then, numerous applications of LIPs for delivery and targeting of drugs have been pursued (Gregoriadis, 2016). Several liposomal products are currently in clinical use after being approved for human use as therapeutics for treatment of different types of diseases, such as cancer, infections, severe pain , etc., the most recent being the mRNA vaccines against COVID-19 (Antimisiaris et al., 2021) (Fig. 2).
Over the years, major breakthroughs in the liposome field have contributed significantly to the production of liposomal drugs, such as: (i) The discovery of polyethylene-glycol-(PEG)-coated LIPs (or PEGylated) that can avoid rapid uptake by RES macrophages and thus circulate for prolonged time periods (compared to non-PEGylated LIPs), thus having much broader therapeutic applicability. (ii) The discovery of the active-loading method for amphipathic drug molecules. (iii) The discovery of first and (most importantly) second generation ionizable lipids that facilitated oligonucleotide packaging / stabilization in stable non-toxic vesicular structures, as well as their early endosomal escape, ensuring the preservation of their activity; and (iv) The development of microfluidic mixing technologies for scalable manufacturing of LIPs and lipid nanoparticles. The two first breakthroughs were particularly important and led to the approval of many PEGylated-liposomal anticancer drug products in the ‘90s and 2000s. The last two discoveries, together with the recent breakthroughs in RNA therapeutics, led to the development of tone liposomal siRNA therapeutic product Onpattro® (for treatment of transthyretin induced amyloidosis), by Alnylam, and two mRNA vaccines against SARS-CoV-2, the BNT162b by Biontech/Pfizer, and mRNA-1273 by Moderna (Antimisiaris et al., 2021).
Liposome types and categories
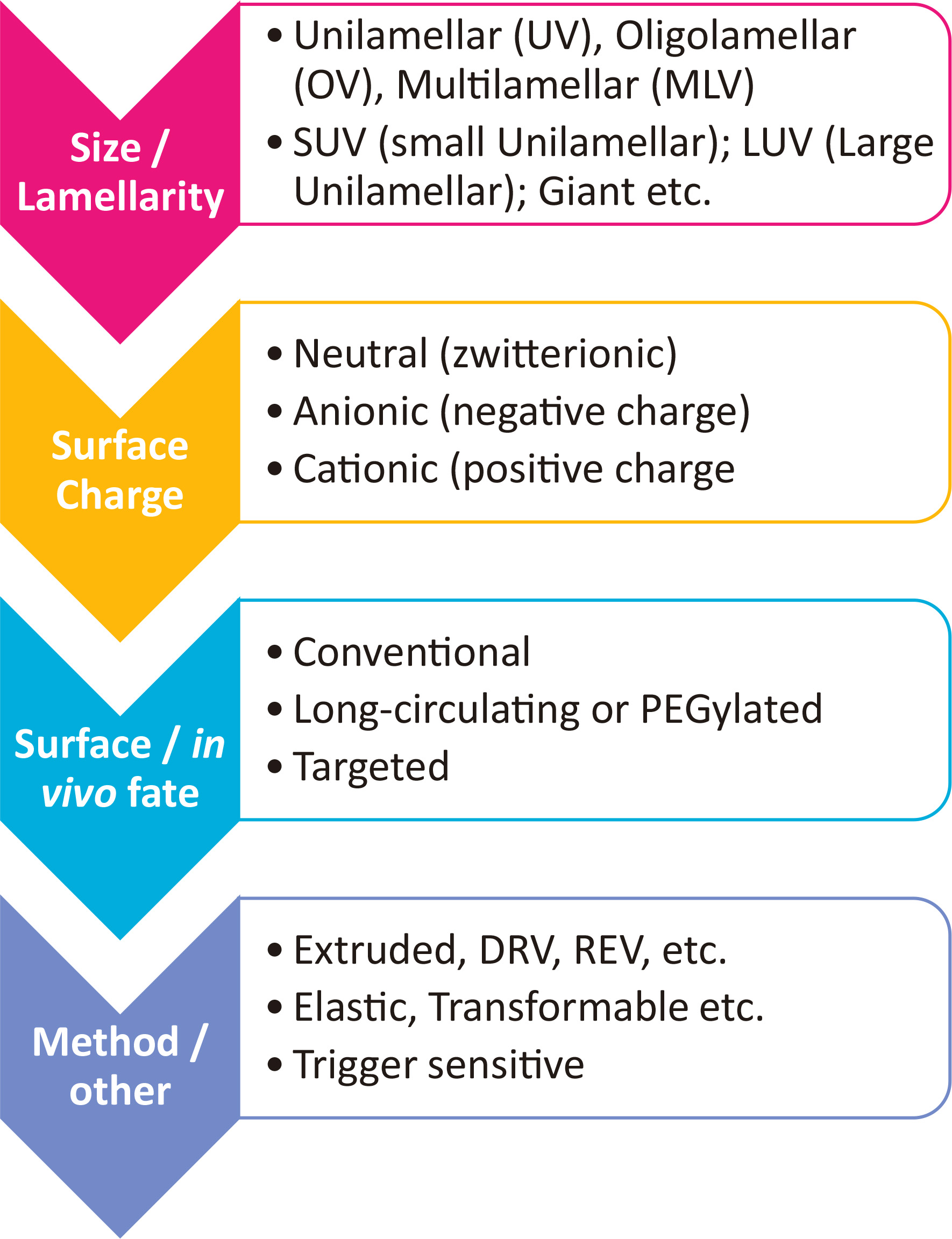
Numerous liposome types and technologies for their manufacturing are currently available. LIPs are classified according to their size, lamellarity and/or structure, surface charge, surface properties, in vivo fate, and method of production (Fig. 3).
In general, liposome sizes (mean diameter) may range between 50 nm and 4-5 um. However, we should clarify that unilamellar vesicles are those currently employed for almost all liposomal drugs, due to the fact that they can be manufactured as highly monodisperse (in terms of vesicle mean diameter) dispersions; they are classified as small unilamellar vesicles [SUV’s] (<200nm) or large unilamellar LIPs [LUV’s] (>200nm). Multilamellar vesicles (MLV) contain polydisperse populations of vesicles that may have mean diameters between 50 – 5000 nm, while giant LIPs (unilamellar or multicompartmental) may also be formulated (Antimisiaris et al., 2008).
LIPs may also be classified according to their zeta-potential or surface charge, as neutral or zwitterionic (no charge), anionic (negative charge) and cationic (positive charge). Cationic LIPs are used in gene delivery applications, due to their capability to form complexes (lipoplexes or lipid-nanoparticles) with (oppositely) highly negative charged oligonucleotides, as mentioned in more detail elsewhere (Antimisiaris et al., 2021; Antimisiaris et al., 2008; Antimisiaris et al., 2017). However, due to the toxicity of cationic LIPs, in the last 5 years, cationic lipids have been replaced with the so called “ionizable” lipids, the charge of which is modulated by pH, resulting in type of LIPs that can retain oligonucleotides at pH 7.40 and rapidly release (following endocytosis) at the lower pH of the cell cytoplasm (Antimisiaris et al., 2021; Antimisiaris et al., 2017).
Another important parameter when selecting an appropriate liposome type for drug delivery is the in vivo fate of LIPs. MLV-LIPs can retain encapsulated aqueous soluble drugs for longer than unilamellar LIPs, since in the case of the former, the drugs are protected by more membranes; however, their large size and wide size distribution minimize their potential applications, as mentioned above. On the other hand, LIPs with nano-dimensions are the most applied LIP-types due to their: (i) longer blood circulation periods; (ii) their increased capability to diffuse through biological membrane or barriers; and (iii) their ability to increase LIP-associated-drug uptake by cells (Antimisiaris et al., 2021; Gregoriadis, 2016; Antimisiaris et al., 2008). According to the EPR effect (enhanced permeation and retention) effect, nano-sized LIPs (mean diameter < 200nm) have the capability to diffuse through the vasculature in tumour tissues more than in normal tissue vasculature, due to the larger junctions between the endothelial cells in the former. Nevertheless, it is well known today that the surface properties of LIPs are perhaps even more important than their size, to ensure prolonged circulation in blood, a pre-requisite for targeting cancer tumors by the EPR-effect (passive targeting). In this respect, LIP are categorized as conventional LIPs and sterically-stabilized LIPs (or long circulating, or “stealth”), depending on their half-life in blood (Fig. 3). Conventional LIPs are rapidly taken up by the macrophages of the reticuloendothelial system (RES) and thus rapidly accumulate in the liver and spleen following injection in the bloodstream; sterically-stabilized or long-circulating LIPs circulate in the blood for much longer time periods (compared with conventional LIPs) due to their decreased interactions with blood components as a result of their surface coating with hydrophilic polymers. The most used polymer for construction of sterically-stabilized LIPs is polyethylene glycol (PEG). PEGylated SUV or LUV LIPs are the types of most liposomal drugs developed in the last 30 years (Antimisiaris et al., 2021).
The in vivo fate of LIPs can also be modulated by active targeting (opposed to passive targeting); targeted LIPs (or ligand-targeted LIPs) have targeting ligands on their surface, such as monoclonal antibodies, peptides, aptamers, small molecules etc., with high affinity towards receptors that are overexpressed on the target cells. For targeted-LIP construction, the ligands are usually attached to the distal end of PEG chains, for optimal identification by the targeted receptors (Antimisiaris et al., 2021; Antimisiaris et al., 2008).
Some other LIP types that have special functions, owing to particular structural components, are the elastic LIPs (ethosomes, transferosomes, niosomes, transformable, invasomes, etc.), which are particularly useful for topical administration, since they can squeeze through narrow gaps between epithelial cells (such as the keratinocytes of the outer layer of epidermis). The components that confer elasticity to the membranes or elastic LIPs are referred to as “edge activators” and are usually surfactants (Antimisiaris et al., 2021; Antimisiaris et al., 2008).
Another LIP type are triggered–release LIPs; these can be conventional, long circulating, targeted, etc. LIPs that, due to special structural components they contain, demonstrate sensitivity towards particular environmental triggers. Environmental triggers may be temperature, pH, light, etc., and the particular LIPs are then referred to as temperature sensitive (or thermo-sensitive), pH-sensitive, and light- or photo-sensitive, respectively. Triggered release LIPs have the ability to release encapsulated drugs at specific tissues of cells or intracellular compartments, due to physiologically existing differences (in pH or ionic strength), or due to externally activated triggers (such as thermal triggers, light triggers, magnetic fields, etc.) (Antimisiaris et al., 2021; Antimisiaris et al., 2008).
Applications of LIPs for drug delivery and targeting
The applications of LIPs for the delivery of drugs are amplified first of all due to the fact that they are highly biocompatible, having very low, if any, toxicity, and secondly due to their biodegradability, since they are composed of phospholipids and cholesterol. The potential to load into LIPs very high amounts of any type of drug, lipid soluble, aqueous soluble, amphiphilic, with low MW or high MW, is an additional advantage (Fig. 1).
As drug carriers, LIPs may confer particular actions to enhance the therapeutic potential of the active substances they accommodate. In more detail, LIPs may: (i) protect labile active substances from distortive environments at the site of administration, or in the blood, or at the site of their pharmacological action; (ii) enhance the permeability of drugs through biological barriers, and/or enhance their cellular uptake; (iii) modulate the drug pharmacokinetics and/or prolong their biological half-life (Antimisiaris et al., 2021; Antimisiaris et al., 2008).
Popular recent examples of the protection and increased cell membrane permeability of actives loaded into LIPs are the recently approved mRNA vaccines; their delivery problem was solved by accommodation into LIPs that protect their rapid decomposition and drastically increase their cellular uptake (Antimisiaris et al., 2021).
PEGylated LIPs confer the most important therapeutic advantages, since by modulating the pharmacokinetics of drugs (compared with free drugs), they may reduce drug toxicity and side effects. The possibility to reduce drug administration frequency (if the LIP-associated drug is released slowly, or its half-life is increased), is another potential therapeutic advantage of liposomal drugs. Particularly, PEGylated LIPs, due to their long blood circulation in combination with the EPR-effect (mentioned above), have numerous applications in cancer therapeutics. Conversely, although ligand-targeted LIPs have been intensively exploited over the last 30 years in many pre-clinical and clinical studies, no ligand-targeted LIP formulation is currently approved as a therapeutic (Antimisiaris et al., 2021).
Depending on the intended therapeutic application, the particular active substance to be delivered, and the intended route of administration, the best LIP type can be identified between the different types mentioned in Fig. 3.
Finally, since many LIP manufacturing methods are currently available, it is easy to select the best method, based on the physicochemical properties of the drug or drugs to be delivered (Antimisiaris et al., 2021; Antimisiaris et al., 2008). When LIP formulations are intended for topical delivery of drugs, their rheological properties, their viscosity and their mucoadhesive properties should be adjusted. The latter is usually achieved by either adding gelling agents (polymers etc.) in LIP dispersions, or by embedding the LIPs in pre-formed ointments of hydrogels, etc. Other additives such as surfactants, penetration enhancer, and antimicrobial preservatives and/or antioxidants, etc. may also be needed. More details about LIP manufacturing methods can be found elsewhere (Marazioti et al., 2008; Shah et al., 2020).
Growth factors in tissue regeneration
Tissue regeneration and role of growth factors
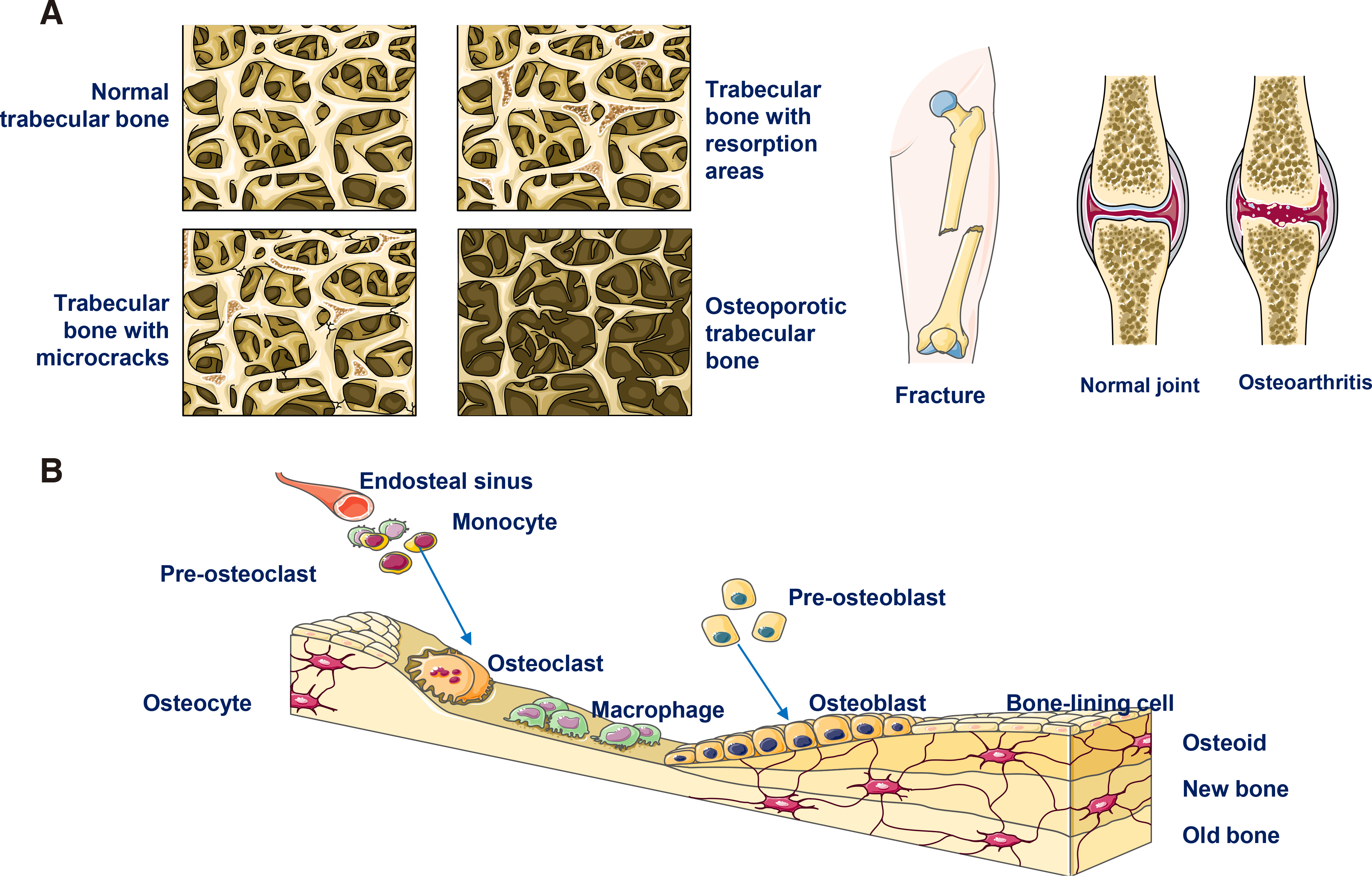
Tissue regeneration is required when tissue defects occur due to various reasons, such as malignancy-related resection surgeries, traumatic injuries, degenerative diseases (such as osteoporosis and osteoarthritis that result in bone defects and other neural degeneration causing pathologies) and various congenital diseases (Fig. 4A) (Koria, 2012). Currently, different methods and strategies are considered for treatment of tissue defects, the most usual being: (i) transplantation of the defected tissue or the whole organ (if tissue transplantation is impossible); (ii) use of allografts or xenografts that may be implanted at the pathological site to replace the defect; (iii) administration or insertion of synthetic devices consisted of biocompatible biomaterials; (iv) administration of cells and particularly stem cells (in most cases). Unfortunately, all of the stated approaches are accompanied by serious drawbacks (Fig. 4B). Indeed, immune rejections of transplanted tissues or organs are possible, especially when xenografts are used, while the surgery required for their functional insertion is usually complicated, posing serious health risks, or even threats to life. Furthermore, transplantation may confer chronic inflammation, jeopardizing the final outcome of the solution. Lastly, the availability of tissues, organs and xenografts for transplantation is usually low, rendering the solution unavailable for many patients, while the use of allografts may also not be possible. Therefore, in many cases, alternative strategies are explored (Koria, 2012; Kulebyakin et al., 2020; Sharma et al., 2021).
To-date, the alternative approach considered in most cases is to replicate as far as possible the natural regenerative pathways and their microenvironment, in order to induce, augment and/or accelerate regeneration. Consequently, in order to replicate the natural regenerative pathways, methods for exogenous administration of growth factors (GFs) or GF combinations are considered, in some cases also in combination with stem cells (SCs) (Koria, 2012; Sharma et al., 2021). The latter is based on the knowledge that tissue regeneration/renewal is controlled (on the molecular level) by different types of bioactive agents, such as short peptides and chemokines, neurotransmitters, and, last but not least, GFs.
GFs are large soluble bioactive peptides secreted by cells, and function as critical signaling molecules affecting numerous cellular functions. During embryonic development, GFs (e.g. FGF, Wnt, PDGF, etc.) interact with other molecules such as extracellular matrix (ECM) components, and trigger the mechanisms for migration of different cell-types to specific locations for initiation of organogenesis. The role of GFs in shaping the structural arrangement of cells in organs is just as important as that of other extracellular substances during organogenesis. After birth, GFs are absolutely vital for tissue renewal/regeneration and healing, since they can modulate/regulate the particular cellular processes involved in tissue regeneration, such as cell growth and differentiation, cell metabolism, chemotaxis, migration, and apoptosis. GFs function as signaling molecules, transferring information between cells and extracellular matrix (ECM) and mediating optimal tissue regeneration by promoting cells to differentiate, proliferate and migrate; additionally, GFs induce ECM synthesis. Furthermore, GFs are key players in angiogenesis, succeeding in enhancing/accelerating the vascularization of the regenerating new tissue, a crucial parameter for optimum (damaged) tissue renewal. The processes for GF biogenesis and their activation are particularly complex, and more details of the latter can be found elsewhere (Koria, 2012; Kulebyakin et al., 2020).
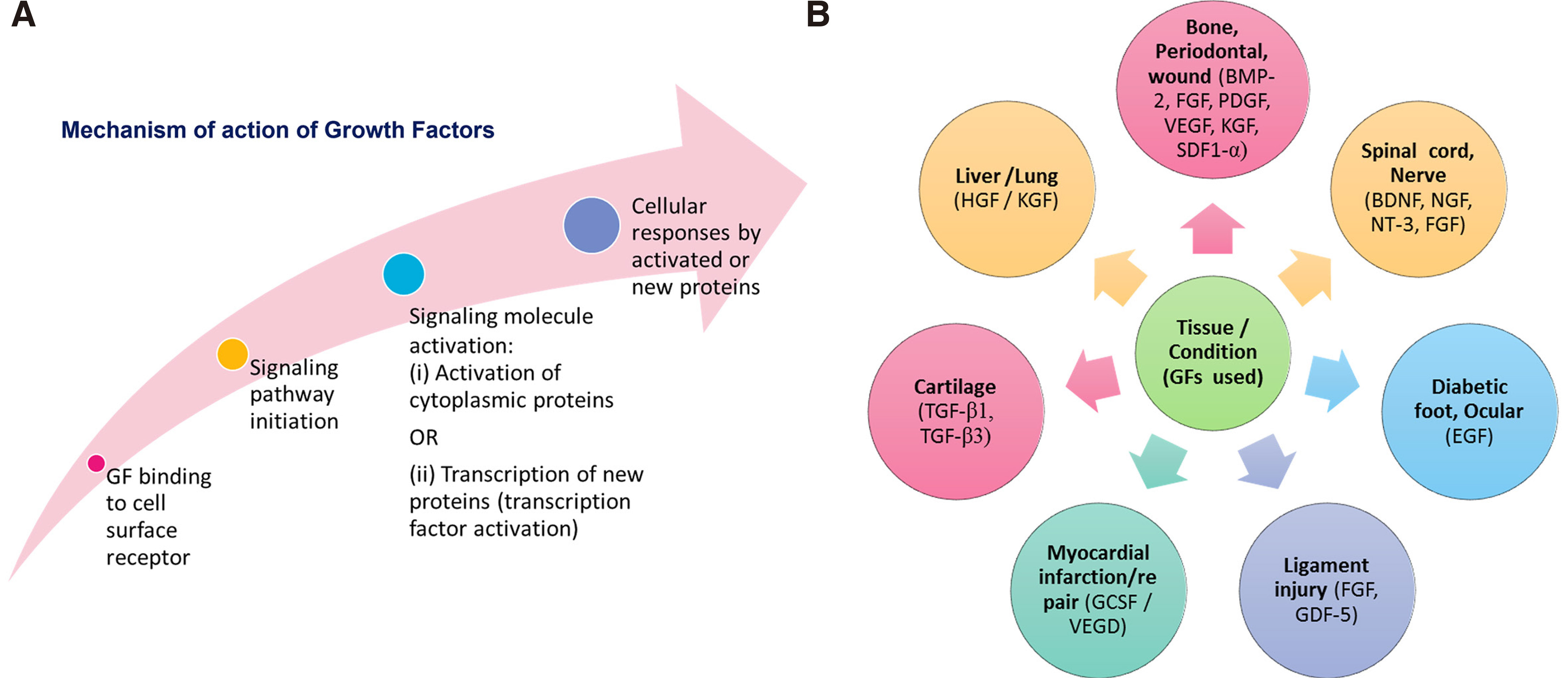
Regarding the mechanisms of action of GFs and how they manage to alter the functionality of cells, it should be pointed out that although GFs are not endocytosed; they regulate cell functions by appropriate conversion of signaling pathway after binding to specific transmembrane receptors. The different steps of possible GF mechanisms of action are schematically presented in Fig. 5A. An important point is that the binding of a particular GF to its transmembrane receptor on cells is highly specific; this ensures that a particular message is transferred to distinct cell types.
GFs are thus established through evolution as unique systems that provide tissue formation during development, and, subsequently, throughout life, they support cell integration, homeostasis, and tissue regeneration. From all the aforementioned functions of GFs, it is logical to conclude that exogenous GFs can be administered for augmentation of the self-healing capacity of patients, and more particularly when acceleration of tissue regeneration and/or healing is needed. In fact, numerous GFs have been applied for healing/regeneration of various tissue types and/or treatment of various pathological conditions or injuries, as seen in Fig. 5B. Some examples include exogenous delivery of BMP-2, FGF, PDGF etc. for bone or periodontal wound regeneration, BDNF, NGF, NT-3, etc. for spinal cord injury treatment, HGF for treatment of liver cirrhosis, etc.
However, it is important to bear in mind that GF action is not always predictable, since when tissue damage occurs, they may become “the cure and the cause”, as recently reported (Kulebyakin et al., 2020). Indeed, it is possible for the same GFs to produce positive or negative outcomes, depending on where and when they manifest their actions within the organism. In more detail, the same cells may respond to same GF treatment in a different way under different conditions, conferring different cellular phenotypes due to activation of differing intracellular pathways. Thus, the spatio-temporal characteristics of GF delivery are crucial for their final outcome as regenerative treatments.
In addition to the aforementioned fact that GFs are required at specific phases during the procedure of regeneration, which means that their retention at the site in a functional state until the particular phase of the regeneration process in which they are useful is particularly crucial (spatio-temporal delivery), other important drawbacks for realization of optimal tissue regeneration by exogenous GF delivery exist, such as: (i) the short in vivo half-life of GFs due to their rapid degradation by proteases and consequent loss of activity; (ii) the rapid clearance of exogenous GFs when administered as free molecules, due to diffusion; (iii) the serious toxicities caused by high concentrations of GFs, when administration of high concentrations is used as a strategy to overcome their rapid clearance and degradation.
GF delivery is complex, and they are usually delivered within biocompatible biomaterials in order to retain the GFs at the diseased site, control their release kinetics (in order to minimize chances of toxicity due to high GF concentrations), and protect the GFs from detrimental action of proteases present at the site of administration and/or action. Such biocompatible biomaterials may be solid scaffolds, hydrogels or nanostructures, depending also on the administration site and method of administration. In some cases, injectable biomaterials are preferred, and in others, solid biocompatible scaffolds, which, in addition to delivering the GF, may also function as fillers of the tissue voids, preserving (at least partly) the defected tissue’s mechanical functions, if required (as in cases of bone or defects). During selection of the optimal biomaterial for GF delivery, one needs to consider whether administration of stem cells together with the GFs is required, or if the migration of cells at the diseased site (realized due to the action of specific GFs) will be sufficient for successful regeneration. Furthermore, in addition to the selection of the appropriate material and structure of the GF delivery-assisting biomaterial, the method used for the attachment of the GFs on the biomaterial is also significant. Simple adsorption, covalent immobilization, or association of the GF within the biomaterial during scaffold manufacturing (i.e. during electrospinning for production of polymeric fibers), are the methods used in most cases. Of course, the method of attachment of GFs to delivery carriers should ensure optimal release and function of the GF.
In recent years, another strategy being applied as a method to overcome the problems involved in exogenous delivery of GFs is the delivery of plasmids encoding the protein, for in situ production of the particular GF protein. Again, the identification of an optimal delivery strategy for the selected gene is a great challenge.
Taking into account all the aforementioned required characteristics of biomaterials for optimal delivery of GFs (or genes encoding the GFs), together with the advantages and functions of LIPs as drug carriers mentioned in part 1 and Fig. 1, it is clear that LIPs may indeed be ideal carriers for exogenous delivery of GFs.
Liposomal growth factors
Herein, we present the research efforts carried out in the last 20 years for liposomal delivery of GFs, and the potential of liposomal GFs for treatment of defected tissues or tissue regeneration.
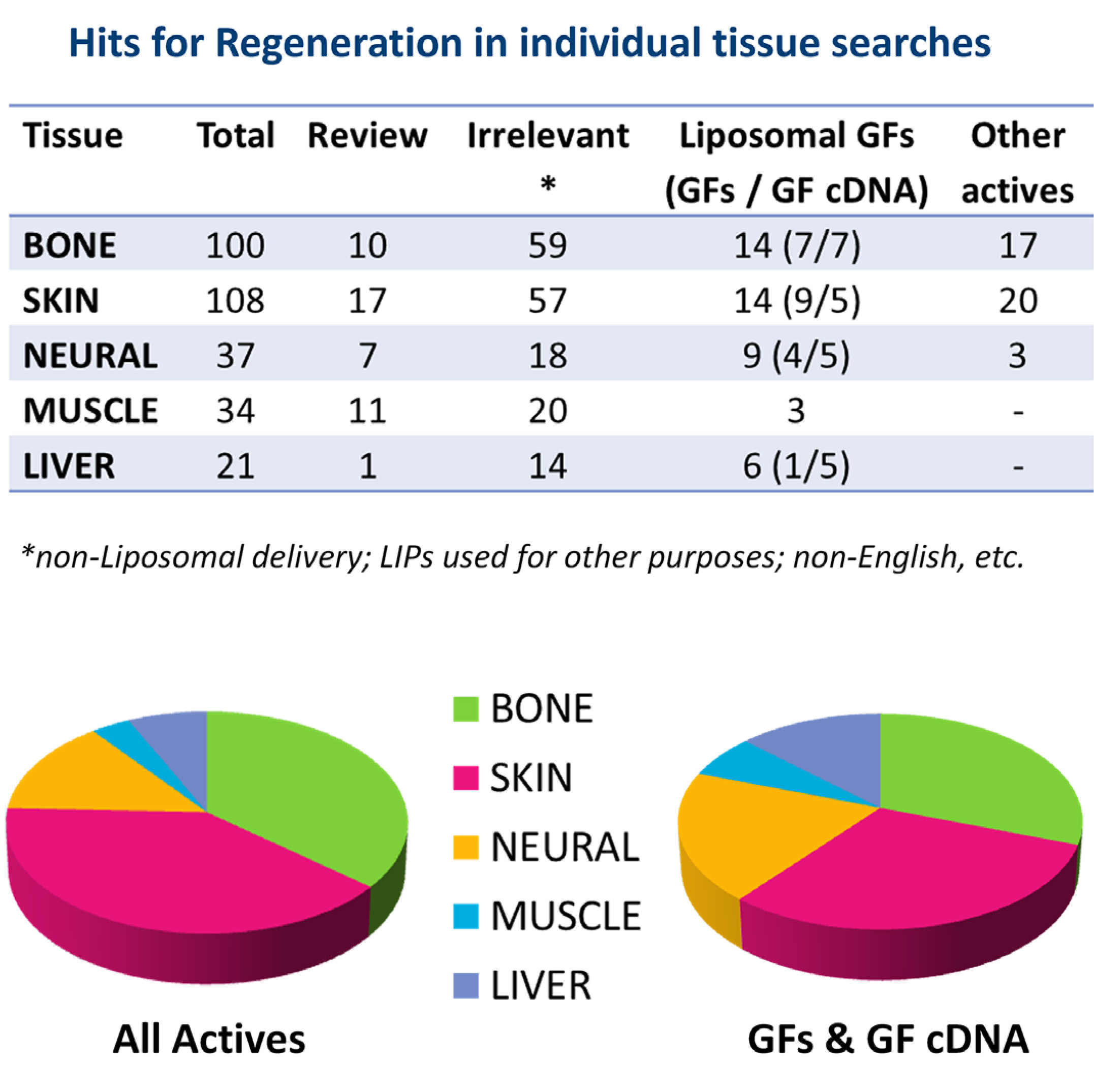
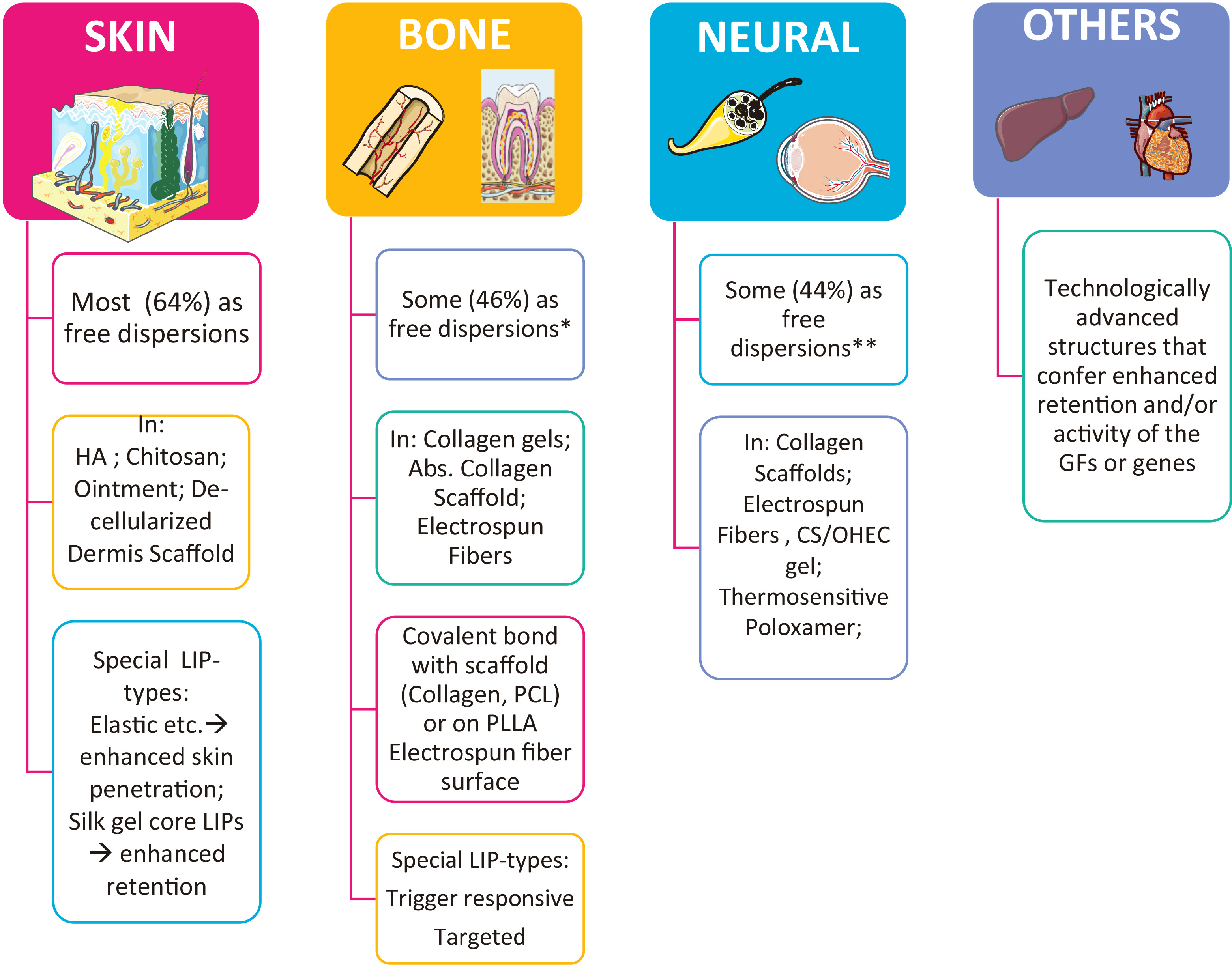
With regard to the methodology of the bibliographic search for reports on liposomal GF development and performance, we searched Pub Med database (https://pubmed.ncbi.nlm.nih.gov, assessed on 21-03-2021) for the period between 2001and 2021. Initially, we used the key words: “Growth factors” AND “liposome” AND “tissue” AND “regeneration”. The former search resulted in 121 hits. In order to extend the pool of hits (in case the first search missed some relevant studies), another search was conducted using as key words: <(Growth factors OR bioactive lipids OR drugs)> AND <liposome> AND <tissue> AND <regeneration>, which resulted in 267 hits. The hits from the last search were reviewed for their relevancy to the subject of this article, and it was seen that the specific tissue types involved in most of the relevant reports were bone, skin, neural, muscle and liver. In case some reports were missed, we additionally conducted separate searches for each of the individual tissues, and finally reviewed the hits of all individual searches. The total, relevant, irrelevant and review article hits, for each tissue, are reported in Fig. 6. As seen, the cumulative total hits for all searches are 300, slightly higher than the hits (267) of the general search (using the word “tissue” instead of specific tissue names).
It is interesting to report that of the 300 total hits (Fig. 6), only 132 (<50%) where found to be relevant with the topic, and of the relevant hits, 46 were review articles, and 86 were research articles. Of the latter, 86 research articles, 46 (53.5%) concerned liposomal delivery of GFs or GF cDNA, and the other 40 were about liposomal delivery of other types of actives with regeneration activity, such as proteins, small molecule drugs etc., revealing that other types of molecules are also highly considered for tissue regeneration, probably due to the difficulties encountered for exogenous delivery of GFs. Furthermore, the fact that the majority of the reported studies involving liposomal GF (as well as other active substance) delivery are for skin and bone regeneration (Fig. 6), reveals the importance of retaining the GFs (or other actives) at the site of action, which is probably easier in the case of skin, where topical delivery is possible, and bone, where the liposomal GFs can be retained on implants and/or biomaterials positioned at the site during surgical procedures.
Selected reports on the use of liposomal GFs (or other actives) for regeneration of individual tissues from those identified by the methodology mentioned above are discussed below, separately for each tissue. We tried to include the most recent examples from all the strategy types applied in each particular tissue.
Liposomal GFs for skin regeneration and wound healing
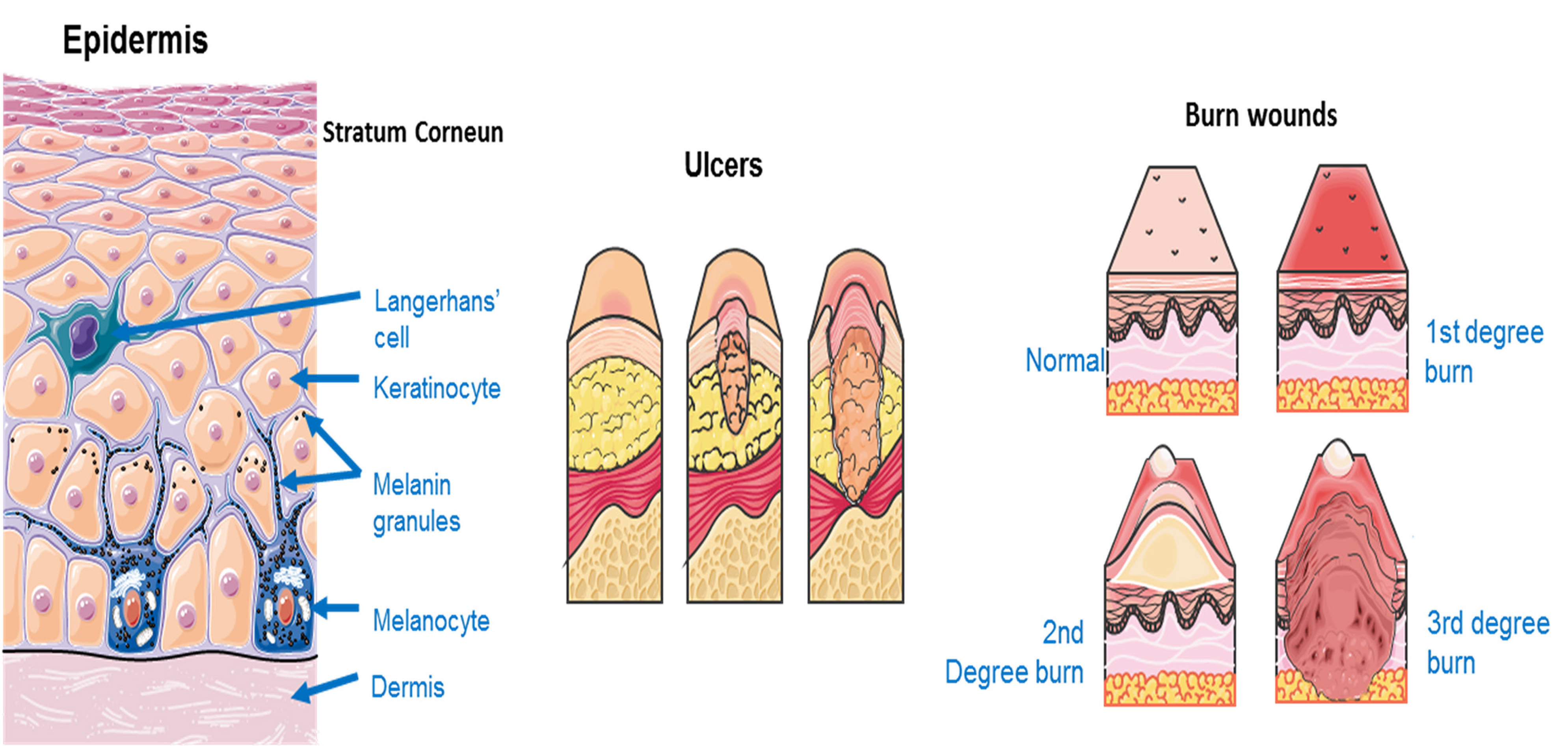
Liposomal GFs for skin regeneration are mostly used for healing of wounds, such as diabetic ulcers, trauma wounds or burn wounds that can be less or more severe as seen in Fig. 7.
As mentioned in section 1, the liposome types used as drug carriers intended for skin delivery (dermal or transdermal delivery), are different from conventional LIPs regarding the composition of their lipid membrane, as already mentioned above (section 2.1) (Antimisiaris et al., 2021; Antimisiaris et al., 2008).
Usually, such LIPs have components in their membrane, the so-called edge activators, that make them more flexible or elastic, or, in other words, able to squeeze through the narrow passages between the keratinocytes of the stratum corneum (Antimisiaris et al., 2021). Ethanol, surfactants (such as tween, span, etc.) and surface active natural substances have been applied for the purpose, and the resulting LIPs are referred to as deformable, elastic, ethosomes, transferosomes, invasomes etc. (Antimisiaris et al., 2021; Gregoriadis, 2016; Antimisiaris et al., 2008).
Furthermore, in case of topical administration, the liposomal GFs are usually embedded in emulsions, creams or hydrogels, in order to sustain their retention on the site of administration (skin or wound). In addition to delivery of GFs in liposomal form for increased GF stability, other strategies considered for enhancing skin regeneration by liposomal GFs include the use of GF/drug combinations, the use of GF combinations, or combinations of GFs with permeation enhancers (that facilitate the penetration of LIP-GFs to deeper skin layers).
Selected examples of liposomal actives for skin regeneration, from the relevant hits of the search conducted, are tabulated in Table 1, and discussed in more detail below.
Table 1
Examples of liposomal GFs for skin regeneration and/or wound healing
| Liposome or delivery system type/ Composition | GF or Active | Models used for study |
Method of LIP attachment on biomaterial |
Ref |
| MLV - Chol/DPPC 1:1 mol/mol; 4.44 μm |
EGF |
2nd degree burn wounds on rats |
Chitosan gel (2%) | Değim et al., 2011 |
| DPPC/Chol, 1:1 mol/mol; 4.44 μm | Non-attached | Alemdaroğlu et al., 2008 | ||
| DSPC/Chol/DSPA 7:2:1mol & Rho-lipid 1 mole% (tracking) 150 nm | SDF-1 | Diabetic murine excisional wound assay | No-cell dermis scaffolds | Olekson et al., 2015 |
| PC/Chol and non-ionic surfactants; 200-250nm | Catalase | Thermally injured rat skin model |
Non-attached |
Abdel-Mageed et al., 2018 |
| DOPC/DOPG /Chol (3:3:4 mol/mol); 117.2 nm |
Glycyl-L-histidyl-L-lysine (GHK)-Cu |
Shallow 2nd degree burn model on mice (scald burn) | Wang et al., 2017 | |
| DMPC; labeled with DiI (0.01 mol%) | Wnt3a protein | Ear wound model in mice | Whyte et al., 2013 | |
| PC/Chol/ laurocapam Silk fibroin (SF) hydrogel core 103nm | bFGF | Mouse skin; mice with deep second scald; | Xu et al., 2018 | |
| DOTAP /Tween 80 (6:1 w/w); 16 nm. & SPC/Tween 80 (10, 20, 30%); 87 nm | EGF; All- trans retinoic acid (TRA) |
HaCaT cells; Deep partial-thickness burn model in rats |
LIPs incorporated in ointment | Lu et al., 2019 |
| HPC/Chol/DOTAP (8:4:1 mol/mol); 107 nm | EGF, IGF-I, PDGF-A | Artificial skin; scratch assay; diabetic wound; | LIPs combined with HA | Choi et al., 2017 |
|
DMRIE/Chol (1:1 mol/mol) |
IGF-I; KGF c DNA | Acute dermal/epidermal wound rats (scald burn) |
Non-attached |
Jeschke abd Hendon, 2007 |
| PDGF cDNA | Yorkshire pigs with fully excised burns / autograft; | Branski et al., 2010 |
In relation to GF skin delivery by LIPs, Degim et al. loaded EGF in LIPs in order to treat rat 2nd degree burn wounds. EGF-containing MLV LIPs were additionally embedded in 2% chitosan gel, and in vivo results demonstrated that the LIPs enhanced epithelization and increased the epidermal thickness of the burned skin faster, compared with control formulations (Değim et al., 2011). In another case, Alemdaroglu et al. used LIPs that carry EGF with the aim of investigating their potential to heal burn wounds. MLV type LIPs containing EGF were prepared and applied to rats with second-degree standard burn wounds. The experiments proved the efficacy of EGF LIPs in treating the burn wounds (Alemdaroglu et al., 2008). Olekson et al. used LIPs loaded SDF-1 in order to investigate its effects on skin regeneration in rat diabetic wounds. They formulated nano-dimension SDF-1 LIPs, and embedded the LIPs into commercially available (for treatment of skin wounds) micro porous, cell-free dermis scaffolds. The bioactivity of acellular dermis loaded with SDF-1 LIPs was tested in vivo in a mouse diabetic wound model. Results showed that SDF-1 LIPs could increase the performance of several acellular matrices applied for tissue engineering (Olenson et al., 2015). More recently, the potential for increasing the stability of catalase by encapsulating it in a type of LIPs called niosomes, which consist of lipids, Chol, and non-ionic surfactants (in the current case Brij 30, 52, 76, 92 or 97), was investigated. There was evaluation of whether increased stability of niosomal-catalase could confer increased skin regeneration in a rat model of burn injury. The rats were exposed to hot water in order to produce second-degree burn injuries and were then immediately treated with either plain catalase or niosomal catalase. The latter group showed significant decrease of the lesion size compared to free-catalase treated rats as well as untreated rats. Niosome-encapsulated Catalase was found to enhance the rate of wound healing by protecting against oxidative stress (Olekson et al., 2015).
In another recent study, Wang et al. encapsulated in LIPs a smaller molecule, with known wound repair activity, the tripeptide Glycyl-L-histidyl-L-lysine (GHK) and Cu2+ complex (GHK-Cu). The effects of the LIPs on the proliferation of human umbilical vein endothelial cells (HUVECs) and on the healing of scald wounds in vivo (in mice) were studied. GHK-Cu promotes angiogenesis, and increases skin oxygen and auxiliary antioxidant enzymes. LIPs encapsulating GHK-Cu, or free GHK-Cu were spread daily on burn-wounds of mice, and it was seen that the wound healing effect of the GHK-Cu-LIPs was superior compared with the effect achieved with the free GHK-Cu. In more detail, in the first stages of healing, a better wound integrity and reduced inflammatory reactions were observed in the group treated with GHK-Cu-LIPs, while at the later stages of healing, retarded scar-formation and faster tissue repair were demonstrated in the same group, compared with other groups (non-treated and treated with free GHK-Cu) (Wang et al., 2017).
Wnt signaling pathways are pathways initiated by protein-assisted signal transfer into cells, through receptors located on the cell surfaces. Whyte et al. applied liposomal Wnt3a to a non-healing ear wound in mice in order to study the effect of Wnt signaling on the skin repair process. LIPs were labeled with DiI and loaded with Wnt3a. LIP-Wnt3 applied topically on non-healing wounds was shown to enhance the endogenous Wnt signaling, and finally conferred improved wound healing, compared with free-Wnt3 (Whyte et al., 2013).
In another case, Xu et al. used a more sophisticated system comprising of LIPs to stabilize GFs that were combined with a permeation enhancer for increased GF permeability. The skin-permeable LIPs incorporated bFGF and were used to treat 2nd degree burns developed on mice skin. The novel LIPs (bFGF-SF-LIP), had a silk fibroin (SF) hydrogel core as a strategy to stabilize bFGF. Aurocapam was added in the LIPs as permeation enhancer (PE) (PE-bFGF-SF-LIPs). bFGF’s skin-permeability was significantly enhanced (reaching the dermis) by PE-bFGF-SF-LIPs. Furthermore, after the novel treatment, the morphology of the hair follicles at the wound zone was visually improved and hair regrowth was also observed in the deep second scald mice model. Mechanistic studies revealed that the proposed treatment acted mainly by inhibiting the formation of scar and promoting vascular growth in the skin dermis (Xu et al., 2018).
One example of the strategy to use GF and drug combinations for skin regeneration is the study reported by Lu et al. where retinoic acid loaded elastic LIPs (TRA DLs), together with a second type of elastic cationic LIPs loaded with EGF, both embedded in an ointment, were used. The TRA LIPs consisted of soya lecithin (sPC) and varying concentrations of Tween 80 (10%, 20%, 30% [w/w]); the second EGF-loaded liposome was a cationic deformable liposome type consisted of the cationic lipid DOTAP and the surfactant Tween 80 (lipid/tween ratio was 6:1 (w/w)). Together with in vitro tests, a partial-thickness burn wound model on rats was used. Results indicated that the combination of the two liposomal formulations resulted in synergism and conferred increased HaCaT cell proliferation and migration (compared to all control treatments). In vivo, the ointment containing the two LIPs stimulated wound closure, formation of skin epidermis and collagen production, improving the quality of healing, compared with the non-liposomal control formulation (Lu et al., 2019).
An example of the strategy of employing GF combinations for skin regeneration is the work of Choi et al., which applied cationic elastic LIPs (ELIPs) to deliver three GFs: PDGF-A, IGF-I, and EGF. The cationic elastic LIPs were comprised of hydrogenetad-PC and Chol with or without DOTAP; the GFs were fused by using low-molecular-weight protamine (LMWP), and the nanodelivery system was complexed with hyaluronic acid to ensure sustained topical delivery. The cationic GFs-HA complex-containing ELIPs were shown to significantly accelerate wound closure, reducing the size of the wound, in a diabetic mouse model (Choi et al., 2017).
As mentioned, another approach for regeneration is to apply gene delivery for in situ production of the GF-proteins. LIPs can be used for this purpose, since the delivery of genes is problematic due to their low integrity/stability and non-ideal physicochemical properties (Antimisiaris et al., 2021; Antimisiaris et al., 2017). In this context, Jeschke and Herndon used LIPs to transfer IGF-I cDNA and KGF cDNA in order to test whether they can facilitate the wound healing process in rats. The results showed that after subcutaneous injection of liposomal cDNA complexes in thermally injured rats, significantly improved re-epithelization ensued. Moreover, the IGF-I / KGF cDNA combination increased VEGF expression and neovascularization, compared with IGF-I cDNA, or KGF cDNA alone (and other non-LIP controls). Additionally, due to increased neovascularization conferred, the combined LIP treatment accelerated re-epithelization and the regeneration of the dermis and epidermis. The combination treatment also significantly increased the expression of KGF, IGF-I, collagen type IV, FGF and VEGF, but did not modulate collagen type I and III expression (Jeschke and Herkdon, 2007). Another example of LIP-assisted delivery of cDNA for skin regeneration is the study by Branski et al. in which LIPs were used for delivery of PDGF-cDNA in a porcine wound model, in order to test its effect on wound re-epithlialization and skin regeneration. LIPs incorporating PDGF cDNA plasmid were injected subcutaneously, and the results showed that liposomal cDNA gene transfer improved regeneration of dermis and epidermis (Branski et al., 2010). The results of the two later studies indicate that liposomal gene transfer can be a successful therapeutic strategy for wound healing.
In conclusion, among the cases discussed above for skin regeneration by liposomal formulations of GFs or other types of active substances, several methods to prolong the retention of LIPs on the delivery site have been applied, such as the incorporation of the LIPs in chitosan or hyaluronic acid gels (Değim et al., 2011; Choi et al., 2017), ointments (Lu et al., 2019), or even in more sophisticated scaffold systems, such as microporous acellular dermis (Olenson et al., 2015). Another method used is the construction of special type silk-fibroin-core LIPs for enhanced retention of the GF in the LIPs; LIPs also incorporated a penetration enhancer as a method to increase the penetration depth for treating deep (second-degree) burns (Xu et al., 2018).
Liposomal GFs for bone regeneration
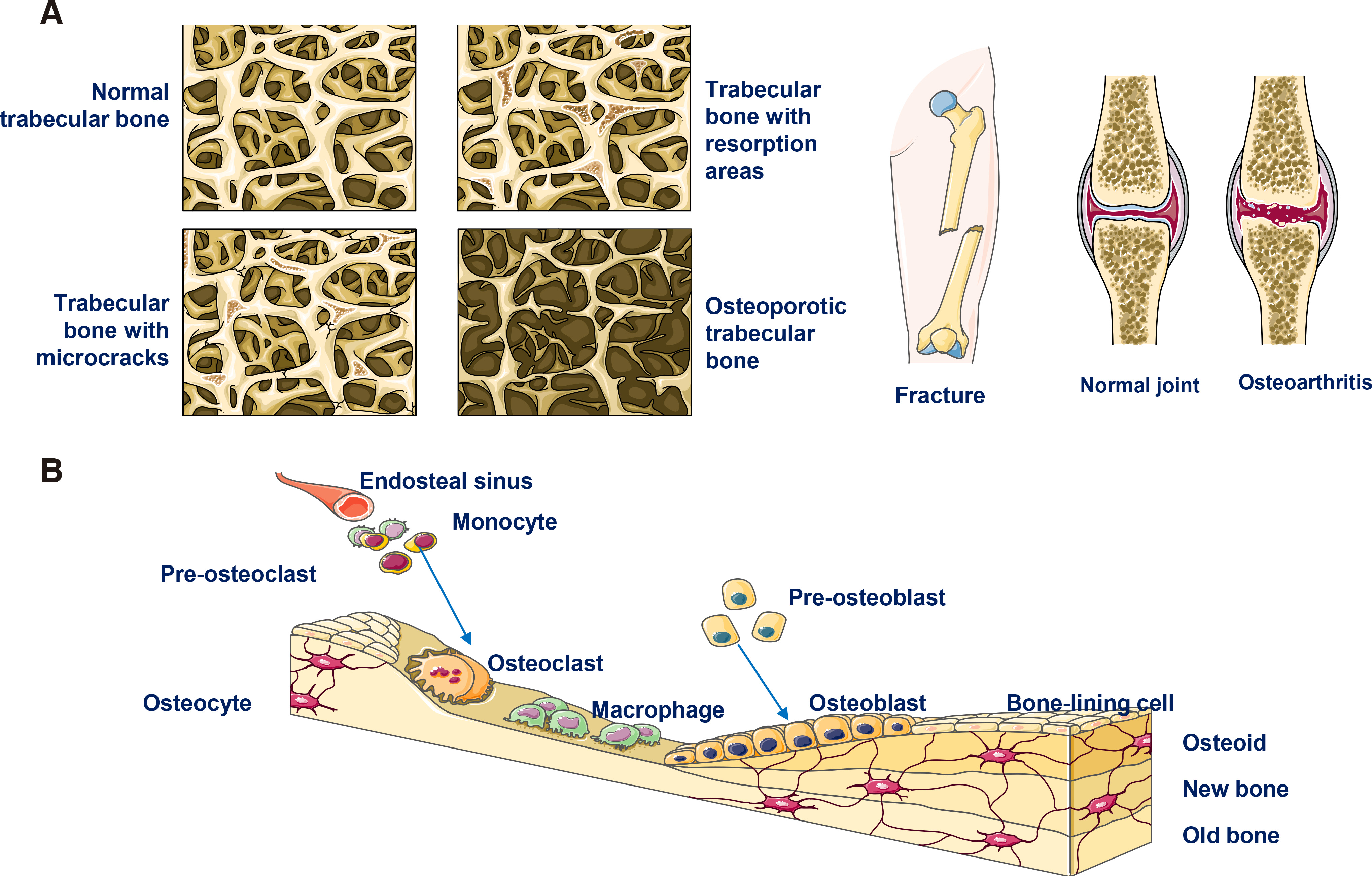
Bone defects often occur due to bone loss caused by different diseases, such as osteoarthritis and osteoporosis, which are usually associated with aging. In addition to age-related pathologies, other bone defect causes are congenital malformations, cancer-related surgeries, injuries/trauma, infections and periodontitis (see Fig. 8A).
Generally, bone tissue has a significant renewal capability and continuously regenerates throughout adult life, remaining an active organ (Fig. 8B). However, under particularly adverse conditions, and most likely when bone defects are large, natural regeneration cannot be achieved; in these cases, clinical interventions are required.
Numerous studies have concluded that bone regeneration is a complex procedure that involves repeated induction and conduction of intracellular and extracellular molecular signaling pathways (in a well-organized manner). The latter sequence requires different types of bioactive substances and cells. For bone fractions, two main mechanisms for repair are possible: the first involves direct tissue remodeling with minimal callus formation, and the second (most dominant for fracture healing) applies indirect remodeling with callus formation achieved by a combination of intra-membranous and endo-chondral ossification.
The most important signaling pathways for bone regeneration include the Wnt/β-catenin pathway, the BMP/TGF-β pathway, the Notch signaling pathway, the MAPK pathway, the PI3K/Akt/mTOR pathway, PDGF signaling, IGF signaling, FGF pathway, etc. An interesting strategy used in several cases for efficient bone regeneration by increasing osteoblast numbers and/or osteoblast maturation, is to target the (previously mentioned) involved signaling pathways (Majidinia et al., 2018). In general, the bone regeneration process is divided into three phases: (i) the inflammation phase, during which a peak in the levels of TNFα, IL-1, IL-6, IL-11, and IL-18 is observed at about 24 h.; (ii) the renewal phase, which includes chondrogenesis, and when several GFs play important roles, such as PDGF, TGF-b1, IGFs, FGF2, and BMPs; and (iii) the remodeling phase, during which osteoblasts and osteoclasts differentiate in order to form osteoprogenitor cells (Majidinia et al., 2018).
As seen in Fig. 6, a high percentage of the studies on liposomal GFs for tissue regeneration concern bone regeneration (approx. 40%). In several cases, it is proven that LIPs can be used as successful carriers for controlled delivery of GFs during bone healing. Furthermore, the liposomal GFs can be associated or immobilized on scaffolds or biomaterials by covalent bond formation, or may be embedded in scaffolds during sophisticated manufacturing technologies, such as electrospinning. Some examples of the most recent cases found in the literature about liposomal GFs for bone regeneration are tabulated in Table 2, and discussed in more detail below. In addition to the cases of liposomal GFs, we also discuss some cases of small molecules or proteins used for regeneration, as well as some example of the strategy to deliver plasmids for intracellular production of particular GF-proteins, using LIPs or lipid-nanoparticles.
Table 2
Examples of liposomal GFs for bone or osteochondral regeneration
| Liposome or delivery system type/composition | GF or active | Models used for study |
Method of liposome attachment on biomaterial |
Tissue | Ref |
| DPPC/lysophosphatidilcholine, 10:1, SUV | EGF | Wistar rats; maxillary second molar extracted | Non-attached (in tooth socket) | Tooth | Marquez et al., 2013 |
| PC, DOPC, DPPC, Chol,PE, CL & triolein or trioctanoin | TGF-β1, β2, β3; BMP-2, 13; IGF-1; EGF ; TGF-α; TenascinC | Knee defects in Gottingen mini pigs | Non-attached | Cartilage | Hunziker et al., 2001 |
| DSPC/ Chol/DSPE-PEG (45:45:10 mol/mol); 145 nm; trigger sensitive | RhBMP-2 | Male Swiss Webster mice with bilateral defects in hind limbs | Absorbable collagen sponge (ACS) | Bone | Crasto et al., 2016 |
| DPPC/ LPC, 10:1 (mol); 100nm | BMP-4 ; TGF- β 1 | Wistar rats; maxillary second molar extracted | Non-attached (in tooth socket) |
Tooth |
Ferreira et al., 2013 |
| DPPC/LPC (10:1); 100nm | IGF or/and PDGF-BB | Wistar rats; maxillary second molar extracted | Non-attached (in tooth socket) | Abreu et al., 2013 | |
| DPPC/Chol/PEG- maleimide (64:35:1); 129nm | BMP-2 | MSCs; male Wistar rats | HS-Maleimide bonds |
Bone |
Mohammadi et al., 2018 |
| DMPC | WNT | Skeletal injury in mice | Non-attached | Minear et al., 2010 | |
| DMPC:Chol; 90:10 mol; 100nm | WNT3A | BALB/C female mice; Axin2Cre ERT2 /+;R26RmTmG/+; Axin2LacZ/+ | In collagen gel | Liu et al., 2019 | |
| DMPC:Chol (90:10 mol) | WNT3A | In collagen gel | Tooth | Yuan et al., 2018 | |
| MLV 6% Asolectin + phospholipids | bFGF & insulin | Osteochondral defect bearing mini-pigs | In electrospun PVA nanofibers | Osteochondral | Filová et al., 2013 |
| SA/PC/DOPE/ Chol/PEG; 16:45:10:29:0 - 3 | BMP-9 pDNA | Fibroblasts; Swiss albino mice | Non-attached |
Bone |
Vhora et al., 2018 |
|
HSPC/DOPE-DOTAP/Chol/PEG, 16:42:10:29:3; + Pept.targ. ligand; 92-108 nm |
pDNA expressing BMP-9 | Ovariectomized SD rats (OVX) rat model of osteoporosis. | Simple impregnation | Vhora et al., 2019 | |
| DPPC/PEG/PEG-Mal 86:10:2 | PTHrP 107–11 | Pre-osteoblastic MC3T3-E1 cells | HS-Maleimide | López-Noriega et al., 2014 | |
| DPPC/Chol/Rho-Lipid/ PEG-NH2; 195-199 nm | Aspirin | hMSCs; BALB/c-nu mice | Polydopamine –NH2 | Li et al., 2019 | |
| PC/Chol+pyrophosphorylated Chol (targeting) 180 nm | Salvianic acid A | Female CD-1 mice with closed femur fractures | Non-attached; local delivery | Delayed union | Liu et al., 2018 |
Concerning liposomal delivery of GFs, Marquez et al. used liposomal EGF (EGF-LIPs) for bone healing in rats after maxillary second molar extraction. The LIPs and controls (EGF solution, blood clot, empty LIPs) were placed in the tooth sockets; no particular attachment method for the LIP-GF was applied. Results showed that the group receiving EGF-LIPs had faster recovery than all control groups. Moreover, increased type III collagen and fibronectin expression was observed three and seven days post-surgery in the group receiving EGF-LIPs, probably due to the capacity of LIPs for controlled delivery of EGF (avoiding rapid clearance of the GF from the site), which resulted in mesenchymal cell stimulation and, finally, in osteoblast differentiation. Furthermore, the faster recovery observed could also be partly due to the fact that EGF-LIPs protect the GF from early degradation by proteases (Marquez et al., 2013). In another study, Hunziker et al. encapsulated different GFs in LIPs, preparing 18 different LIP formulations for optimization. The ability of TGF-beta superfamily members (such as TGF-β2, TGF-β3, BMP-2 and BMP-13), as well as TGF-α,Tenascin-C, IGF-1, and EGF, to induce chondrogenesis was evaluated in cartilage defect models (defects in knees of minipigs) in vivo, for confirmation of previous studies carried out with TGF-β1 LIPs embedded in a fibrin matrix. It was proven that TGF-β1, -β2, and -β3 have similar effects on chondrogenesis, and that ΒΜP-2 and BMP-13 induced chondrogenesis in about 90% of the defected tissue, in a similar way as TGF-β1; however, cell proliferation was higher in the previous case. None of the other GFs tested demonstrated chondrogenic effects. By comparing the activities of free and LIP GFs, researchers suggest that LIPs potentate the activity of the GFs. It was also found that LIPs encapulating BMP proteins cause fewer side effects (that are initiated by leakage of formulations into cavities of the joint and/or into connective tissue subsynovial spaces) compared with LIPs with TGFs (all types studied). Thus, it was concluded that BMP encapsulating LIPs are more suitable as treatments (Hunziker et al 2001).
Crasto et al. developed a more sophisticated delivery approach for GFs, by constructing novel LIP nanocomplexes (NC) of rhBMP-2 that release the GF in response to a non-thermogenic ultrasound trigger, as a strategy to avoid the potential risks associated with high rhBMP-2 doses. Initially, it was verified in vitro that the NCs release rhBMP-2 in analogy to the duration and pressure of the applied ultrasound. After implantation of absorbable collagen sponge implants (ACS) containing either the rhBMP-2 NCs or free rhBMP-2 into hindleg muscle, it was proven that the LIP-rhBMP-2 NCs induced local bone formation only after exposure to ultrasound; additionally, spatio-temporal control over rhBMP-2 bioavailability was possible. Conversely, free rhBMP-2 rapidly diffused out of the ACS implant, in a burst release mode. Therefore, by using triggered-release LIPs, the release of GF could be synchronized with bone-forming precursor cell migration, allowing the use of lower doses of BMP2, and reducing the high cost of GFs and the associated risks of high dose GF delivery, while also successfully enhancing bone regeneration (Crasto et al., 2016).
In some cases, combinations of GFs in LIPs were tested for their bone regeneration potential. In this context, Ferreira et al. developed LIPs that encapsulate BMP-4 and TGF-β1 and evaluated their bone regeneration potential (alone and in combination) in rats whose maxillary second molar had been extracted. The formulations were introduced into the tooth sockets and histological evaluations followed by morphometric analysis were carried out; in addition, the levels of collagen type III and fibronectin (proteins determining the early phase of bone repair) were measured. The results showed higher blood vessel numbers and a higher percentage of bone trabeculae in the groups that received the LIP GFs separately or in combination, compared with the corresponding groups that received free GFs or empty LIPs. Furthermore, it was verified that the increased protein expression (collagen type III and fibronectin) occurred in the early phases of bone regeneration. In conclusion, it was indicated that GFs carried by LIPs could successful enhance healing of tooth sockets, when delivered either separately or in combination (Ferreira et al., 2013). In another study, an isoform of PDGF (PDGF-BB) and IGF-1 was encapsulated in LIPs and tested separately and in combination (in the same LIPs) for its bone-healing potential in rat tooth sockets (once again, rats following maxillary second molar extraction). Significantly higher numbers of blood vessels and bone trabeculae (%), as well as immunoreactivity levels, were demonstrated in rats treated with the PDGF-BB LIPs or theIGF-1 LIPs or their combination, compared with all control treatments (free GFs, empty LIPs etc.). All GF-LIP treated groups had statistically similar results, suggesting that GFs carried by LIPs enhance tooth socket healing in rats when used separately or in combination (Abreu et al., 2013).
In another case, the GF-encapsulated LIPs were covalently attached on a biomaterial scaffold. Additionally, in order to stimulate mesenchymal stem cells (MSC) osteogenic differentiation of, maleimide-group functionalized BMP-2 LIPs were attached to electrospun PLLA nanofibers that were functionalized by sulfhydryl groups, for this purpose. Hydroxyapatite (HA) nanoparticles were used to coat the electrospun nanofibers as a way to simulate bone minerals. It was shown that the covalent attachment of BMP-2 LIPs on the nanofibers could sustain BMP-2 release for 21 d; additionally, cell proliferation and adherence on fibers was possible without showing any cytotoxicity. In vitro results confirmed increased MSC proliferation and differentiation on the LIP-containing scaffolds. RUNX2 and OCN (osteogenesis-related gene) expressions were both higher in LIP-scaffold-HA than in the non-LIP scaffolds. Thus, LIPs can significantly modify BMP-2 release kinetics from primary ossification centers, and promote osteogenic differentiation of MSCs, after being covalently attached on scaffolds (Mohammadi et al., 2018).
Another group of reports involves liposomal delivery of Wnt proteins for bone regeneration. Wnt signaling pathway is particularly important for bone regeneration and homeostasis. It has been observed that Wnt ligands promote bone growth, supporting the theory that Wnt factors could be applied externally for bone healing. Since Wnt ligands are insoluble in water, Minear et al applied Wnt-LIPs for bone healing and showed faster healing after LIP delivery to skeletal defects of mice (compared to free ligands). The mechanism proposed was the stimulation of proliferation and differentiation of skeletal progenitor cell into osteoblasts, indicating that Wnt-LIPs could prove useful in activating other systems to achieve rapid healing and homeostasis (Minear et al., 2010). Others also evaluated Wnt-LIPs for bone healing. Liu et al. used WNT3A encapsulating LIPs and empty LIPs (as control) in ovariectomized (OVX) mice (osteoporotic-like phenotype) to evaluate alveolar bone repair. New osteoid matrix was observed at day seven, in the LIP-treated groups only. Histological assessment confirmed that osteotomies were filled with fibroblasts in groups treated with empty LIPs, while they were filled with bone in WNT3A-LIP treated groups. Osteoclast activity (cathepsin K expression) was repressed by WNT3A-LIP treatment, and osteogenic gene expression (RUNX2 and Osterix expression) was enhanced. Bone volume/total volume ration as well as bone mineral density (BMD) were increased in response to WNT3A-LIPs. At day 21, post-osteotomy mice treated with WNT3A-LIP were healed, while those treated with empty LIPs were not. In conclusion, WNT3A-LIP treatment accelerated bone healing in an OVX model of alveolar bone repair (Liu et al 2019). In another case, WNT3A LIPs were tested for their potential to heal the trauma in tooth extraction sockets (empty LIPs were used as controls). Formulations were injected in the periodontal ligament (PDL) activated by tooth extraction in order to evaluate whether a single dose is sufficient to increase rate of bone formation. CT and histology studies revealed that the sockets treated with the WNT3A–LIPs had more than 200% bone increase, compared with controls, and also significantly higher bone volume/total volume ratio, and the new bone had better lamellar organization. This demonstrated that amplification of Wnt signaling via LIP proteins can significantly accelerate already robust bone-healing processes (Yuan et al., 2018).
Another interesting potential application of LIP-GFs explored by Filová et al. is the potential development of alternatives of the known strategy for regeneration of chondral or osteochondral defects, referred to as MACI from matrix-associated autologous chondrocyte implantation. The matrices used in the clinic include collagen I/III membranes, hyaluronan derivatives, and fibrin, fibrin/hyaluronate scaffolds. In the current study, a sophisticated cell-free composite scaffold composed of hyaluronate, fibrin and type I collagen, mixed with polyvinyl alcohol (PVA) nanofibers that were enriched during electrospinning with LIP bFGF and Insulin, was developed to serve as a cell-free alternative of MACI. The system was tested for osteochondral regeneration in minipigs. In vivo studies proved that MSC recruitment from bone marrow was enhanced due to the controlled release of insulin and βFGF from the LIPs. 12-weeks after scaffold implantation, enhanced osteochondral regeneration was evident in the defects treated with the LIPs-containing system, compared with untreated defects that contained high amounts of fibrous tissue. In conclusion, the novel scaffold containing PVA nanofibers enriched with LIP-GFs successfully enhanced the migration of MSC in tissue defects, and finally osteochondral regeneration (Filová et al., 2013).
Among the strategies for GF-assisted bone regeneration, some recent reports involve LIPs or lipid nanoparticles (LNPs) or lipoplexes for delivery of GF genes. In one case, bone-targeted LNPs were additionally developed and compared with non-targeted LIPs. In more detail, in an initial study, Vhora et al. developed PEGylated stearyl amine (pegSA) lipoplexes of BMP-9 encoding plasmid DNA, and compared the effect of naked pDNA and lipoplexes. Efficient gene delivery to C2C12 cells was demonstrated by the lipoplexes, and osteogenic differentiation (via BMP-9 expression) was proved by enhanced calcium deposition; naked pDNA failed to show any calcium mineralization. In vivo studies for acute toxicity demonstrated the safety of the developed lipoplexes. In conclusion, this first study by the group proved that lipoplexes could be used for delivery of BMP-9 gene to treat osteoporosis by stem cell transfection (Vhora et al., 2018). In a second study, the same group synthesized different ionizable lipids and prepared LIPs and then lipid nanoparticles (LNPs) that encapsulate pDNA coding for BMP-9, and evaluated the potential for treatment of osteoporosis in an ovariectomized rat model. Targeted LIPs and LNPs (tLIP and tLNP) were also prepared by attaching a peptide to targeted osteoblastic lineage cells, on the vesicle surface. All LNP-types were shown to protect the pDNA form DNases and serum and demonstrated low hemolytic potential and cytotoxicity, as well as high transfection activity and non-significant ROS induction. In vivo experiments (in OVX rats) showed that both LNPs significantly increased bone thickness (cortical) and reduced the diameter of the endo-osteal, compared with the vehicle group, and were also shown to be safe treatments for bone regeneration. The tLNPs performed significantly better, compared with the non-targeted ones and naked pDNA. To summarize, targeted BMP-9 gene entrapping LNPs show potential as systemically delivered treatment for osteoporosis (Vhora et al., 2019).
In the next part, three examples of advanced systems for delivery of small molecules or peptides with bone regeneration activities are discussed. In the first case, an osteoconductive collagen hydroxyapatite scaffold was designed for bone repair; collagen is an attractive tissue engineering material, since it is highly biocompatible and bio absorbable. The surface of the collagen-based scaffold was functionalized for covalent attachment of thermoresponsive LIPs loaded with a pentapeptide (PTHrP 107–111) with pro-osteogenic and anti-osteoclastic activity, which were covalently attached to the scaffold, in order to construct an externally triggered drug eluting system. The system released the drug on-demand when an external thermal stimulus (42 °C, 20 min) was applied. This externally controlled release of PTHrP 107– 111 had a pro-osteogenic effect, as proven by increased alkaline phosphatase activity (osteogenic marker), and corresponding increased expressions of osteocalcin and osteopontin. Thus, the externally developed controlled drug elution scaffold-based system can be used to tune the delivery of different drugs and/or GFs for regeneration of numerous types of tissue defects (López-Noriega et al., 2014).
In a more recent study, aspirin-LIPs were found to promote hMSC immunomodulation and osteogenesis. When the aspirin-LIPs were combined with polycaprolactone (PCL) scaffolds, they were found to induce enhanced hMSC osteogenic differentiation. PCL and PCL-aspirin-LIP scaffolds were both implanted subcutaneously together with hMSC into nude mice for estimation of osteo-inductivity. The aspirin-LIP PCL scaffold exhibited higher osteogenic activity after six weeks, compared with the plain PCL scaffolds, suggesting the potential applications of such aspirin-LIP containing scaffolds in bone regenerative medicine (Li et al., 2019).
Finally, one of the challenges in orthopedic practice is delayed fracture union, with only a few non-surgical treatment options. Liu et al recently developed bone-targeting LIPs (BTL) loaded with a potent bone anabolic agent, salvianic acid A (SAA), as a novel strategy for delayed fracture union treatment. Pyrophosphorylated Chol was used as the targeting ligand, and the LIPs demonstrated strong binding affinity in vitro (hydroxyapatite) and in vivo to bones. In a delayed fracture union mouse model (prednisone-induced), BTLs significantly improved the fracture callus formation and its micro-architecture, compared with the same doses of free SAA, non-targeted LIPs or non-treatment. Biomechanical analyses further verified the high therapeutic efficacy of the BTLs, suggesting the novel targeted LIP type as a promising candidate for delayed bone fracture union treatment (Liu et al 2018).
In conclusion, LIP GFs have been assessed as strategies to accelerate and improve bone tissue regeneration, for treatment of osteoporosis, injury related defects (such as tooth extraction), fractures and delayed fraction unions, etc. It is particularly interesting that several advanced liposomal technologies have been evaluated for bone regeneration. Indeed, bone-targeted LIPs (Vhora et al., 2019; Liu et al., 2018) as well as triggered-release LIPs (Crasto et al., 2016; López-Noriega et al., 2014) have been evaluated. Furthermore, some LIP formulations were attached or embedded onto or within biomaterial (PLLA, PVA, PCL) scaffolds using technologically advanced methodologies, such as covalent immobilization of functionalized LIPs (Mohammadi et al., 2018; López-Noriega et al., 2014; Li et al., 2019), or electrospinning of the LIPs together with polymers [in core-shell structure] during formation of scaffolds consisted of electrospun fibers (Filová et al., 2013). Interestingly, the results of all the above studies demonstrated improved regeneration when such advanced technologies were utilized, indicating that the specific strategies have high potential for delivery regeneration-inducing substances. Another very important result is that in all the above studies, the liposomal systems (even the most sophisticated ones) were shown to be biocompatible and non-cytotoxic, opening the way to clinical testing.
Liposomal GFs for neural regeneration
Neural regeneration is a huge challenge, due to the severity of the resulting pathologies and their corresponding effects upon patients (optic neuropathies lead to blindness; spinal cord injuries lead to paralysis; brain injuries lead to paralysis or other severe consequences) and their related societal and economic impacts. Although few in number, some reports concerning liposomal GFs for neural regeneration have been found in the literature (Fig. 6). Most of the corresponding LIP formulations are developed for spinal cord injuries (SCI) or brain injuries, or are more general reports on the potential of LIP formulations for regeneration of neural cells, while one concerns optic nerve damage repair. With regard to the therapeutic strategy, some reports consider delivery of GFs or their corresponding genes, while others address delivery of other types of active molecules (drugs, proteins of phytochemicals), with regenerative activity. In the following part, the examples of LIP substances for neural regeneration presented in Table 3 are discussed in more detail.
Table 3
Examples of liposomal GFs for neural regeneration (spinal cord, optic nerve)
|
Liposome or delivery system type/ Composition |
Growth Factor or GF combination | Models used for study | Method of LIP attachment | Tissue | Ref |
| PC/Chol; targ. Tetrapeptide CAQK; 176 nm; | Docetaxel (DTX); BDNF;(in LIPs) aFGF (on hydrogel); |
Adult female Sprague-Dawley rat SCI model |
In thermo-sensitive poloxamer gel | Neural, spinal cord | |
|
LIPs (Taxol) |
Taxol (LIP); NT3 (bound to Collagen | In collagen Scaffold | |||
| DC/Chol | pEGFP-GDNF cDNA | Male Sprague-Dawley SCI- model |
Non-attached |
||
|
PS/PC/Chol (1:4.8:2) w/w Ligand= HVJ |
hHGF cDNA | Wistar rat ischemic- reperfusion nerve injury (IRI)-model | Neural- sciatic & tibial nerves | ||
| PC/Chol/PEG/CHO (160:40:4) w/w/w | IL-4 plasmid; NGF |
SCI model (Sprague Dawley rat) |
In electrospun fibers |
Neural- spinal cord |
Xi et al., 2018 |
| Paclitaxel-LIPs | Paclitaxel | In collagen scaffold | |||
|
PC/Chol 10:1; 179 - 200 nm |
Asiaticoside; graphene oxide [in scaffold] | NIH/3T3, PC12 cells | In CS/OHEC hydrogel | peripheral nerve | Zheng et al., 2020 |
| DOPS; 250 nm | Saposin C |
primary fibroblasts; PSAP−/− mice |
Non-attached | Genetic defect | Chu et al., 2005 |
| DPPC/Chol/ PEG/OPP/PAP2 or PAP4; 55:40:5:1:1 mol %) ~100 nm | CNTF, IGF-1, osteopontin-mimic; phosphatase-tension homologue inhibitors |
C57BL/6J mice NMDA optic nerve damage mouse model |
Optic nerve | Eriksen et al., 2018 |
Two of the most recent studies of use of the LIP system for treatment of SCI (Table 3) concern GF/ drug combinations. In the first case, Wang et al. studied the combined effect of docetaxel (DTX), and BDNF-encapsulating LIPs were decorated with a tetrapeptide (cysteine-alanine-glutamine-lysine, CAQK) as a ligand for scar targeting. The t-LIPs were embedded in a heparin-modified poloxamer (thermosensitive) hydrogel (HP) where aFGF was also bound. The system was used for SCI site local administration. By using in vivo fluorescence imaging and magnetic resonance imaging, the potential synergistic effects and the mechanisms of the combination treatment were evaluated in a SCI model developed in rats (in vivo) and on primary neurons (in vitro). The combined action of GFs and DTX conferred neural regeneration and improved neuronal survival and plasticity. Additionally, axonal regeneration was achieved by altering the microtubule function and promotion of mitochondria transport along regenerating axons. This novel multifunctional and scar-targeted therapeutic strategy is particularly promising for clinical treatment of SCI (Wang et al., 2018). In the second case, Li et al. used sponge-like collagen scaffold modified with Taxol-LIPs and collagen-bound NTF3 as a method for neural regeneration and functional improvement in a traumatic brain and SCI injury model in adult female Sprague Dawley rats. Following implantation in the SCI model, the combination scaffold augmented myelin-derived inhibition on neurite outgrowth, and neuronal differentiation. It also promoted neuronal and axonal regeneration, as well as the formation of synaptic connections. Optimal neuro- and electrophysiological recovery and hind-limb loco-motor improvement were observed in rats receiving the multifunctional implant. In conclusion, the novel scaffold was proven to realize neural networks for functional recovery, constituting a promising strategy for SCI treatment (Liu et al., 2021).
Another strategy applied for neural regeneration is the LIP delivery of GF-encoding plasmid DNA. In one case, Lu et al. directly injected pEGFP-GDNF cDNA-encapsulating cationic LIPs ino the SC gray matter of rats (SCI model). Increased EGFP-GDNF expression was observed between 1-4 weeks post-injection in glial and neuronal cells, indicating successful transfection of SC neuron and glial cells by GDNF gene. Additionally, regeneration of corticospinal tract axons and scores of locomotion in the LIP-treated were higher than in the control group between 1–4 weeks post-treatment. These data suggest that delivery of LIP GDNF cDNA may successfully enhance axonal regeneration and elocomotion, offering potential for SCI treatment (Lu et al., 2002). In another study, an ischemic reperfusion injury (IRI) rat model was used to evaluate the potential of HGF to treat ischemic neuropathy. Tsuchihara et al. delivered HGF encoding plasmid by LIPs into the peripheral nervous system (Tsuchihara et al., 2020). In this case, hemagglutinin virus of Japan (HVJ) was attached to the LIPs as a targeting ligand. HGF gene transfer demonstrated improved blood flow and skin temperature, thermal hyperalgesia and mechanical allodynia, and the threshold of plantar stimuli in the hind paw. Liposomal HGF gene injection also significantly improved the conduction velocity of sciatic nerve and muscle action. Endoneural microvessel numbers were significantly increased in sciatic and tibial nerves, promoting nerve regeneration; neovascularization was also observed in peripheral nerves. In conclusion, the delivery of targeted LIPs encapsulating HGF gene enhanced angiogenesis and nerve regeneration, and could therefore play a role in treatment of acute IRI (Tsuchihara et al., 2020).
In another recent study instead of a GF gene, the delivery of IL-4 plasmid and NGF was considered for SCI treatment. In more detail, Xi et al. developed aldehyde-modified cationic LIPs (aLIPs) encapsulating IL-4 plasmid for delivery to SCI-model Sprague Dawley (SD) rats. The aLIPs-encapsulating the IL-4 plasmids were embedded in electrospun (ES) fibers, which also incorporated NGF (in a core/shell structure); the aLIPs were released in acidic environments (triggered release), thus releasing the NGF in a sustained manner (due to the porosity of the fibers). When the environment-responsive electrospun fibers with immunoregulatory activity were implanted into acute SCIs, neural differentiation of MSCs was promoted. Due to combined action of the released NGF, and IL-4 plasmid, acute inflammation response was down-regulated, scar-tissue formation was reduced, and enhanced injury-site angiogenesis and neural differentiation were observed. Additionally, improved functional recovery was observed in the treated mice. The specific strategy also provided novel insights in the controversy between immune response and neural regeneration, thus representing an alternative treatment for acute-SCI (Xi et al., 2020).
Other examples of LIP systems for treatment of SCI involve liposomal drugs (such as Paclitaxel (PTX) or other non-GF molecules with regenerative activity, such as Asiaticoside and Saposin-C). In more detail, Li et al. developed a functional microchannel scaffold consisting of collagen that was loaded with PTX-LIPs as a strategy to control PTX release in the diseased tissue site where the scaffold was implanted (complete transection lesion sites in SCI rat model), since it was known that PTX is able to augment intrinsic axon regeneration and to also reduce scarring after SCI. The studies demonstrated that PTX could rescue myelin-inhibited NSC differentiation and thus enhance neuronal differentiation, compared with that achieved normally. Implantation of NSC-laden collagen scaffold (containing the PTX-LIPs) into the SCI resulted in axon extension, sensory and motor regeneration in neurons, and also led to improvement of motion recovery of the hindlimbs. Finally, mechanistic studies showed that the neural regeneration activity of PTX implicated the signaling pathway of Wnt/β-catenin (Li et al., 2018). In another case, a sophisticated system comprising a CS/OHEC hydrogel loaded with asiaticoside-LIPs and reduced graphene oxide (rGO) was developed by Zheng et al. to be used as an artificial nerve conduit for neural regeneration in peripheral nerve defects. In the knowledge that, when hollow nerve conduits are used as systems to repair peripheral nerve defects, the nerve extension is negatively affected by scar tissue, they tried to solve this problem by reformation of the microenvironment. Asiaticoside is a white, crystalline phytomolecule that is extracted from the leaves of the Centella asiatica, known to inhibit fibroblast differentiation/proliferation and inhibit scar tissue development. The hydrogel was suitable for adhesion/proliferation of neural cells in vitro, and was also non-cytotoxic. When applying electrical stimulation via the rGO, differentiation/ proliferation of neural cells was promoted, and nerve regeneration was accelerated. The asiaticoside released could significantly inhibit growth of fibroblasts, as well as collagen secretion. Taken together, these results prove that the novel hydrogel/liposome multifunctional system could be a promising system for regeneration of peripheral nerves (Zheng et al., 2020). Finally, in an earlier study Chu et al. developed Saposin-C encapsulating LIPs as a potential treatment of a genetic disease causing neural degeneration. In more detail, Prosaposin (PSAP) is a protein functioning towards neuron preservation, with the ability to enhance neural regeneration. In humans and mice, genetic PSAP defects are connected with a complex lysosomal storage disease, expressed by high multivesicular body (MVB) accumulation in skin fibroblasts. MVBs are important in cell homeostasis and consequently have implications in neuronal development and growth. Saposin-C is a lipid-binding protein derived from the precursor protein. In this study, the exogenous delivery of saposin-C LIPs into PSAP-/- fibroblasts (cellular model of genetic disease) was seen to reduce MVB accumulation down to normal amounts. Furthermore, the delivery of Saposin-C into brain neuronal cells in PSAP-/- mice (after crossing the blood-brain barrier) was found to be possible after i.v. injection of Sapocin-C-LIPs, indicating their potential for neuron preservation and regeneration (Chu et al., 2005).
The final example of LIP GFs for neural regeneration in Table 3 involves treatment of optic neuropathies. In optic neuropathies, the loss of axons and death of retinal ganglion cells (RGCs) is a common consequence, and the final result is irreversible blindness. Eriksen et al. injected LIPs encapsulating GFs and other bioactive molecules into the vitreous in a mouse model of optic nerve damage (N-methyl-D-aspartic acid induced RGC death). Specifically, the LIPs encapsulated a lipopeptide N-fragment to mimic osteopontin, IGF-1, CNTF and lipopeptide phosphatase tension homologue inhibitors (for the ATP domain or c-terminal tail) Results demonstrated that one intravitreal injection of LIP GFs (and other substances), conferred reduced RGC death and lowered the reduction of the electrophysiological activity of the retina. Furthermore, when the LIPs were combined with transplantation of pluripotent SC-derived RGCs, further improvement of the electrophysiological outcome was noticed. Thus, it was concluded that LIPs delivering several signaling pathway modulators, such as GFs and other types of substance, may indeed augment neuroprotection as well as the electrophysiological result of transplanted cells, indicating the potential of such LIP GFs for treatment of optic neuropathies (Eriksen et al., 2018).
In conclusion, it is evident from the examples presented above that when liposomal GFs are planned for neural regeneration, in most cases (66% of the cases included in Table 3), the formulations are associated with special types of biomaterials, such as absorbable collagen scaffolds (Liu et al., 2021; Li et al., 2018), electrospun fibers (Xi et al., 2020), complex hydrogel types (Zheng et al., 2020), or even in thermos-sensitive hydrogels for triggered release (on demand) (Wang et al., 2018). This is logical, since most pathologies requiring neural regeneration are located in body areas where it is extremely difficult to retain liposome dispersions.
Liposomal GFs for regeneration of other tissues
In this last section, addressing examples of LIP-assisted tissue regeneration, some examples of the hits from the bibiographic search carried out (Fig. 6), concerning the regeneration of different muscle-types or liver (tabulated in Table 4), are discussed.
Table 4
Examples of liposomal GFs for regeneration of other tissues
|
Liposome or delivery system type / Composition |
GF or combination | Models used for study | Method of LIP attachment | Tissue | Ref |
| DPPC with octadecylamine-hyaluronic acid [HA] (grafted on surface); 400 nm | TNFα (LIP); ADSCs |
mouse ischemic hindlimb model |
Bound on ADSCs by HA | hindlimb muscle | Leong et al., 2020 |
| PC/Chol 2:1 (w/w); 480 nm | VEFG; +UCs and SMCs | rabbit model of bladder injury |
Sandwiched between UCs and SMCs on nanofiber- PLCL-scaffolds |
smooth muscle (bladder) | Ling et al., 2017 |
| PG/ gangliosides / HVJ (on surface) | hHGF gene (in LIP); neonatal rat cardiomyocytes | Lewis rat model of proximal left anterior descending coronary artery (LAD) | Non-attached | Myocardium | Miyagawa et al., 2002 |
| DPPC/Bio-PEG/DC-Chol (Bio-PEG-DSPE LIPs); Bio-MB + Avidin | HGF | rat model of hepatic fibrosis | LIPs complexed with Bio-MB by avidin | Liver (hepatocyte) | Zhang et al., 2013 |
Three examples of studies involving LIP GF-assisted regeneration of muscles, involving hindlimb, bladder and cardiac muscle, are mentioned. In the first study, Leong et al. used TNFα LIPs tethered on the surface of ADSCs as nano-stimulators for in situ augmentation of the cell secretory activity. The LIP-tethered ADSCs exhibited enhanced secretion of Prosteoglandin E2 (PGE2) and proangiogenic VEGF, and decreased secretion of antiangiogenic pigment epithelium-derived factors. As expected, the novel LIP-stimulated ADSCs promoted vascularization (in vitro in a 3D microvascular chip). Additionally, improved walking and muscle mass was observed in mice (murine ischemic hindlimb model) treated with the tethered ADSCs, compared with those receiving non-tethered-ADSCs. This surface tethering strategy for in situ stimulation of SCs is proposed as an alternative to the costly and cumbersome preconditioning process of SCs intended for tissue regeneration (Leong et al., 2020). In another study, VEGF-LIPs were used for construction of a complex 3D scaffold consisting of UCs (urothelial cells), VEGF, and SMCs (smooth muscle cell), which was developed as an implantable system for blood vessel regeneration and bladder repair. The system was evaluated for its potential for tissue regeneration in a rabbit model of bladder injury. The VEGF-LIPs were sandwiched between the two cell types, in order to provide protection and controlled release of the GF, to avoid repeated injections of VEGF. Experimental results of in vivo studies revealed the potential of the proposed system to improve the bladder functions of the rabbits (smooth muscle function, urodynamics, urothelial permeability) compared with the control group, which underwent direct suturing after bladder injury (Ling et al., 2017).
The strategy of LIP gene delivery for cardiomyoplasty enhancement was evaluated in an earlier study by Miyagawa et al. LIPs encapsulating a gene for hHGF was proposed as a cellular cardiomyoplasty approach to regenerate the impaired myocardium, and tested in vivo in a model of proximal left anterior descending coronary artery (ligation model, LAD) in Lewis rats. A sophisticated method for LIP gene delivery was employed by generating LIPs containing gangliosides that were used to encapsulate the hHGF gene; their surface was coated with hemagglutinating virus of Japan (HVJ) (to ensure cellular uptake). In vivo, after LAD ligation, three different treatment groups were evaluated: rats treated with neonatal rat cardiomyocytes; rats treated with HVJ-LIPs encapsulating hHGF gene; and rats treated with both. Cardiac performance was significantly improved in the rats treated with both the cells and the LIPs and a marked increase in myocardial perfusion, neovascularization and significant fibrosis reduction, were also noticed in the same group. In conclusion, it was proven that hHGF gene transfection, with the aid of this specific LIP type, augmented cellular cardiomyoplasty. Thus, such combined therapy (LIP-GF gene plus SCs) may offer potential for treatment of myocardial-infarction-caused heart failure (Miyagawa et al., 2002).
Hepatic fibrosis is caused by liver aggression. By delivery of hHGF via a system comprised of ultrasound microbubbles and cationic nano-LIPs, the potential to treat hepatic fibrosis was evaluated in a hepatic fibrosis (bile duct ligation) rat model by Zhang et al. In more detail, a novel gene delivery system was constructed by biotin-tagged cationic LIPs (Bio-CNLP) and biotin-tagged ultrasound micro-bubbles (Bio-MB), forming complexes after addition of avidin. The complex was additionally mixed with an HGF gene (pCDH-HGF plasmid), and this targeted gene therapy system finally demonstrated significant anti-fibrotic effects, proven by significant reduction of hydroxyproline and by liver histology evaluation (Zhang et al., 2013).
In all the cases presented above, the liposomal formulations are constructed in different types of technologically advanced structures that confer enhanced retention and/or activity of the GFs or genes, suggesting that regeneration of the specific tissue-types (muscle, liver) is a particular challenge, requiring such sophisticated approaches.
Summary of methods used for administration of LIP GFs
The conclusion drawn from all the cases of LIP GFs discussed in parts 3.1 – 3.4 is that in addition to the delivery of liposomal GFs as injectable freestanding formulations when the particular application permits, in several cases, depending mostly on the defected tissue/site location, different methods of embedding, attaching or encapsulating the liposomal formulations could be applied. The goal is to provide protection of the GF and also to regulate its release in a way that is adjusted to the particular GF’s mode of action. The different methods of LIP GF administration are schematically depicted in Fig. 9, which summarizes all the examples/cases discussed in the previous part of this chapter, per tissue.
Finally, with the advances in the discovery of new ionizable lipids, the strategy of delivering plasmids encoding particular GSs seems to be a very promising alternative approach for bone regeneration, and one likely to be further exploited in preclinical and clinical settings.
Clinical studies and Future perspectives for Liposomal GFs
Several clinical trials concerning the applications of GFs in tissue regeneration are ongoing, planned or completed. A search of the NIH clinical trial database (https://clinicaltrials.gov/ ; NIH Clinical trial Database, assessed on 30/3/2021), using the key words “growth factor” and “regeneration”, resulted in 44 studies (three studies were found when using as key words “growth factor” and “tissue regeneration”). With the key words “growth factor” and “fracture”, 22 studies were found, indicating that about half of the studies of the first search related to bone regeneration. In fact, almost all the other studies (from the first search) involved wound healing/skin regeneration, indicating that strong correlation between preclinical studies of liposomal GFs, where most reports involved efforts at skin and bone regeneration (Fig. 6), and clinical studies of GFs in general.
In most clinical settings, limited benefits have been reported by GFs that are administered as solutions for direct injection into the body or for topical administration, as expected, given their low bioavailability at the pathological site (resulting from diffusion and rapid clearance) (Koria, 2012; Sharma et al., 2021). Thus, association of GFs with scaffolds, biomaterials or delivery systems has been proposed as the best solution, as mentioned earlier. Indeed, it is not surprising that all the GFs currently approved by the US FDA, are formulated in association with some type of delivery system, usually a biomaterial or a scaffold. Examples of such systems are the hydrogel formulation of PDGF (becaplermin) under the name REGRANEX Gel, for treatment of diabetic foot ulcers, and collagen sponge impregnated BMP-2 for treatment of bone fractures (Koria, 2012; Sharma et al., 2021).
Despite the fact that much preclinical work involves the use of liposomes as advanced delivery systems for GF administration, only a few clinical trials on the exact subject were found in the NIH database (https://clinicaltrials.gov/ ; NIH Clinical trial Database, assessed on 30/3/2021). The first study, NCT00592189, which was completed in 2014 (started in 2008), investigates whether the use of fetal membrane (human amnion) as transient wound coverage in wound repair could be safe and efficacious. Furthermore, and most closely connected with the topic of this article, the investigators evaluated whether the incorporation of liposomal gene constructs into the amnion would enhance the functionality of human amnion and improve wound repair. We found no more details of the specific study about the gene-encoding protein, and no results were released. Another more recent study (currently recruiting subjects) concerns ART352-L, a liposomal formulation of recombinant human Wnt3A protein, developed by Ankasa Regenerative Therapeutics, Inc. (Ankasa). ART352-L is applied ex vivo, to harvested autologous bone grafts (autograft) as a strategy to enhance the osteogenic properties of the autograft prior to re-implantation in orthopedic surgeries. Study NCT04378543 is a phase 1/2 open label safety evaluation of ART352-L treated autologous bone grafts, in patients undergoing posterolateral lumbar spinal fusion to treat single level degenerative spondylolisthesis. The primary objective of the study is to evaluate the safety and tolerability of ART352-L treated local bone autografts in patients being treated for this condition, with a secondary objective being the evaluation of the rates of early and overall spinal fusion. Additionally, changes in patient mobility and quality of life following treatment with ART352-L will be evaluated. Most of the other clinical studies involving liposomal formulations of growth factors are for treatment of cancer.
By way of epilogue, it is our belief that the numerous recent successful examples of using liposomal GFs (or other types of active substances with tissue regeneration activities), presented above will lead to new clinically tested and finally approved innovative products in the near future, which will materialize the advantages of liposomal drug delivery systems and overcome the current limitations of GF delivery. The fact that clinical investigation of liposomal GFs or relevant genes for tissue regeneration has been already initiated is particularly encouraging. In fact, the recent success in delivering mRNA by lipid-nanoparticles (Antimisiaris et al., 2021; Antimisiaris et al., 2008), will definitely contribute to technological advances in successful delivery of GF encoding genes.
Declarations
Funding
SGA, AM and PM acknowledge funding form the European Union’s Horizon 2020 research and innovation programme under grant agreement No874896. E.N, F.G. and S.G.A. acknowledge support of this work by the project "Preclinical development of INNOvative FORmulations of antibiotics for intraocular administration for the treatment/prevention of postoperative endophthalmtis -Innofor I" (MIS 5031792), which is implemented under the Special Service of the Operational Program Competitiveness Entrepreneurship and Innovation. The project is funded by the Operational Programme "Competitiveness, Entrepreneurship and Innovation" (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Abbreviations
ADSCs, adipose-derived stem cells ; aFGF, acidic fibroblast growth factor ; BDNF, brain-derived neurotrophic factor ; bFGF, Basic fibroblast growth factor ; BMP, bone morphogenetic protein; ; CBNF3, collagen-binding neurotrophic factor 3 ; Chol, Cholesterol ; CNTF, Ciliary neurotrophic factor ; CS/OHEC, oxidized hydroxyethyl cellulose ; CS, chitosan ; DC-Chol, 3β-{N-[2-(Dimethylamino) ethyl]carbamoyl}cholesterol ; DMPC, 1,2-dimyristoyl-sn-glycero-3-phosphocholine ; DMRIE, 1, 2-dimyristyloxypropyl-3-dimethyl-hydroxy ethyl ammonium bromide ; DOPC, 1,2-dioleyl-sn-glycero-3-phosphocholine ; DOPG, 1,2-dioleyl-sn-glycero-3-phosphoglucerol ; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine ; DSPA, 1,2-distearoyl-sn-glycero-3-phosphatidate monosodium salt ; DSPC, 1,2,-disteraroyl-sn-glycero-3-phosphatidylcholine ; EGF, epidermal growth factor ; FGF, fibroblast growth factor ; GCSF, granulocyte colony-stimulating factor ; GDF, growth differentiation factor ; HGF, hepatocyte growth factor ; hHGF, human hepatocyte growth factor ; HVJ, hemagglutinating virus of Japan ; IGF-I, Insulin-like growth factor-I ; KGF, Keratinocyte growth factor ; LIP, Liposome ; NGF, nerve growth factor ; NMDA, N-methyl-D-aspartic acid ; NT, neurotrophin ; PC, Lecithin, Phosphatidyl-choline ; PDGF, Platelet-derived growth factor ; PLCL, poly(l-lactide-co-ε caprolactone) ; PLLA, Poly L-lactic acid ; RGCs, retinal ganglion cells ; rGO, reduced graphene oxide ; Rh, recombinant human ; Rhod-lipid, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); ; SA, Stearyl amine ; SDF, stromal-derived factor ; TGF, transforming growth factor ; TGF-β, transforming growth factor-beta ; TRA, All- trans retinoic acid ; VEGF, vascular endothelial growth factor ;References
Abdel-Mageed H. M., Fahmy A. S., Shaker D. S., Mohamed S. A. (2018). Development of novel delivery system for nanoencapsulation of catalase: formulation, characterization, and in vivo evaluation using oxidative skin injury model . Artificial Cells, Nanomedicine, and Biotechnology 46: 362-371.
Abreu F. A. M. , Ferreira C. L., Silva G. A. B., Paulo C. O., Miziara M. N., Silveira F. F., Alves J. B. (2013). Effect of PDGF-BB, IGF-I Growth Factors and their Combination Carried by Liposomes in Tooth Socket Healing. Brazilian Dental Journal 24: 299-307.
Alemdaroğlu C., Degim Z., Celebi N., Şengezer M., Alömeroglu M., Nacar A. (2008). Investigation of epidermal growth factor containing liposome formulation effects on burn wound healing. Journal of Biomedical Materials Research Part A 85A: 271-283.
Antimisiaris S. G., Kallinteri P., Fatouros D. G., (2008). Liposomes and Drug Delivery. In Pharmaceutical Manufacturing Handbook: Production and Processes. (Ed. Gad S. C., ) John Wiley & Sons Inc., New Jersey.
Antimisiaris S.G., Marazioti A., Kannavou M., Natsaridis E., Gkartziou F., Kogkos G., Mourtas S. (2021). Overcoming barriers by local drug delivery with liposomes. Advanced Drug Delivery Reviews 174: 53-86.
Antimisiaris S., Mourtas S., Papadia K. (2017). Targeted si-RNA with liposomes and exosomes (extracellular vesicles): How to unlock the potential. International Journal of Pharmaceutics 525: 293-312.
Branski L. K., Masters O. E., Herndon D. N., Mittermayr R., Redl H., Traber D. L., Cox R. A., Kita K., Jeschke M. G. (2010). Pre-clinical evaluation of liposomal gene transfer to improve dermal and epidermal regeneration. Gene Therapy 17: 770-778.
Choi J. U., Lee S. W., Pangeni R., Byun Y., Yoon I.S., Park J. W. (2017). Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomaterialia 57: 197-215.
Chu Z. (2005). Saposin C: Neuronal Effect and CNS Delivery by Liposomes. Annals of the New York Academy of Sciences 1053: 237-246.
Crasto G. J., Kartner N., Reznik N., Spatafora M. V., Chen H., Williams R., Burns P. N., Clokie C., Manolson M. F., Peel S. A.F. (2016). Controlled bone formation using ultrasound-triggered release of BMP-2 from liposomes. Journal of Controlled Release 243: 99-108.
Değim Z., Çelebi N., Alemdaroğlu C., Deveci M., Öztürk S., Özoğul C. (2011). Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. International Wound Journal 8: 343-354.
Eriksen A. Z., Eliasen R., Oswald J., Kempen P. J., Melander F., Andresen T. L., Young M., Baranov P., Urquhart A. J. (2018). Multifarious Biologic Loaded Liposomes that Stimulate the Mammalian Target of Rapamycin Signaling Pathway Show Retina Neuroprotection after Retina Damage. ACS Nano 12: 7497-7508.
Ferreira C. L., Abreu F. A. M. , Silva G. A. B., Silveira F. F., Barreto L. B. A., Paulino T. P., Miziara M. N., Alves J. B. (2013). TGF-β1 and BMP-4 carried by liposomes enhance the healing process in alveolar bone. Archives of Oral Biology 58: 646-656.
Filová E., Rampichová M., Litvinec A., Držík M., Míčková A., Buzgo M., Košťáková E., Martinová L., Usvald D., Prosecká E., Uhlík J., Motlík J., Vajner L., Amler E. (2013). A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. International Journal of Pharmaceutics 447: 139-149.
Gregoriadis G. (2016). Liposomes in Drug Delivery: How It All Happened. Pharmaceutics 8: 19.
Hunziker E. B., Driesang I. M. K., Morris E. A. (2001). Chondrogenesis in Cartilage Repair is Induced by Members of the Transforming Growth Factor-Beta Superfamily. Clinical Orthopaedics and Related Research 391: S171-S181.
Jeschke M. G., Herndon D. N. (2007). The combination of IGF-I and KGF cDNA improves dermal and epidermal regeneration by increased VEGF expression and neovascularization. Gene Therapy 14: 1235-1242.
Koria P. (2012). Delivery of Growth Factors for Tissue Regeneration and Wound Healing. BioDrugs 26: 163-175.
Kulebyakin K. Y., Nimiritsky P. P., Makarevich P. I. (2020). Growth Factors in Regeneration and Regenerative Medicine: “the Cure and the Cause”. Frontiers in Endocrinology 11: 384.
Leong J., Hong Y.T., Wu Y.F., Ko E., Dvoretskiy S., Teo J. Y., Kim B. S., Kim K., Jeon H., Boppart M., Yang Y. Y., Kong H. (2020). Surface Tethering of Inflammation-Modulatory Nanostimulators to Stem Cells for Ischemic Muscle Repair. ACS Nano 14: 5298-5313.
Li X., Fan C., Xiao Z., Zhao Y., Zhang H., Sun J., Zhuang Y., Wu X., Shi J., Chen Y., Dai J. (2018). A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials 183: 114-127.
Li Y., Bai Y., Pan J., Wang H., Li H., Xu X., Fu X., Shi R., Luo Z., Li Y., Li Q., Fuh J. Y. H., Wei S. (2019). A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. Journal of Materials Chemistry B 7: 619-629.
Ling Q., Wang T., Yu X., Wang S.G., Ye Z.Q., Liu J.H., Yang S.W., Zhu X.B., Yu J. (2017). UC-VEGF-SMC Three Dimensional (3D) Nano Scaffolds Exhibits Good Repair Function in Bladder Damage. Journal of Biomedical Nanotechnology 13: 313-323.
Liu D., Shu M., Liu W., Shen Y., Long G., Zhao Y., Hou X., Xiao Z., Dai J., Li X. (2021). Binary scaffold facilitates in situ regeneration of axons and neurons for complete spinal cord injury repair . Biomaterials Science 9: 2955-2971.
Liu Y., Jia Z., Akhter M. P., Gao X., Wang X., Wang X., Zhao G., Wei X., Zhou Y., Wang X., Hartman C. W., Fehringer E. V., Cui L., Wang D. (2018). Bone-targeting liposome formulation of Salvianic acid A accelerates the healing of delayed fracture Union in Mice. Nanomedicine: Nanotechnology, Biology and Medicine 14: 2271-2282.
Liu Y., Li Z., Arioka M., Wang L., Bao C., Helms J.A. (2019). WNT3A accelerates delayed alveolar bone repair in ovariectomized mice. Osteoporosis International 30: 1873-1885.
López-Noriega A., Ruiz-Hernández E., Quinlan E., Storm G., Hennink W. E., O'Brien F. J. (2014). Thermally triggered release of a pro-osteogenic peptide from a functionalized collagen-based scaffold using thermosensitive liposomes. Journal of Controlled Release 187: 158-166.
Lu K.J., Wang W., Xu X.L., Jin F.Y., Qi J., Wang X.J., Kang X.Q., Zhu M.L., Huang Q.L., Yu C.H., You J., Du Y.Z. (2019). A dual deformable liposomal ointment functionalized with retinoic acid and epidermal growth factor for enhanced burn wound healing therapy. Biomaterials Science 7: 2372-2382.
Lu K.W., Chen Z.Y., Jin D.D., Hou T.S., Cao L., Fu Q. (2002). Cationic Liposome-Mediated GDNF Gene Transfer after Spinal Cord Injury. Journal of Neurotrauma 19: 1081-1090.
Majidinia M., Sadeghpour A., Yousefi B. (2018). The roles of signaling pathways in bone repair and regeneration. Journal of Cellular Physiology 233: 2937-2948.
Marquez L., de Abreu F. A. M., Ferreira C. L., Alves G. D., Miziara M. N., Alves J. B. (2013). Enhanced bone healing of rat tooth sockets after administration of epidermal growth factor (EGF) carried by liposome. Injury 44: 558-564.
Minear S., Leucht P., Jiang J., Liu B., Zeng A., Fuerer C., Nusse R., Helms J. A. (2010). Wnt Proteins Promote Bone Regeneration. Science Translational Medicine 2: 29ra30.
Miyagawa S., Sawa Y., Taketani S., Kawaguchi N., Nakamura T., Matsuura N., Matsuda H. (2002). Myocardial Regeneration Therapy for Heart Failure. Circulation 105: 2556-2561.
Mohammadi M., Alibolandi M., Abnous K., Salmasi Z., Jaafari M. R., Ramezani M. (2018). Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomedicine: Nanotechnology, Biology and Medicine 14: 1987-1997.
Olekson M. A. P., Faulknor R., Bandekar A., Sempkowski M., Hsia H. C., Berthiaume F. (2015). SDF-1 liposomes promote sustained cell proliferation in mouse diabetic wounds. Wound Repair and Regeneration 23: 711-723.
Shah S., Dhawan V., Holm R., Nagarsenker M. S., Perrie Y. (2020). Liposomes: Advancements and innovation in the manufacturing process. Advanced Drug Delivery Reviews 154-155: 102-122.
Sharma P., Kumar A., Dey A. D., Behl T., Chadha S. (2021). Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sciences 268: 118932.
Tsuchihara T., Nukada H., Nakanishi K., Morishita R., Amako M., Arino H., Nemoto K., Chiba K. (2020). Efficacy of nonviral gene transfer of human hepatocyte growth factor (HGF) against ischemic-reperfusion nerve injury in rats. PLOS ONE 15: e0237156.
Vhora I., Lalani R., Bhatt P., Patil S., Misra A. (2019). Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. International Journal of Pharmaceutics 563: 324-336.
Vhora I., Lalani R., Bhatt P., Patil S., Patel H., Patel V., Misra A. (2018). Colloidally Stable Small Unilamellar Stearyl Amine Lipoplexes for Effective BMP-9 Gene Delivery to Stem Cells for Osteogenic Differentiation. AAPS PharmSciTech 19: 3550-3560.
Wang Q., Zhang H., Xu H., Zhao Y., Li Z., Li J., Wang H., Zhuge D., Guo X., Xu H., Jones S., Li X., Jia X., Xiao J. (2018). Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 8: 4429-4446.
Wang X., Liu B., Xu Q., Sun H., Shi M., Wang D., Guo M., Yu J., Zhao C., Feng B. (2017). GHK-Cu-liposomes accelerate scald wound healing in mice by promoting cell proliferation and angiogenesis. Wound Repair and Regeneration 25: 270-278.
Whyte J. L., Smith A. A., Liu B., Manzano W. R., Evans N. D., Dhamdhere G. R., Fang M. Y., Chang H. Y., Oro A. E., Helms J. A. (2013). Augmenting Endogenous Wnt Signaling Improves Skin Wound Healing. PLoS ONE 8: e76883.
Xi K., Gu Y., Tang J., Chen H., Xu Y., Wu L., Cai F., Deng L., Yang H., Shi Q., Cui W., Chen L. (2020). Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nature Communications 11: 4504.
Xu H.L., Chen P.P., Wang L.F., Tong M.Q., Ou Z., Zhao Y.Z., Xiao J., Fu T.L., Wei-Xue (2018). Skin-permeable liposome improved stability and permeability of bFGF against skin of mice with deep second degree scald to promote hair follicle neogenesis through inhibition of scar formation. Colloids and Surfaces B: Biointerfaces 172: 573-585.
Yuan X., Pei X., Zhao Y., Tulu U.S., Liu B., Helms J.A. (2018). A Wnt-Responsive PDL Population Effectuates Extraction Socket Healing. Journal of Dental Research 97: 803-809.
Zhang S., Wen K., Wu W., Li W., Zhao J. (2013). Efficacy of HGF carried by ultrasound microbubble-cationic nano-liposomes complex for treating hepatic fibrosis in a bile duct ligation rat model, and its relationship with the diffusion-weighted MRI parameters. Clinics and Research in Hepatology and Gastroenterology 37: 602-607.
Zheng F., Li R., He Q., Koral K., Tao J., Fan L., Xiang R., Ma J., Wang N., Yin Y., Huang Z., Xu P., Xu H. (2020). The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Materials Science and Engineering: C 109: 110560.