Int. J. Dev. Biol. 67: 39 - 48 (2023)
Conditions for transplantation of primordial germ cells in the yellowtail tetra, Astyanax altiparanae
Original Article | Published: 1 August 2023
Abstract
Biotechniques, including surrogate propagation derived from primordial germ cell (PGC) transplantation, are valuable tools for the reconstitution of endangered fish species. Although promising, there are no previous studies reporting such approaches using neotropical fish species. The aim of this study was to establish germline chimeras in neotropical fish by using the yellowtail tetra Astyanax altiparanae as a model species of the order Characiformes. Germline chimeras were obtained after transplantation of PGCs cultivated under different conditions: saline medium and supplemented with DMEM, amino acids, vitamins, glutamine, pyruvate, and fetal bovine serum, and subsequently transplanted into A. altiparanae triploids and triploid hybrids from the cross between A. altiparanae (♀) and A. fasciatus (♂). The results indicate ectopic migration in host embryos after transplantation of PGCs cultivated in saline medium. However, PGCs cultivated in supplemented medium migrated to the region of the gonadal ridge in 4.5% of triploid and 19.3% in triploid hybrid. In addition, the higher expression of dnd1, ddx4 and dazl genes was found in PGCs cultivated in supplemented culture medium. This indicates that the culture medium influences the maintenance and development of the cultivated cells. The expression levels of nanos and cxcr4b (related to the differentiation and migration of PGCs) were decreased in PGCs from the supplemented culture medium, supporting the results of ectopic migration. This is the first study to report the transplantation of PGCs to obtain germline chimera in neotropical species. The establishment of micromanipulation procedures in a model neotropical species will open new insights for the conservation of endangered species.
Keywords
biotechnology, genebank, germline chimera, primordial germ cells, teleost, transplant
Introduction
Faced with the need to develop biotechnologies that act in the conservation and reconstitution of endangered fish species, as well as assisting production practices, several methodologies have been developed. These include the generation of germline chimeras that compromise the transplantation of different cell types, such as PGCs embryonic (Saito et al., 2008; Yasui et al., 2011), spermatogonial (Franěk et al., 2019) and oogonial stem cells (Pšenička et al., 2015) from adults. The heterologous propagation of gametes via germline chimeras in fish can be used as a tool for the propagation of species in which reproduction is more critical, such as endangered species with high commercial value or with a long period of gonadal maturation (Robles et al., 2017; Siqueira-Silva et al., 2018). Studies in domestic species often point to the need for more advanced studies in more critical species, such as endangered fish.
The use of primordial germ cells (PGCs) in genebanks and species reconstitution has become a very promising approach (Goto and Saito, 2019; Siqueira-Silva et al., 2018). This is because they are the only precursor cells of the entire germ line and subsequent gamete formation, and they preserve maternal constituents such as germplasm and mitochondrial DNA (Yamaha et al., 2007; Yoshizaki et al., 2003). Although PGCs have favorable characteristics for their use, transplantation involving this cell type covers more complex paths, since it requires previous knowledge of donor and receptor reproduction and also embryo development (Arashiro et al., 2018; Silva et al., 2017) for the successful application of germline chimera production technology (Kawakami et al., 2010; Saito et al., 2011; Yasui et al., 2011). This includes the characterization of the origin, specification, and migration routes of PGCs in different species (Coelho et al., 2021, 2019; Linhartova et al., 2014; Saito et al., 2006).
PGCs can be transplanted into a sterile host organism, such as triploids generated by chromosome manipulation (Adamov et al., 2016; Arai and Fujimoto, 2013; Nascimento et al., 2017). The germline chimera will then produce gametes from the donors species (xenogenesis) (Saito et al., 2008; Yamaha et al., 2003; Yasui et al., 2011).
The yellowtail tetra, Astyanax altiparanae, is known as “lambari” and “tambiú”. The species is distributed in rivers and streams of the upper Paraná River basin of Brazil; it can reach 5-20 cm in length and weigh up to 60 g as an adult (Lobón-Cerviá and Bennemann, 2000; Porto-Foresti et al., 2005). As an omnivorous species, it easily accepts commercial pellets for consumption and adapts easily in captivity (Hayashi et al., 2004). Gonadal maturation is precocious; within four months, this species reaches the adult stage, starting reproduction. It is a species with high fecundity and easy larviculture (Porto-Foresti et al., 2005; Yasui et al., 2020).
Astyanax altiparanae presents simpler in vitro fertilization procedures, which makes it a good model species for development of biotechnologies. Recently, research efforts have been carried out on basic techniques for artificial reproduction of A. altiparanae (Yasui et al., 2015), in addition to studies of fertilization events, sperm morphology and motility, and embryonic development (Pereira-Santos et al., 2016). With this set of information, it was possible to establish a chromosomal manipulation protocol (Adamov et al., 2016; Piva et al., 2018), in order to ensure the sterility of recipients, and this has been used as an important factor for the success of PGC transplantation. Based on these previous steps, the yellowtail tetra can be considered an important experimental model for the development of biotechniques involving PGC transplantation to produce germinative chimeras. Other Characiformes species may be produced from the procedures established in the yellowtail tetra.
Results
For transplantation, regardless of the medium in which the PGCs were cultivated, post-transplant survival showed significant differences during embryonic development and in the percentages of normal and abnormal larvae, compared with the control groups. Only the condition where transplants had triploid recipient associated with cells cultivated in saline solution was there no significant differences between groups (Tables 1, 2, 3, and 4).
Table 1
Embryonic development of the control groups (donor and recipient) and of the GFP-positive transplanted triploid recipients, using primordial germ cells (PGCs) from A. altiparanae, derived from the culture in saline solution
| Groups | n | Unfertilized | 2-cell | Blastula | Gastrula | Somite | Hatch | Larvae | |
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | ||||||||
| Donor | 91 | 0.0 ± 0.0% | 100 ± 0.0% | 99.0 ± 0.6% | 99.0 ± 0.6% | 98.0 ± 1.2% | 95.8 ± 1.0% | 90.0 ± 5.8% | 10.0 ± 5.8% |
| Receptor | 100 | 0.0 ± 0.0% | 100.0 ± 0.0% | 100.0 ± 0.0% | 99.0 ± 0.6% | 93.0 ± 4.0% | 74.0 ± 1.2% | 86.3 ± 3.3% | 13.7 ± 3.3% |
| Transplanted | 86 | - | - | 94.8 ± 1.2% | 78.8 ± 6.7% | 56.0 ± 12.4% | 47.5 ± 11.7%(36) | 52.4 ± 11.0%(20) | 47.6 ± 11.0% (16) |
| P-value | 0,1255 | 0,1899 | 0,1871 | 0,1340 | 0,2217 | 0,2217 | |||
Numbers between brackets represent real numbers of PGC-GPF positive larvae.
Table 2
Embryonic development of the control groups (donor and recipient) and of the GFP-positive transplanted triploid hybrids using primordial germ cells (PGCs) from A. altiparanae, derived from the culture in saline solution
| Groups | n | Unfertilized | 2-cell | Blastula | Gastrula | Somite | Hatch | Larvae | |
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | ||||||||
| Donor | 146 | 0.7 ± 0.0% | 99.3 ± 0.6% | 98.6 ± 0.6% | 97.1 ± 1.2% | 89.6 ± 1.9%a | 88.9 ± 2.1%a | 97.1 ± 2.4%a | 2.9 ± 2.4%a |
| Receptor | 150 | 1.4 ± 0.0% | 98.6 ± 1.1% | 97.3 ± 2.2% | 92.0 ± 4.1% | 84.0 ± 1.6%a | 82.0 ± 1.6%a | 95.9 ± 1.4%a | 4.1 ± 1.4%a |
| Transplanted | 125 | - | - | 89.2 ± 8.0% | 83.1 ± 9.1% | 63.4 ± 3.7%b | 58.5 ± 4.5%b (76) | 73.6 ± 6.3%b (56) | 26.4 ± 6.3%b (20) |
| P-value | 0.5164 | 0.4220 | 0.0026 | 0.0025 | 0.0246 | 0.0246 | |||
Numbers between brackets represent real numbers of PGC-GPF positive larvae.
Table 3
Embryonic development of the control groups (donor and recipient) and of the GFP-positive transplanted triploid recipients, using primordial germ cells (PGCs) from A. altiparanae, derived from the supplemented culture medium
| Groups | n | Unifertilized | 2-cell | Blastula | Gastrula | Somite | Hatch | Larvae | |
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | ||||||||
| Donor | 188 | 7.3 ± 0.0% | 92.8 ± 4.4% | 92.7 ± 4.4% | 80.0 ± 4.3% | 78.0 ± 3.3%b | 76.9 ± 4.2%b | 96.1 ± 2.5%b | 3.9 ± 2.5%a |
| Receptor | 152 | 7.2 ± 0.0% | 92.8 ± 3.1% | 82.8 ± 2.8% | 72.3 ± 1.6% | 62.9 ± 2.0%ab | 49.2 ± 8.1%ab | 66.8 ± 12.6%ab | 33.2 ± 12.6%ab |
| Transplanted | 94 | - | - | 96.4 ± 1.8% | 87.3 ± 7.6% | 43.6 ± 8.5%a | 34.3 ± 10.1%a (22) | 20.8 ± 12.3%a (6) | 79.2 ± 12.3%b (16) |
| P-value | 0,108 | 0,312 | 0,0286 | 0,0462 | 0,0153 | 0,0153 | |||
Numbers between brackets represent real numbers of PGC-GPF positive larvae.
Table 4
Embryonic development of the control groups (donor and recipient) and of the GFP-positive transplanted triploid hybrids using primordial germ cells (PGCs) from A. altiparanae, derived from the supplemented culture medium
| Groups | n | Unifertilized | 2-cell | Blastula | Gastrula | Somite | Hatch | Larvae | |
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | ||||||||
| Donor | 196 | 7.2 ± 0.0% | 92.8 ± 4.5% | 92.3 ± 4.2% | 82.9 ± 6.3% | 81.1 ± 5.1% | 77.7 ± 4.6% | 91.4 ± 3.1%b | 8.6 ± 3.1%a |
| Receptor | 193 | 1.0 ± 0.0% | 99.0 ± 0.8% | 89.5 ± 6.4% | 88.0 ± 7.1% | 65.6 ± 10.2% | 55.0 ± 14.0% | 68.7 ± 6.3%ab | 31.3 ± 6.3%ab |
| Transplanted | 62 | - | - | 93.1 ± 2.9% | 86.6 ± 3.0% | 68.7 ± 10.7% | 53.8 ± 10.6%(31) | 41.8 ± 13.8%a (12) | 58.2 ± 13.8%b (19) |
| P-value | 0,8999 | 0,8710 | 0,5983 | 0,3899 | 0,0499 | 0,0499 | |||
Numbers between brackets represent real numbers of PGC-GPF positive larvae.
A total of 86 transplants were performed by using PGCs cultivated in saline solution into triploid recipients, where 36 transplanted embryos subsequently hatched (47.5 ± 4.5%) and of which 20 (52.4 ± 11.0%) were normal (Table 1 and Fig. 1). For the 125 transplants that were performed with triploid hybrid recipient, 76 (58.5 ± 4.5%) hatched, and 56 (73.6 ± 6.3%) were normal. (Table 2 and Fig. 2).
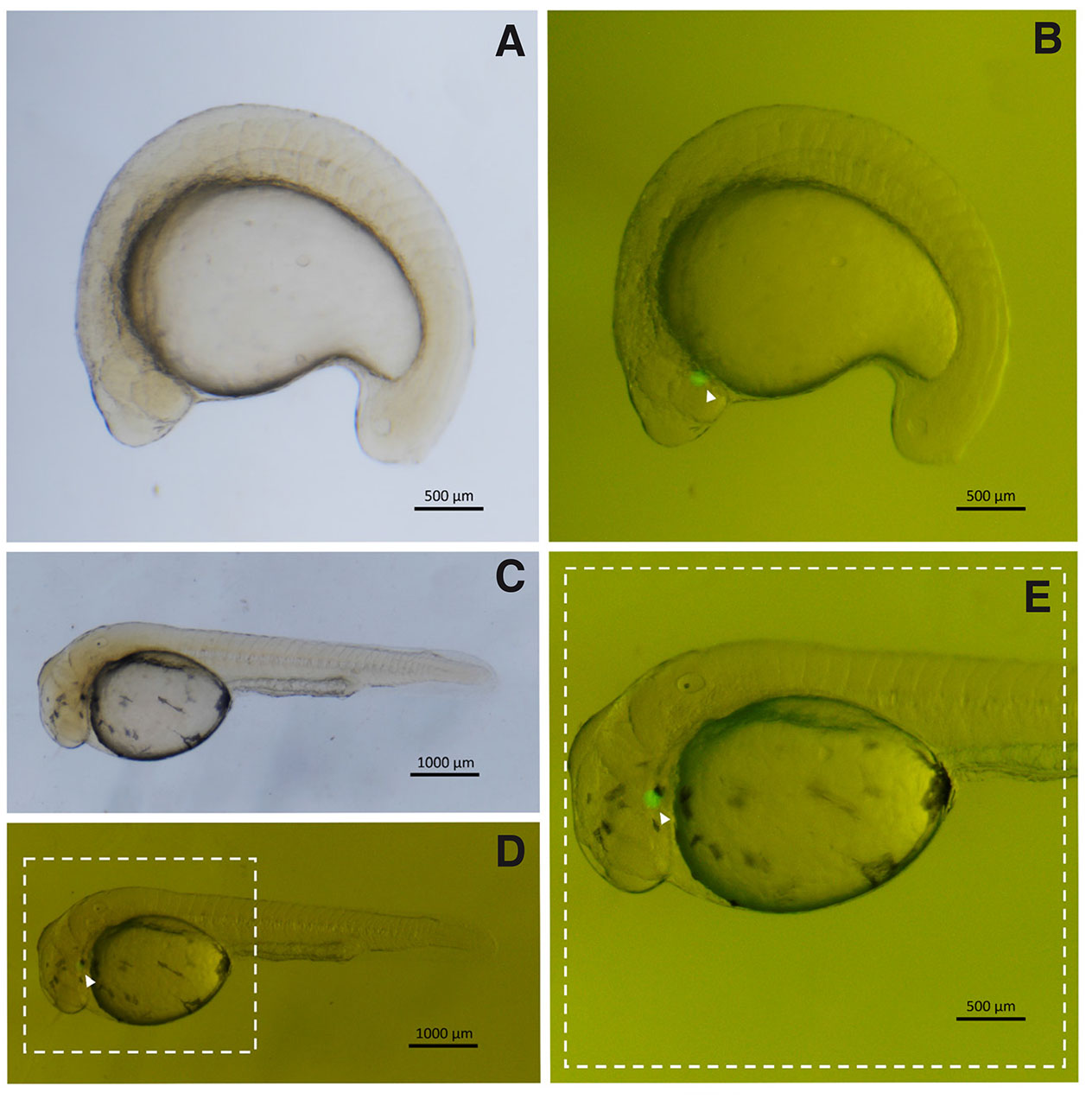
Fig. 1. Triploid embryos transplanted with GFP positive primordial germ cells (PGCs) from A. altiparanae, cultivated in saline.
(A,B) Embryos with 20 somites. (C,D) Embryo in the hatching phase. (E) Detail of the highlighted region in (D). (PGCs, arrowhead). (B,D) Images captured under fluorescence of (A,C), respectively.
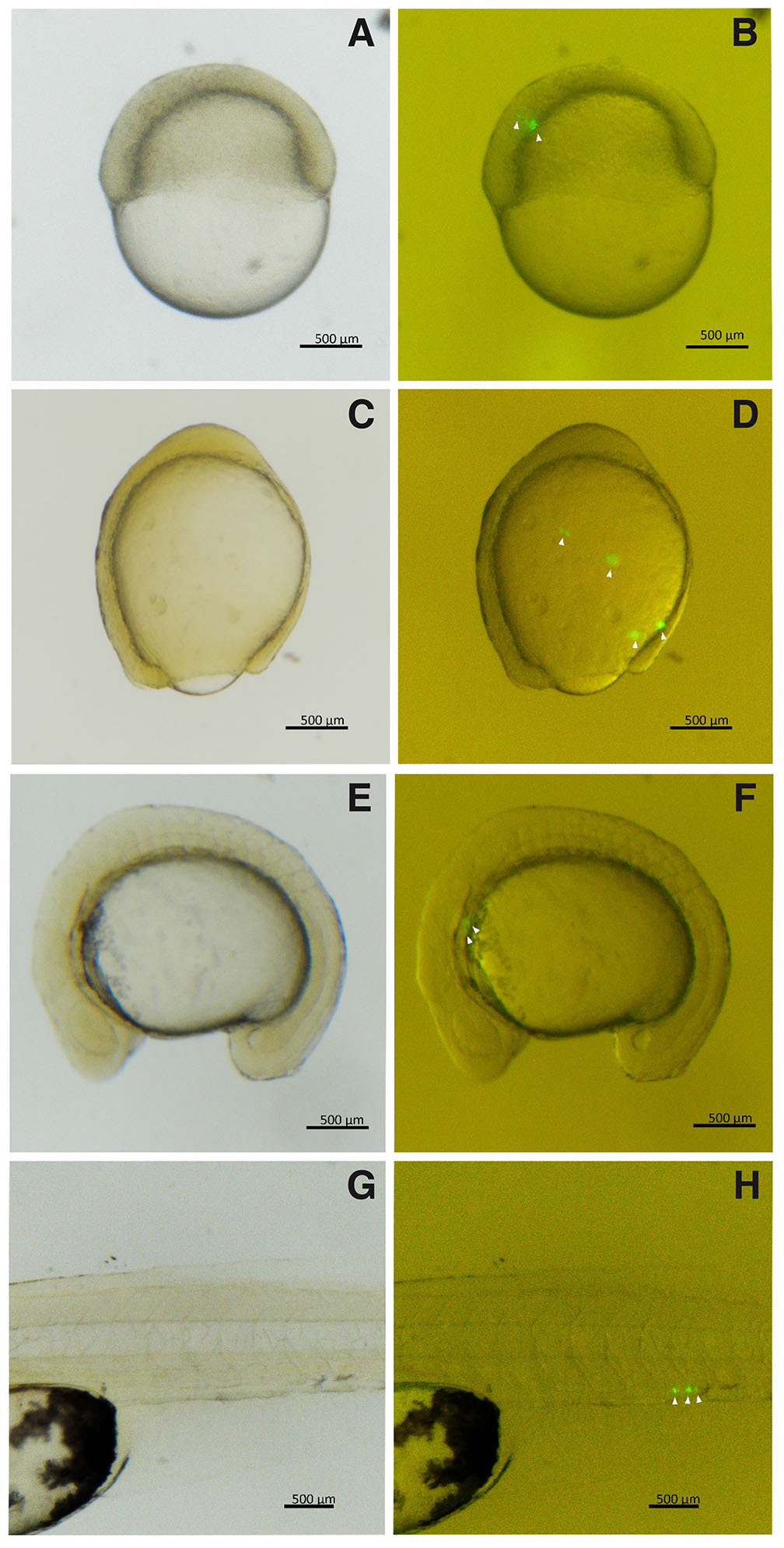
Fig. 2. Triploid hybrid embryos transplanted with A. altiparanae GFP positive primordial germ cells (PGCs), cultivated in saline.
(A,B) Embryos in gastrula stage with 30% epiboly. (C,D) Embryos in gastrula stage with 90% epiboly. (E,F) Embryos with 16 somites. (G,H) Larvae with 1-day post-hatch. (PGCs, arrowhead). (B,D,F and H) Images captured under fluorescence of (A,C,E, and G), respectively.
In the experiment using PGCs cultivated in supplemented medium and triploid receptors, 94 transplants were performed, where 22 embryos hatched (34.4 ± 10.1%), and 6 presented normal morphology (20.8 ± 12.3%) (Table 3 and Fig. 3). At the same time, 62 transplants were performed for PGCs cultivated in supplemented culture medium and transplanted into triploid hybrid recipients, where 31 embryos hatched (53.8 ± 10.6%) with 12 being normal (41.8 ± 13.8%) (Table 4 and Fig. 4).
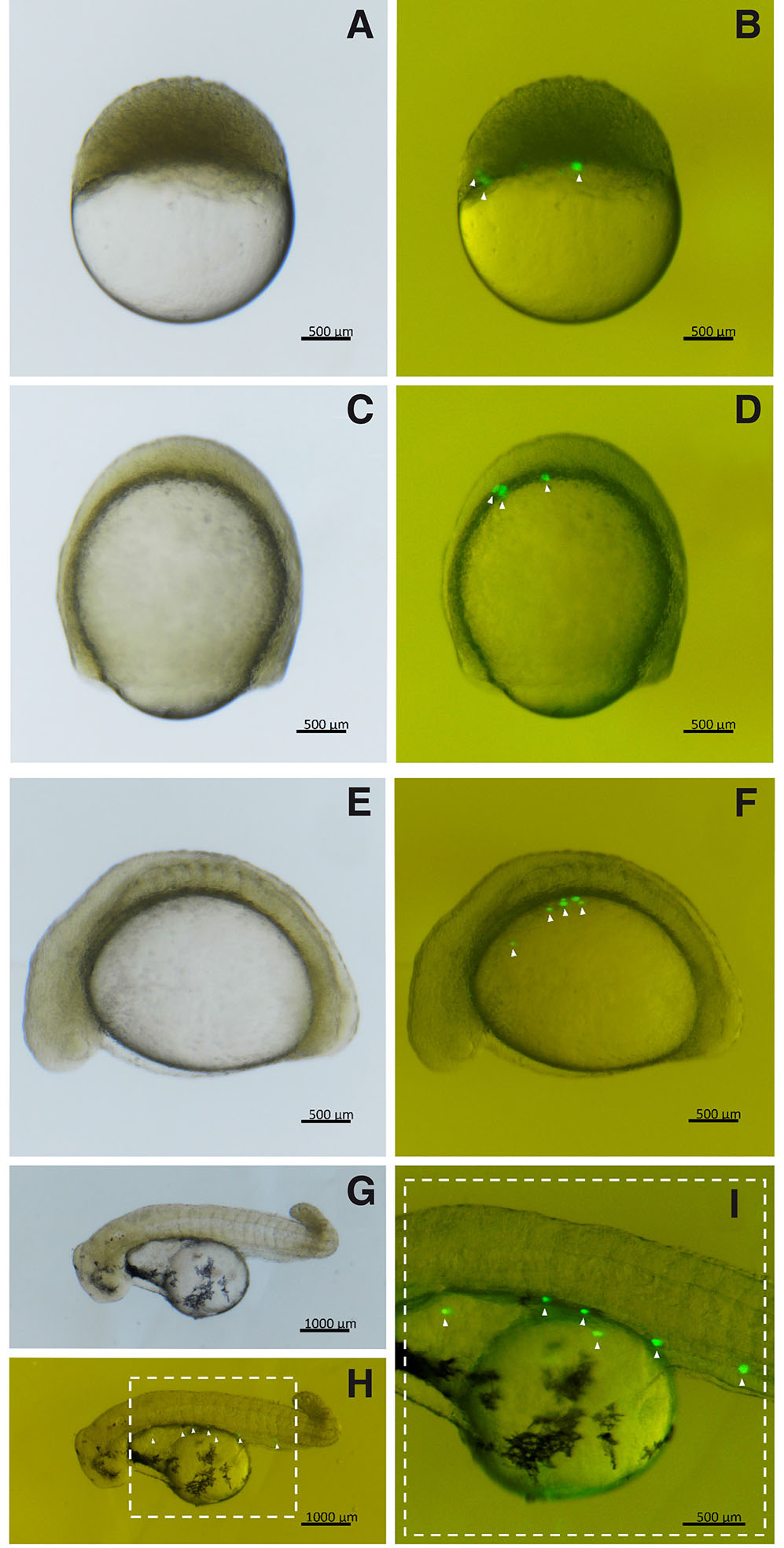
Fig. 3. Triploid embryos transplanted with PGCs from A. altiparanae, grown in supplemented cell culture medium.
(A,B) Embryos in the final stage of blastula. (C,D) Embryos in gastrula stage with 90% epiboly. (E,F) Embryos with 12 somites. (G,H) Embryos at hatch. (I) Detail of the highlighted region in (H). (PGCs, arrowhead). (B,D,F, and H) are images captured under fluorescence of (A,C,E and G), respectively.
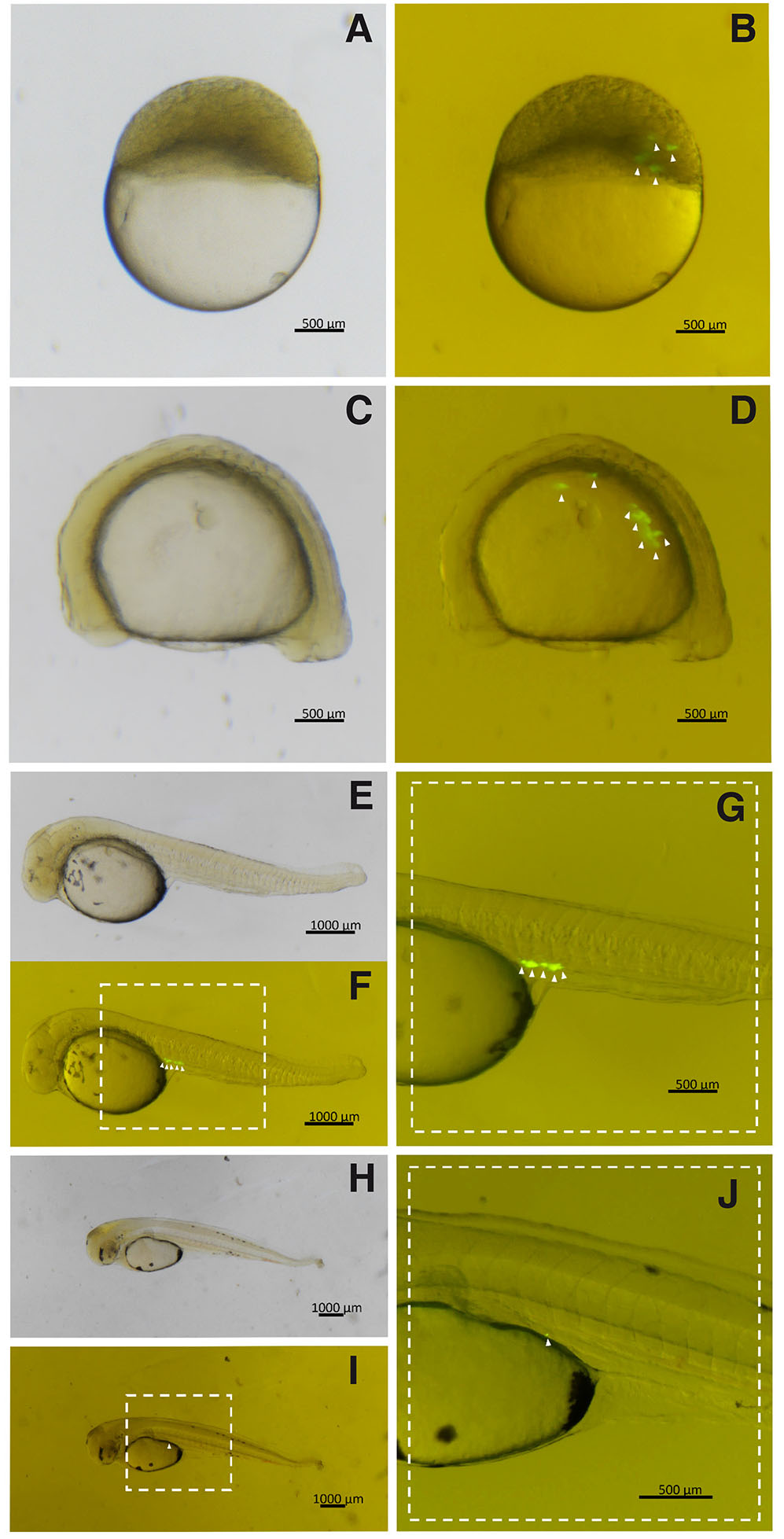
Fig. 4. Triploid hybrid embryos transplanted with PGCs from A. altiparanae, grown in supplemented cell culture medium.
(A,B) Embryos in early gastrula stage. (C,D) Embryos with 8 somites. (E,F) Embryos at hatch. (G) Detail of the highlighted region in (F). (H,I) – Larva with 1-day post-hatch. (J) Detail of the highlighted region in (I). (PGCs, arrowhead). (B,D,F, and I) are images captured under fluorescence of (A,C,E, and I), respectively.
In experiments carried out using the transplantation of PGCs cultivated in saline medium, it was observed that the PGCs transplanted in all embryos presented ectopic migration, regardless of the type of receptor, and GFP-positive cells were visualized in the cephalic region, yolk, and musculature (Figs. 2 and 3). Only for triploid hybrid recipients, the migration of PGCs from the donor was verified, being very close to the region of formation of the gonadal ridge (Fig. 3). Transplants were performed using 1 to 3 donor PGCs, and cell division was observed in 2 (5.5%) embryos of triploid recipient and in 1 (1.3%) hybrid triploid embryos. For these embryos in the hatching phase, between 4 to 6 GFP positive cells were observed. The presumptive chimeric embryo did not observe cell division.
In transplantation experiments of PGCs cultivated in supplemented medium, directed migration to the region of gonadal ridge formation was observed in 1 (4.5%) triploid transplanted embryo, and this embryo presented abnormal hatching (Table 3 and Fig. 3). However, when transplantation was performed in triploid hybrid receptors, GFP-positive cells with migration directed to the region of the gonadal ridge were observed in 6 embryos (19.3%), where 2 of them presented normal hatching and 4 abnormal hatching (Table 4 and Fig. 4).
Cellular proliferation of transplanted cells was observed in 3 (13.6%) transplanted triploid embryos and in 5 (16.1%) triploid hybrids, regardless of the final migration site of the PGCs. At hatching, 4 to 6 GFP positive cells were observed, except for two transplanted triploid hybrids. PGCs in the region of the gonadal ridge that hatched with 9 and 12 cells presented embryos with a drastic drop in GFP expression 1-day post-hatching (Fig. 4).
Gene expression
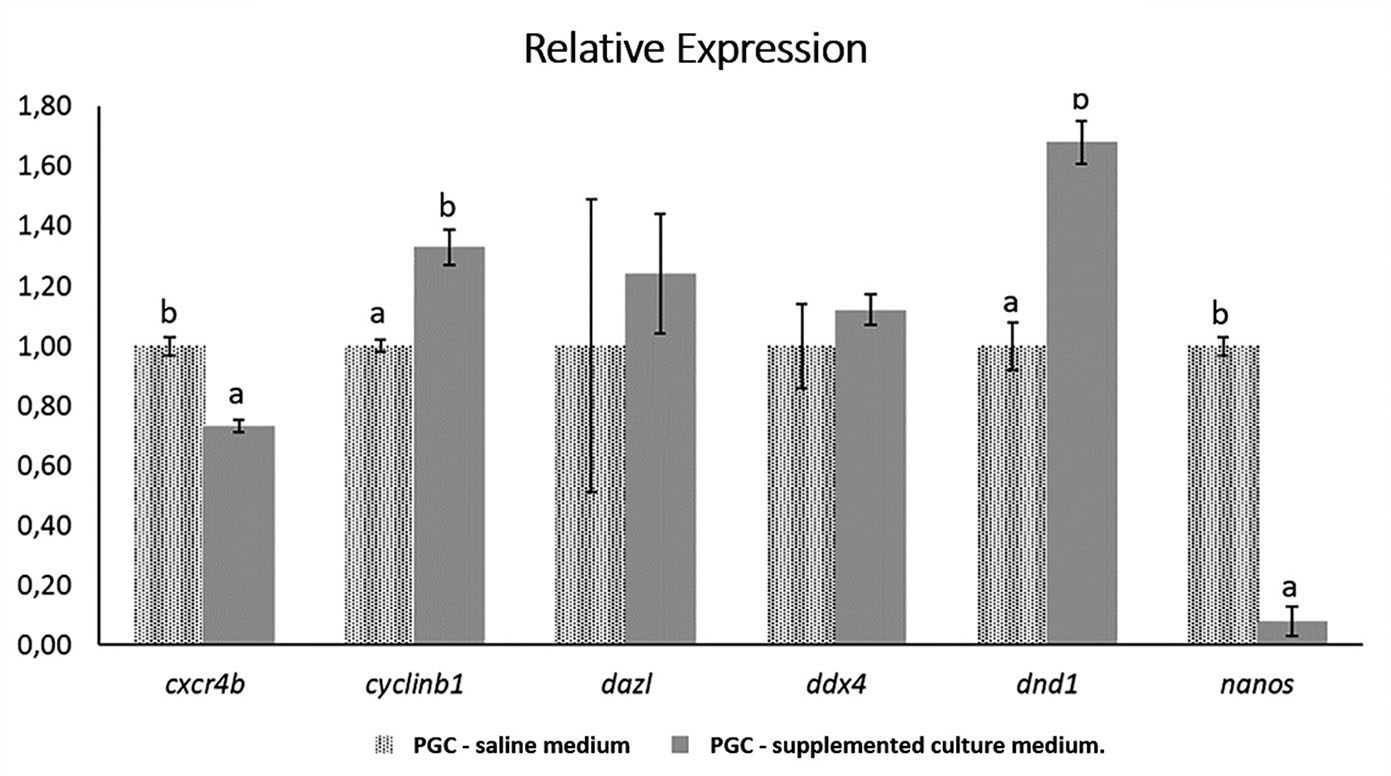
The relative gene expression results demonstrate differences in the expression levels of the genes evaluated between different samples (Fig. 5). An increase in the expression level of genes cyclin B1, dnd1, dazl, and ddx4 was observed in PGCs cultivated in supplemented medium, while the expression levels of cxcr4b and nanos genes showed a decrease (Fig. 5). The dazl and ddx4 genes did not show significant differences.
The greatest increase in gene expression level was observed for the dnd1 gene, where there was about 68% higher in PGC in supplemented medium than in saline. Cyclin B1 had an increase of about 33% greater in PGCs for the supplemented medium compared to PGCs from the saline medium. Although no significant difference was found, the dazl gene showed, on average, about 24% increase in expression in the supplemented medium, while ddx4 showed about 12% increase in expression in relation to the saline medium. These results indicate a trend in the increase of gene expression in the PGCs of the genes evaluated in the supplemented medium in relation to the saline medium (Fig. 5).
Differing from the tendency to increase the level of gene expression in the genes evaluated for the supplemented medium, the cxcr4b and nanos genes showed a decrease in the expression level in PGCs from the supplemented medium compared with PCGs from the saline medium. For cxcr4b, a decrease of around 23% was observed in the level of gene expression in the supplemented medium in relation to the saline medium; and for nanos, the fall was drastic, approximately 92%. (Fig. 5).
Expression of target genes in tissues
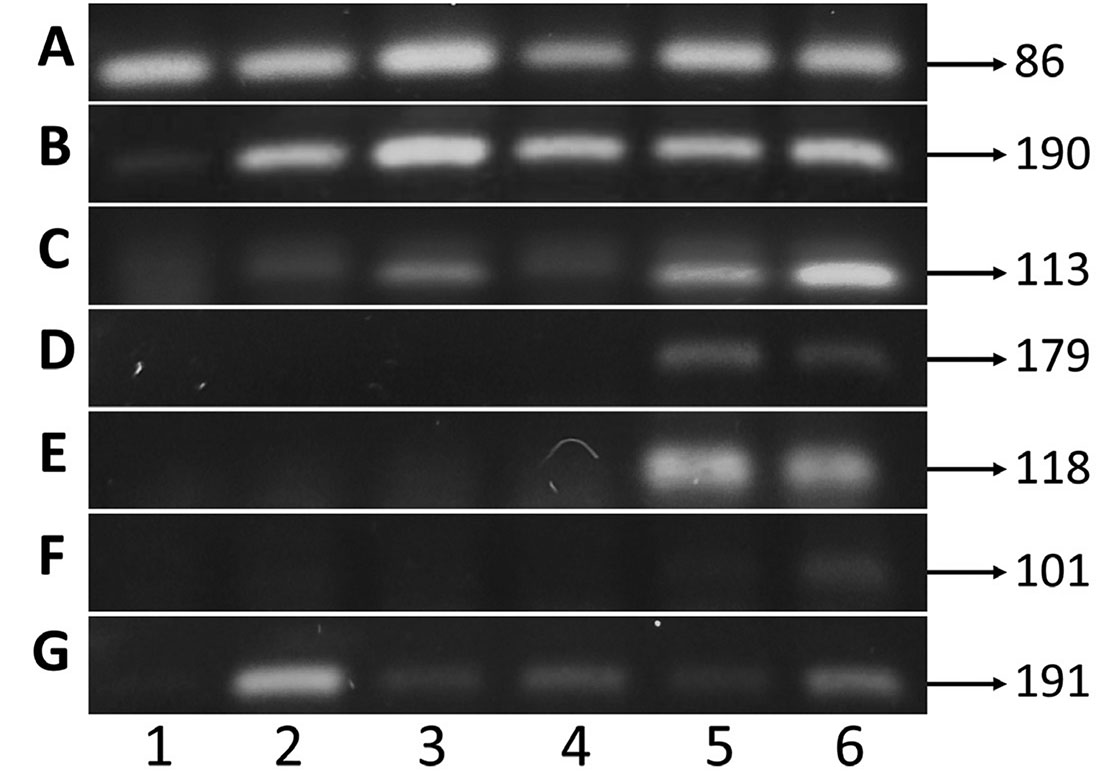
In adult A. altiparanae, cxcr4b, cyclin B1, and nanos (Fig. 6 C,D,H) were expressed in a wide range of tissues, including brain, kidney, liver, and gonads. cxcr4b and cyclin B1 expression was observed in muscle only (Fig. 6 C,D). Dazl and ddx4 are specific for male and female gonads (Fig. 6 E,F). Dnd1 showed weak expression in both male and female gonads (Fig. 6G).
Discussion
The transplanted PGCs presenting effective migration showed similar behavior to the PGCs of the species A. altiparanae (unpublished data). In the screening studies of PGCs in A. altiparanae embryos at the 6 to 19 somites stage, the stage used for transplantation, 3 to 12 PGCs were visualized placed in groups or spread over the yolk extension. While in the hatching phase, between 3 to 13 PGCs were found positioned mostly in the yolk's extension, where some cases were in the upper part of the yolk. These data are similar to those found in transplant receptors.
Successful transplantation is well known to be related with the correct migration of donor cells to the region of gonadal formation in the recipients. In addition, there is also an adequate time range for transplantation, due to their ability to migrate, which can decrease over time (Takeuchi et al., 2003; Yamaha et al., 2007).
The efficiency of germ cell transplantation is also related to previous sterilization of the recipient. The infertility of the recipient species contributes to the decrease in competition between endogenous and transplanted germ cells. Previous sterilization work was carried out with the studied species, using hybridization and chromosomal manipulation techniques. Nascimento et al., 2017 shows that triploid females are sterile, on the other hand, triploid males were not sterile. In later studies, using triploid hybrids of crossing A. altiparanae x A. fasciatus generated sterile offspring, with progenies that did not present a germ lineage in the gonads (Piva et al., 2018). This is in agreement with the results observed in our studies using triploid receivers and triploid hybrids.
In species frequently used in micromanipulation (experimental), such as Danio rerio, Carassius auratus, Misgurnus anguillicaudatus, Danio albolineatu, and trout, the embryos and isolated cells develop in a saline media, such as Ringer's solution. This solution is used for chorion removal, embryonic culture, and PGC culture; it is usually added with penicillin and streptomycin (Higaki et al., 2010; Kawakami et al., 2010; Saito et al., 2008) and fetal bovine serum or albumin (Fujimoto et al., 2006; Naya et al., 2020; Saito et al., 2010; Yoshizaki et al., 2005). However, it has been verified that in neotropical species, the best conditions for embryonic culture vary highly from one species to another, which often makes it difficult to choose the best solution when carrying out the PGCs cultivation (Coelho et al., 2019, 2021; unpublished data). In this study, it was verified that the PGCs presented directed migration when using adequate cell culture medium, enriched with amino acids, vitamins, glutamine, pyruvate, and fetal bovine serum, in addition to being cultivated in a CO2 incubator. This report is important, as it indicates that the success in transplanting and achievement germline chimeras can be influenced by the culture medium.
The conditions and time of culture of these cells may be related to the viability and expression of genes that are important for the maintenance of PGCs and, consequently, in the migration rates. Several genes are related to differentiation, maintenance, and migration of PGCs, and low levels of expression can lead to loss of identity, germ cell apoptosis, or ectopic migration (Barton et al., 2016; Doitsidou et al., 2002; Gross-Thebing et al., 2017; Knaut et al., 2003)
Gene expression in isolated PGCs showed lower level of expression in cxcr4b in PGCs grown in supplemented culture medium. The reduced level of cxcr4b can maintain the mobile behavior of cells; however, they can reach ectopic sites in the embryo due to undirected migration (Doitsidou et al., 2002; Raz, 2003; Raz and Reichman-Fried, 2006), which may also be related to the high number of ectopic migrations observed in the transplants performed in this work. However, even with the reduction of expression in PGCs cultivated in supplemented medium, the levels of cxcr4b in these cells still allowed migration to occur. This fact may indicate that other factors may be involved in migration and must have been optimized with the cultivation in the supplemented medium, such as the increased expression of dnd1, ddx4, and dazl, genes also responsible for the migration of PGCs. This hypothesis can be reinforced by the fact that even with higher levels of cxcr4b in cells cultivated in saline solution and lower levels of dnd1, ddx4, and dazl, the migrations observed were all ectopic.
It was reported that the deficiency of cxcr4b proteins and its ligand sdf-1 and/or dnd1 affect the migration of PGCs, which are located in ectopic positions or activate the apoptosis process (Barton et al., 2016; Gross-Thebing et al., 2017; Herpin et al., 2008; Sánchez-Sánchez et al., 2010; Weidinger et al., 2003). According Gross-Thebing et al., 2017, PGCs depleted of cxcr4b, but still expressing dnd1, can be located ectopically and maintain their morphology. Depletion of dnd1 can cause changes in the morphology of PGCs, leading to a gradual decrease in the expression of germ cell markers, such as vasa (ddx4) and nanos3, or causing apoptosis.
Studies involving mutations of the ddx4 gene in Danio rerio resulted in offspring consisting only of sterile males (Hartung et al., 2014). In Oryzias latipes, the ddx4 gene knockout did not affect PGC proliferation, survival, and motility; however, when used in transplantation, they did not show adequate migration. This indicates that ddx4 inhibition would result in the loss of PGC response to migration signaling towards the gonad (Li et al., 2009). However, the dazl gene knockout significantly reduced the number of PGCs and, in some cases, completely prevented the formation of PGCs (Li et al., 2016).
The nanos gene, also essential for the development of PGCs, at reduced levels can result in premature mitosis of PGCs, ectopic migration, and loss of their identity (Hashimoto et al., 2010; Köprunner et al., 2001). In this study, the expression level of nanos was drastically reduced in PGCs cultivated in supplemented medium when compared to PGCs from saline solution. It has been reported in Drosophila that Nanos and Pumilio proteins are involved in binding the 3'UTR region of cyclin B1 mRNA and repressing its translation. Cyclin B1 is an active protein in the mitotic phase, and its regulation is important so that PGCs do not proliferate prior to migration (Asaoka-taguchi et al., 1999; Hashimoto et al., 2010; Kadyrova et al., 2007). The gene expression results obtained from PGCs indicated a low level of nanos mRNA and a higher level of cyclin B1 in PGCs cultivated in supplemented medium compared with PGCs grown in saline solution. As described in the transplantation studies present in the literature (Saito et al., 2010, 2008), migration of the transplanted cells with little or no cell division was observed. PGCs from saline, which showed higher mRNA levels for nanos and lower for cyclin B1, show lower rates of cell division, ranging from 1.3 to 5.5%. While for the PGCs from the supplemented culture medium that showed a lower level of nanos expression and higher expression of cyclin B1, the cell division found were higher than those of the saline solution, ranging from 13.6 to 16.1%. These cell division and the mRNA levels of nanos and cyclin B1 appear to be related. Despite a decrease in the expression of nanos, an increase in cyclin B1, and an increase in the rate of division of PGCs, the PGCs from the supplemented culture medium were those capable of performing the correct migration to the gonadal ridge. This suggests that this decrease at the mRNA level of nanos still allows for the correct migration of PGCs.
Given the great diversity in reproductive strategies, spawning pattern, gamete size, specific temperature range for embryonic development, and sensitivity for handling in micromanipulation stages, the methodologies developed for the production of germline chimera can vary and require adaptations according with the species employed. Thus, each species may present a different challenge during the application of the transplant technique, which in turn is already invasive.
For the transplantation of PGCs, it is essential to have previous mastery of in vitro reproduction to obtain the labeling and isolation of PGCs, which is difficult in most species at risk of extinction. In addition, PGC transplantation requires developmental standardization between donor and recipient species, in which each species has a specific temperature range for optimal embryo development (Arashiro et al., 2018; Coelho et al., 2019; Pereira-Santos et al., 2016; Silva et al., 2017).
The size and timing of embryonic development of the species may limit the applicability of some transplantation techniques. The present model species Astyanax altiparanae and other species such as Danio rerio, Oryzias latipes, and Misgurnus anguillicaudatus present small eggs and larvae; this makes it difficult to use some methods used in trout (Takeuchi et al., 2003). The embryonic development velocity of neotropical species can vary from 11 to 29 h (Arashiro et al., 2018; Coelho et al., 2019; Pereira-Santos et al., 2016; Silva et al., 2017), differing from the species most commonly used in transplant works; this can take from 72 h to days for hatching (Fujimoto et al., 2006; Iwamatsu, 2004; Kimmel et al., 1995). In salmonids, for example, where cell transplantation techniques are quite advanced (Kobayashi et al., 2007; Nagasawa et al., 2013; Takeuchi et al., 2004), there are intrinsic biological facilities that optimize transplantation, such as slow embryo development (32-83 days, which increases transplant period), larger embryo size (facilitating visualization and micromanipulation), acclimatization in a micromanipulation system (Petri dish with static water), in addition to scientific support, which, as a group established in the basic sciences and in aquaculture, has important information regarding genetics (gene sequences and their expressions), reproduction, physiology, among other important areas for PGC transplantation. In the case of native species, as is the case of the present work, although great progress has been made in recent works (Arashiro et al., 2018; Bertolini et al., 2017; Coelho et al., 2021, 2019; Nascimento et al., 2020, 2017; Pereira-Santos et al., 2016; Piva et al., 2018; Yasui et al., 2020, 2015), basic information to support these advanced chimerism techniques is still lacking.
This is the first study to report PGC transplantation in order to obtain a germline chimera in a neotropical species. Gene expression in isolated PGCs is a good indicator that the supplementation of the culture medium provided better conditions for PGCs, in addition to confirming the identity of isolated cells for transplantation. Knowledge about the behavior of model native species in micromanipulation systems helps in the use of the technique for the conservation of endangered species. However, there is still a need for improvements in the stages of transplantation and knowledge about cell behavior in culture systems, time for transplantation, recipient conditions, the intercellular interaction between germ cells (donated) and somatic cells (receiver), and the performance of related gene maintenance and characterization of primordial germ cells in native species.
Material and Methods
The experimental procedures and analyses were carried out at the Laboratory of Fish Biotechnology, National Center for Research and Conservation of Continental Fish, Chico Mendes Institute of Biodiversity Conservation (ICMBio/CEPTA) - Pirassununga-SP, in line with the Ethics Committee for the Use of Laboratory Animals from the Faculty of Veterinary Medicine and Animal Science – University of São Paulo (CEUA/FMVZ n° 4534110820).
Origin of broodstocks and artificial fertilization
For the collection of Astyanax altiparanae and Astyanax fasciatus gametes, adult fish were selected for artificial propagation based on external characteristics and behavior. Males were selected based on the bony hooks in the anal fin. Females were selected based on reddish coloration in the papilla area and abdominal volume. The spawning of females induced ovulation with two doses of pituitary extract, where the first dose had a concentration of 0.5 mg.kg-1 and the second at 5.0 mg.kg-1 8 hours was administered after the first dose. Males were induced to spermiation with a single dose, simultaneously with the second dose of the female and at the same hormonal concentration (Pereira-Santos et al., 2016; Silva et al., 2017). A couple was used for each replication and the data was presented as an average of the replicates. The fish were anesthetized with clove oil (100 mg. L-1, Biodinâmica, Pinhais, Paraná, Brasil); gametes were collected by stripping, activated by the addition of water, and quickly homogenized by hand. After fertilization, an aliquot of eggs was immediately used for the steps of triploid production and/or mRNA microinjection.
Production of triploid and triploid hybrids recipients
Oocytes of yellowtail tetra A. altiparanae and sperm of A. fasciatus were obtained as mentioned above. Triploidization was achieved by heat shock at 40°C for 2 minutes on embryos after fertilization (Adamov et al., 2016; Pereira-Silva et al., 2016). Triploids of A. altiparanae and triploid hybrids were produced for later experiments.
In vivo traceability of primordial germ cells
PGCs from A. altiparanae were evidenced as described in (Coelho et al., 2021, 2019). An aliquot of newly fertilized eggs (~500) was dechorionated by enzymatic digestion using Holtfreter's solution (10 mM NaCl, 0.67 mM KCl, 0.90 mM CaCl2 e 2.4 mM NaHCO3), containing 0.05% pronase (Sigma #SLMQ2345V, St. Louis, USA). The dechorionated eggs were transferred to an agar-plated Petri dish 1% prepared with Holtfreter's solution. Microinjection of mRNA was achieved by using approximately 10 nL of mRNA solution (GFP-nanos1 3’UTR). The final concentration of 100 ng. μL-1 was microinjected into the blastodiscs at the one-cell stage by using a microinjector (CellTram vario, Eppendorf, Hamburg, Germany) attached to the micromanipulator (M-152, Narishige, Tokyo, Japan) and then visualized through the stereomicroscope (SMZ18, Nikon®, Tokyo, Japan). The number of eggs used for the microinjection procedure varied for each replicate, according to the time spent between fertilization, chorion removal, and mRNA injection. This was influenced also by the gamete quality, development pattern, and temperature. In general, 350 to 500 embryos were microinjected after removal of the chorion.
The injected embryos were assessed until the blastula stage (256-512 cells); the blastoderm was detached from the yolk with the aid of an insulin needle (23 gauges), thus developing into a spherical shape (blastosphere). Blastospheres were washed with Holtfreter's solution for complete removal of the yolk. After removing the supernatant, 400 μL of Holtfreter's solution containing 0.25% sodium citrate solution was added, in which the blastomeres were isolated after gently pipetting. Some embryos were kept intact to serve as a control to assess the stage of development of the isolated cells.
Dissociated blastomeres were cultivated under two different conditions: 1) saline solution: Holtfreter solution containing 0.01% penicillin and 0.01% streptomycin, grown at 28°C in biochemical oxygen demand incubators (B.O.D); and 2) supplemented medium: 50% Holtfreter solution and 50% cell culture medium consisting of DMEM (Dulbecco's Modified Eagle's Medium – high glucose, SIGMA) supplemented with 10% Fetal Bovine Serum (GIBCO), 1X solution of non-essential amino acids MEM (MEM Non – Essential Amino Acid Solution- 100X, SIGMA), 1X of MEM Vitamins Solution (MEM Vitamins Solution – 100X, SIGMA), 1X of glutamax solution (L – Glutamine 100X, SIGMA), 1X of antibiotic and antimycotic solution (Antibiotic Antimycotic Solution 100X, SIGMA), and 1 mM of pyruvate solution (Sodium Pyruvate Solution – 100 mM, SIGMA) grown at 30ºC in a CO2 incubator.
The dissociated cells were cultivated in different media until the segmentation phase (6-15 somites), approximately 7 hours after fertilization, with the embryonic development being compared with the non-injected control group. At this stage of development, GFP-positive PGCs are evidenced and can be selected and used for transplantation and gene expression steps.
Primordial germ cell transplantation
PGC transplantation was performed using one to three GFP-positive clustered cells of A. altiparanae in segmentation stage (6-15 somites). PGCs were aspirated by using a borosilicate micropipette up to 20 μm in diameter (Drummond, Eugene, USA) and transplanted to the lateral marginal region of the blastoderm (Saito et al., 2008; Yasui et al., 2011) of triploid and hybrids triploid embryos (Piva et al., 2018). To perform the transplants, the receptors embryos underwent enzymatic removal of the chorion.
Transplanted embryos and control groups (donor and recipient species) were maintained in a Petri dish (90 x 15 mm) with 100 mL of Holtfreter's solution containing 0.01% penicillin and 0.01% streptomycin at 26°C in a BOD incubator. The embryonic, larval, and migration pattern of the transplanted PGCs were monitored under a trinocular fluorescence stereomicroscope (Nikon SMZ18, Tokyo, Japan) with a filter set for GFP (Excitation wavelength 470/40 nm) at 30-60 min intervals in which partial solution changes were performed. Digital images were captured by a CCD-type camera (Ds-R2i, Nikon, Tokyo, Japan), and digital images were obtained using Nis-Ar Elements software (Nikon, Tokyo, Japan).
Germline chimerism was assessed by fluorescence, observing the cell migration and proliferation of the transplanted GFP-positive primordial germ cells.
Collection for evaluation of gene expression
For gene expression assessment, labelled and isolated PGCs were cultivated in different conditions until the segmentation phase (3 to 10 somites). A pool of PGCs derived from 14 crosses were used, in which the PGCs were cultivated in saline solution and a pool of PGCs referring to 8 crosses, in which the PGCs were cultivated in supplemented medium. GFP positive PGCs were selected, collected, and stored in Trizol at -80°C to be used in the steps of total RNA extraction, cDNA synthesis, and analysis of relative gene expression.
For gene expression assessment in tissues, specimens were euthanized by an overdose of clove oil anesthetic (Biodynamics, Pinhais, Paraná, Brazil). Tissues such as muscle, brain, kidney, liver, and gonads were collected from a single male, except for the ovary sample that came from a single female, both adults of species A altiparanae. The samples were stored in Trizol at -80°C for subsequent extraction of total RNA.
RNA extraction and cDNA synthesis
For the analysis of the relative expression of genes ubiquitina, cxcr4b, cyclin B1, dazl, ddx4, dnd1, and nanos (Table 5), RNA was extracted from isolated primordial germ cell pool and cultivated in either saline medium or in supplemented medium and of tissues of A. altiparanae with Trizol (Ambion® # 15596026, Carlsbad, USA), according to the manufacturer's protocol. For cDNA synthesis, 1 µg of RNA was used, quantifying in a QIAxpert spectrophotometer (Qiagen, Hilden, Germany). Then, the RNA was treated with DNAse1, and the first strand cDNA synthesis was performed by using the SuperScript III First-Strand Synthesis System kit for RT-PCR (Invitrogen # 18080-051, Carlsbad, USA), according to the instructions of the manufacturer.
Table 5
Primers used to evaluate the gene expression of primordial germ cells in Astyanax altiparanae
| Gene | Accession number | Primer sequence | Amplicon size (BP) |
|---|---|---|---|
| ubiquitin | XM_022672008.1 | FORWARD: 5’ – AGATTACCCCTTCAAACCGC -3’ REVERSE: 5’ – GTCAGTCTTGTAGATGCGGG -3’ |
217 |
| cxcr4b | XM_007232384.3 | FORWARD: 5’ – ATTATCTTCCTCCTGGGCGTG-3’ REVERSE: 5’ – TTCGGCAAGTTCCTGTGCG-3’ |
190 |
| cyclin B1 | XM_007236669.3 | FORWARD: 5’ – GCCTATGGAAACCTCTGGCT -3’ REVERSE: 5’ – TGCAGAGCATGGGATTGTCG -3 |
113 |
| dazl | XM_007239709.3 | FORWARD: 5’ – TGCGGTGAAGGAGGTCAAAA-3’ REVERSE: 5’ – GCGGAGTTCTCGTTCTCTCC-3’ |
179 |
| ddx4 | XM_022681259.1 | FORWARD: 5’ – AAGACCACAGGAACTGAGCG-3’ REVERSE: 5’ – CCCGGTCTCCATGAATGCTT-3’ |
118 |
| dnd1 | XM_007253600.3 | FORWARD: 5’ – GGCTGTGAGGTGTTTATCAGTC-3’ REVERSE: 5’ – TCCGCCTCATGATGAACTTCA-3 |
101 |
| nanos1 | XM_022683547.1 |
FORWARD: 5’ – CTCTAGCGGAGTTTCCACCT -3’ REVERSE: 5’ – GTTGGTGGTTCCAGAAAACG -3 |
191 |
Gene expression analysis
Conventional PCR
The specificity of the designed primers (Table 5) was initially evaluated by using conventional PCR using only oocyte samples; later, evaluations were made of the expression level of the primers in relation to tissue samples. The reactions were standardized under the following conditions: 3 µL 10x PCR Rxn Buffer, 0.6 µL 10 mM dNTPs, 0.9 µL 50 mM MgCl2, 1.5 µL 10 mM Primer Mix, 1 µL cDNA, 0.3 µL Taq DNA polymerase at a concentration of 5 U/µL, and 22.7 µL of nuclease-free water, all included in a total final volume of 30 µL. Cycling was standardized in the following procedure: 94°C for 5 min, followed by 35 cycles at 94°C for 45 s, 60°C for 30 s, 72°C for 30 s, and final incubation at 72°C for 10 min. The product of the amplifications was analyzed by 3% agarose gel electrophoresis. For a visual estimate of the size of the DNA fragments, the 1 Kb plus DNA Ladder at a concentration of 0.5 µL (Invitrogen) was applied to one of the wells as a molecular mass marker.
Real Time PCR
The construction of standard curves for genes of interest cxcr4b, cyclinb1, dazl, ddx4, dnd1, and nanos and endogenous control ubiquitina (Table 5) were performed by using serial 1:3 dilution, where the first point of the undiluted curve of oocyte or blastula cDNA was guided by the results of conventional PCR. The curves were made together and in duplicate for each point.
The relative expression of genes with established standard curves was evaluated by real-time PCR for cDNA samples from primordial germ cells cultivated in saline and supplemented medium, diluted in a 1:3 ratio. A negative reaction control (NTC) was used for each gene. All samples were amplified in triplicate. For each reaction, 10 µL of QuantiNova SYBR Green PCR Master Mix (Qiagen), 8 µL of nuclease-free water, 1 µL of primer mix for target genes, and 1 µL of cDNA diluted 1:5 was used. The amplification conditions were as follows: hold 95°C for 2 min, followed by 40 cycles of 95°C for 5 sec and 63°C for 15 sec with a Melt curve between 55°C and 95°C in a RotorGene Q thermocycler (Qiagen Hilden, Germany). The analyses of the relative quantification of gene expression were conducted based on the standard curve method (Larionov et al., 2005).
Statistics
Data were evaluated as mean ± standard error of the mean. All data were checked for normality using the Lilliefors test and then submitted to the Kruskal-Wallis test. Means were compared by using Tukey's non-parametric multiple amplitude test, using the Statistica 10.0 software. A probability of 0.05 was established for all analyses.
Acknowledgements
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) and Sao Paulo Research Foundation (FAPESP) (2010/17429-1 and 2011/11664-1) for the financial support of this research. We also acknowledge CEPTA/ICMBio for generously providing the facilities and experimental fish and Michael Stablein for the review of this manuscript.
References
Adamov N. S. M., Nascimento N. F. , Maciel E. C. S., Pereira-Santos M., Senhorini J. A., Calado L. L., Evangelista M. M., Nakaghi L. S. O., Guerrero A. H. M., Fujimoto T., Yasui G. S. (2016). Triploid Induction in the Yellowtail Tetra, Astyanax altiparanae , Using Temperature Shock: Tools for Conservation and Aquaculture . Journal of the World Aquaculture Society 48: 741-750.
Arai K., Fujimoto T. (2013). Genomic Constitution and Atypical Reproduction in Polyploid and Unisexual Lineages of the <b><i>Misgurnus</i></b> Loach, a Teleost Fish. Cytogenetic and Genome Research 140: 226-240.
Arashiro D. R., Yasui G. S., Calado L. L., do Nascimento N. F., dos Santos M. P., do Santos S. C. A., Levy-Pereira N., Monzani P. S., Siqueira-Silva D. H., Senhorini J. A. (2018). Synchronizing developmental stages in Neotropical catfishes for application in germ cell transplantation. Zygote 26: 135-148.
Asaoka-Taguchi M., Yamada M., Nakamura A., Hanyu K., Kobayashi S. (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nature Cell Biology 1: 431-437.
Barton L. J., LeBlanc M. G., Lehmann R. (2016). Finding their way: themes in germ cell migration. Current Opinion in Cell Biology 42: 128-137.
Bertolini R. M., Senhorini J. A., Nascimento N. F. , Pereira-Santos M., Nakaghi L. S. O., Peres W. A. M., Silva R. C. , Yasui G. S. (2017). First feeding of diploid and triploid yellowtail tetra Astyanax altiparanae: An initial stage for application in laboratory studies . Aquaculture Research 49: 68-74.
Coelho G. C. Z., Arashiro D. R., Disselli T., Pereira-Santos M., Mira-López T. M., Monzani P. S., Senhorini J. A., Fujimoto T., Yasui G. S. (2021). Developmental stages, incubation temperature, and in vivo traceability of primordial germ cell in an important aquaculture species Piaractus mesopotamicus. Aquaculture 535: 736381.
Coelho G. C. Z., Yo I. S., Mira-López T. M., Monzani P. S., Arashiro D. R., Fujimoto T., Senhorini J. A., Yasui G. S. (2019). Preparation of a fish embryo for micromanipulation: staging of development, removal of the chorion and traceability of PGCs in Prochilodus lineatus. The International Journal of Developmental Biology 63: 57-65.
Doitsidou M., Reichman-Fried M., Stebler J., Köprunner M., Dörries J., Meyer D., Esguerra C. V., Leung T. C., Raz E., (2002). Cell 111: 647-659.
Franěk R., Marinović Z., Lujić J., Urbányi B., Fučíková M., Kašpar V., Pšenička M., Horváth Á., (2019). Cryopreservation and transplantation of common carp spermatogonia. PLoS One 14: 1-17.
Fujimoto T., Kataoka T., Sakao S., Saito T., Yamaha E., Arai K., (2006). Developmental Stages and Germ Cell Lineage of the Loach (Misgurnus anguillicaudatus). Zoological Science 23: 977-989.
Goto R., Saito T. (2019). A state-of-the-art review of surrogate propagation in fish. Theriogenology 133: 216-227.
Gross-Thebing T., Yigit S., Pfeiffer J., Reichman-Fried M., Bandemer J., Ruckert C., Rathmer C., Goudarzi M., Stehling M., Tarbashevich K., Seggewiss J., Raz E. (2017). The Vertebrate Protein Dead End Maintains Primordial Germ Cell Fate by Inhibiting Somatic Differentiation. Developmental Cell 43: 704-715.e5.
Hartung O., Forbes M. M., Marlow F. L. (2014). Zebrafish vasa is required for germ-cell differentiation and maintenance. Molecular Reproduction and Development 81: 946-961.
Hashimoto H., Hara K., Hishiki A., Kawaguchi S., Shichijo N., Nakamura K., Unzai S., Tamaru Y., Shimizu T., Sato M., (2010). Crystal structure of zinc-finger domain of Nanos and its functional implications. EMBO reports 11: 848-853.
Hayashi C., Meurer F., Boscolo W. R., Lacerda C. H. F., Kavata L. C. B. (2004). Freqüência de arraçoamento para alevinos de lambari do rabo-amarelo (Astyanax bimaculatus). Revista Brasileira de Zootecnia 33: 21-26.
Herpin A., Fischer P., Liedtke D., Kluever N., Neuner C., Raz E., Schartl M. (2008). Sequential SDF1a and b-induced mobility guides Medaka PGC migration. Developmental Biology 320: 319-327.
Higaki S., Eto Y., Kawakami Y., Yamaha E., Kagawa N., Kuwayama M., Nagano M., Katagiri S., Takahashi Y. (2010). Production of fertile zebrafish (Danio rerio) possessing germ cells (gametes) originated from primordial germ cells recovered from vitrified embryos. REPRODUCTION 139: 733-740.
Iwamatsu T. (2004). Stages of normal development in the medaka Oryzias latipes. Mechanisms of Development 121: 605-618.
Kadyrova L. Y., Habara Y., Lee T. H., Wharton R. P. (2007). Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline . Development 134: 1519-1527.
Kawakami Y., Goto-Kazeto R., Saito T., Fujimoto T., Higaki S., Takahashi Y., Arai K., Yamaha E., (2010). Generation of germ-line chimera zebrafish using primordial germ cells isolated from cultured blastomeres and cryopreserved embryoids. International Journal of Developmental Biology 54: 1493-1501.
Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics 203: 253-310.
Kobayashi T., Takeuchi Y., Takeuchi T., Yoshizaki G. (2007). Generation of viable fish from cryopreserved primordial germ cells. Molecular Reproduction and Development 74: 207-213.
Köprunner M., Thisse C., Thisse B., Raz E. (2001). A zebrafish nanos -related gene is essential for the development of primordial germ cells . Genes & Development 15: 2877-2885.
Larionov A., Krause A., Miller W. (2005). A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6: 62.
Li M., Hong N., Xu H., Yi M., Li C., Gui J., Hong Y. (2009). Medaka vasa is required for migration but not survival of primordial germ cells. Mechanisms of Development 126: 366-381.
Li M., Zhu F., Li Z., Hong N., Hong Y. (2016). Dazl is a critical player for primordial germ cell formation in medaka. Scientific Reports 6: 28317.
Linhartova Z., Saito T., Psenicka M. (2014). Embryogenesis, visualization and migration of primordial germ cells in tench ( Tinca tinca ) . Journal of Applied Ichthyology 30: 29-39.
Lobón-Cerviá J., Bennemann S. (2000). Temporal trophic shifts and feeding diversity in two sympatric, neotropical, omnivorous fishes: Astyanax bimaculatus and Pimelodus maculatus in Rio Tibagi (Paraná, Southern Brazil). Fundamental and Applied Limnology 149: 285-306.
Nagasawa K., Fernandes J. M.O., Yoshizaki G., Miwa M., Babiak I. (2013). Identification and migration of primordial germ cells in Atlantic salmon, Salmo salar : Characterization of Vasa , Dead End , and Lymphocyte antigen 75 genes . Molecular Reproduction and Development 80: 118-131.
Nascimento N. F., Pereira-Santos M., Levy-Pereira N., Monzani P. S., Niedzielski D., Fujimoto T., Senhorini J. A., Nakaghi L. S. O., Yasui G. S. (2020). High percentages of larval tetraploids in the yellowtail tetra Astyanax altiparanae induced by heat-shock: The first case in Neotropical characins. Aquaculture 520: 734938.
Nascimento N. F., de Siqueira-Silva D. H., Pereira-Santos M., Fujimoto T., Senhorini J. A., Nakaghi L. S. O., Yasui G. S. (2017). Stereological analysis of gonads from diploid and triploid fish yellowtail tetra Astyanax altiparanae (Garutti & Britski) in laboratory conditions . Zygote 25: 537-544.
Naya Y., Matsunaga T., Shimizu Y., Takahashi E., Shima F., Endoh M., Fujimoto T., Arai K., Yamaha E. (2020). Developmental potential of somatic and germ cells of hybrids between Carassius auratus females and Hemigrammocypris rasborella males . Zygote 28: 470-481.
Pereira-Santos M., Yasui G. S., Xavier P. L. P., de Macedo Adamov N. S., do Nascimento N. F., Fujimoto T., Senhorini J. A., Nakaghi L. S. O. (2016). Morphology of gametes, post-fertilization events and the effect of temperature on the embryonic development of Astyanax altiparanae (Teleostei, Characidae) . Zygote 24: 795-807.
Piva L. H., de Siqueira-Silva D. H., Goes C. A. G., Fujimoto T., Saito T., Dragone L. V., Senhorini J. A., Porto-Foresti F., Ferraz J. B. S., Yasui G. S. (2018). Triploid or hybrid tetra: Which is the ideal sterile host for surrogate technology?. Theriogenology 108: 239-244.
Porto-Foresti F., Castilho-Almeida R.B., Foresti F., (2005). Biologia e criação do lambari-do-rabo-amarelo (Astyanax altiparanae). In Espécies Nativas Para Piscicultura No Brasil. (Ed. Baldisserto B., Gomes L. C., ) .
Pšenička M., Saito T., Linhartová Z., Gazo I. (2015). Isolation and transplantation of sturgeon early-stage germ cells. Theriogenology 83: 1085-1092.
Raz E. (2003). Primordial germ-cell development: the zebrafish perspective. Nature Reviews Genetics 4: 690-700.
Raz E., Reichman-Fried M. (2006). Attraction rules: germ cell migration in zebrafish. Current Opinion in Genetics & Development 16: 355-359.
Robles V., Riesco M. F., Psenicka M., Saito T., Valcarce D. G., Cabrita E., Herráez P. (2017). Biology of teleost primordial germ cells (PGCs) and spermatogonia: Biotechnological applications. Aquaculture 472: 4-20.
Saito T., Fujimoto T., Maegawa S., Inoue K., Tanaka M., Arai K., Yamaha E. (2006). Visualization of primordial germ cells in vivo using GFP-nos1 3'UTR mRNA. The International Journal of Developmental Biology 50: 691-699.
Saito T., Goto-Kazeto R., Arai K., Yamaha E. (2008). Xenogenesis in Teleost Fish Through Generation of Germ-Line Chimeras by Single Primordial Germ Cell Transplantation1. Biology of Reproduction 78: 159-166.
Saito T., Goto-Kazeto R., Kawakami Y., Nomura K., Tanaka H., Adachi S., Arai K., Yamaha E. (2011). The Mechanism for Primordial Germ-Cell Migration Is Conserved between Japanese Eel and Zebrafish. PLoS ONE 6: e24460.
Saito T., Goto-Kazeto R., Fujimoto T., Kawakami Y., Arai K., Yamaha E. (2010). Inter-species transplantation and migration of primordial germ cells in cyprinid fish. The International Journal of Developmental Biology 54: 1479-1484.
Sánchez-Sánchez A. V., Camp E., Leal-Tassias A., Atkinson S. P., Armstrong L., Díaz-Llopis M., Mullor J. L. (2010). Nanog Regulates Primordial Germ Cell Migration Through Cxcr4b . Stem Cells 28: 1457-1464.
Silva R. C., Pereira dos Santos M., Senhorini J. A., Paes M. C. F., Valentin F. N., Fujimoto T., do Nascimento N. F., Yasui G. S., Nakaghi L. S. O. (2017). The effect of temperature on the initial development of Brycon amazonicus Spix & Agassiz, 1829 as tool for micromanipulation of embryos . Zygote 25: 637-651.
Siqueira-Silva D. H., Saito T., dos Santos-Silva A. P., da Silva Costa R., Psenicka M., Yasui G. S. (2018). Biotechnology applied to fish reproduction: tools for conservation. Fish Physiology and Biochemistry 44: 1469-1485.
Takeuchi Y. (2003). Generation of Live Fry from Intraperitoneally Transplanted Primordial Germ Cells in Rainbow Trout. Biology of Reproduction 69: 1142-1149.
Takeuchi Y., Yoshizaki G., Takeuchi T. (2004). Surrogate broodstock produces salmonids. Nature 430: 629-630.
Weidinger G., Stebler J., Slanchev K., Dumstrei K., Wise C., Lovell-Badge R., Thisse C., Thisse B., Raz E. (2003). dead end, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival. Current Biology 13: 1429-1434.
Yamaha E., Murakami M., Hada K., Otani S., Fujimoto T., Tanaka M., Sakao S., Kimura S., Sato S., Arai K. (2003). Recovery of fertility in male hybrids of a cross between goldfish and common carp by transplantation of PGC (primordial germ cell)-containing graft. Genetica 119: 121-131.
Yamaha E., Saito T., Goto-Kazeto R., Arai K. (2007). Developmental biotechnology for aquaculture, with special reference to surrogate production in teleost fishes. Journal of Sea Research 58: 8-22.
Yasui G. S., Fujimoto T., Sakao S., Yamaha E., Arai K. (2011). Production of loach (Misgurnus anguillicaudatus) germ-line chimera using transplantation of primordial germ cells isolated from cryopreserved blastomeres1. Journal of Animal Science 89: 2380-2388.
Yasui G.S., Porto-Foresti F., Castilho-Almeida R.B. de, Senhorini J.A., Nascimento N.P. do, Foresti F., (2020). Biologia e criação do Lambari-do-Rabo-Amarelo (Astyanax altiparanae). In Espécies Nativas Para Piscicultura No Brasil: 3° Edição Revista, Atualizada e Amplificada. (Ed. Baldisserotto B., ) Fundação de Apoio a Tecnologia e Ciência - UFSM.
Yasui G.S., Senhorini J.A., Shimoda E., Pereira-Santos M., Nakaghi L.S.O., Fujimoto T., Arias-Rodriguez L., Silva L.A. (2015). Improvement of gamete quality and its short-term storage: an approach for biotechnology in laboratory fish. Animal 9: 464-470.
Yoshizaki G., Tago Y., Takeuchi Y., Sawatari E., Kobayashi T., Takeuchi T. (2005). Green Fluorescent Protein Labeling of Primordial Germ Cells Using a Nontransgenic Method and Its Application for Germ Cell Transplantation in Salmonidae1. Biology of Reproduction 73: 88-93.
Yoshizaki G., Takeuchi Y., Kobayashi T., Takeuchi T., (2003). Fish Physiology and Biochemistry 28: 453-457.